Autotaxin Has a Negative Role in Systemic Inflammation
Abstract
:1. Introduction
2. Results
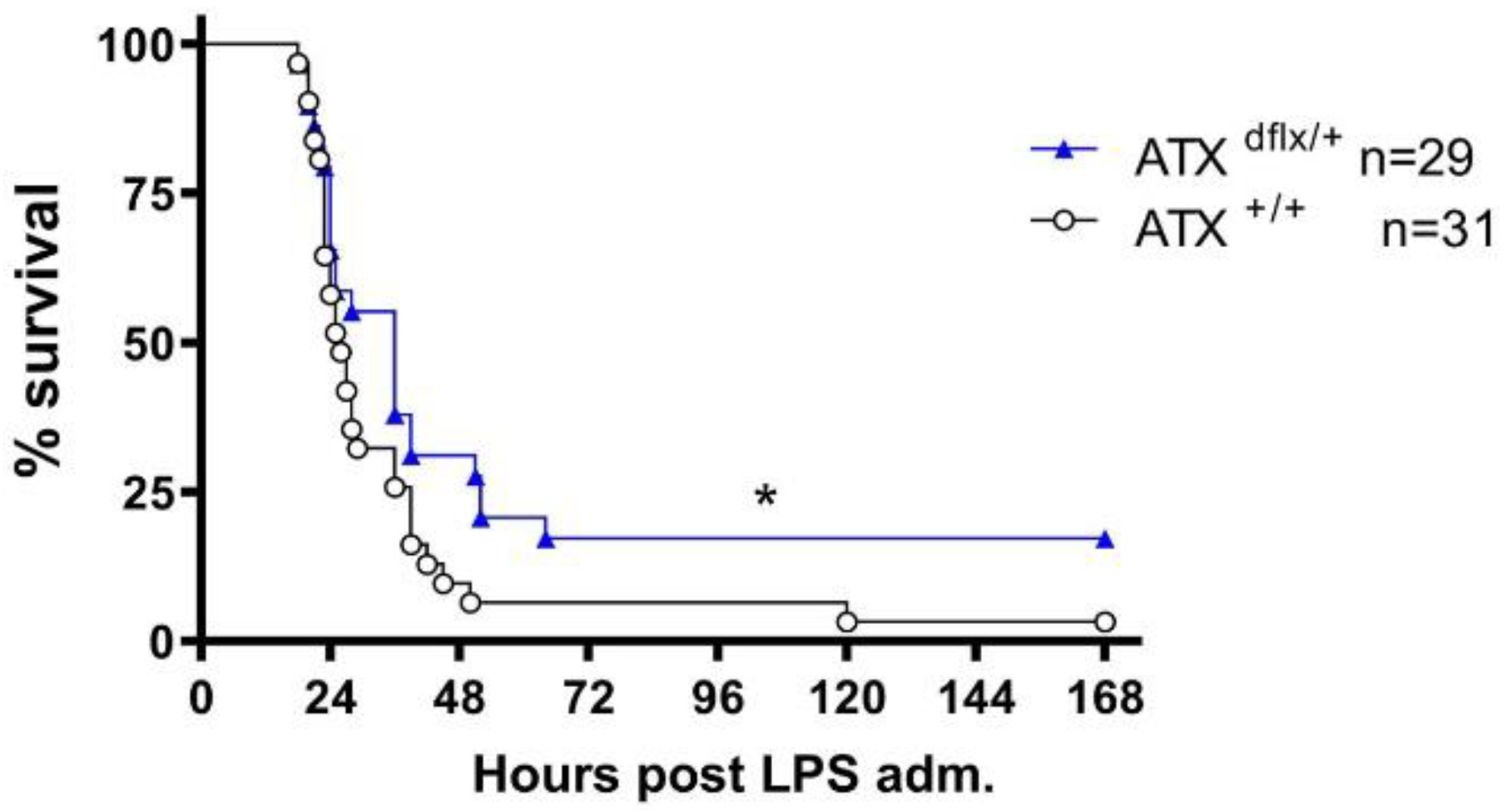
2.1. ATX Haploinsufficiency Protects Mice from LPS-Induced Sepsis
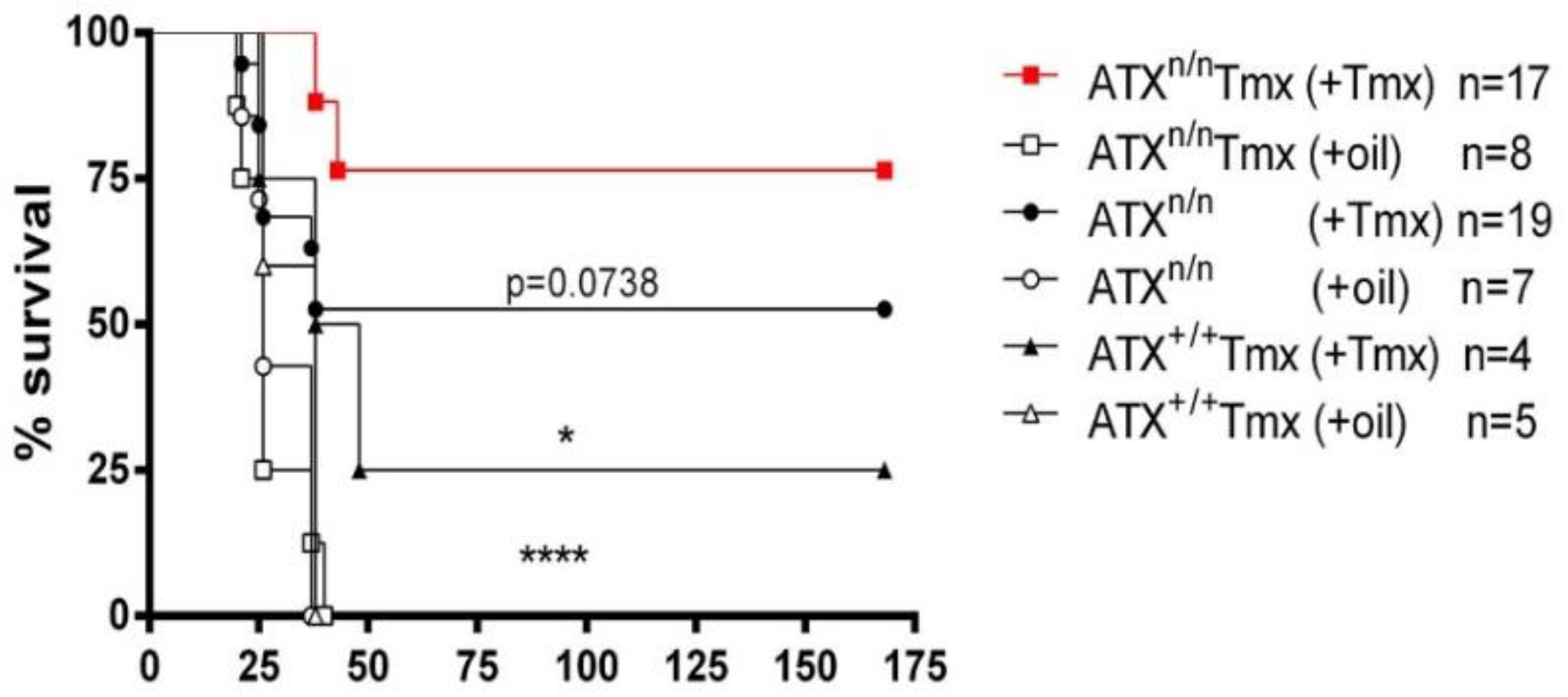
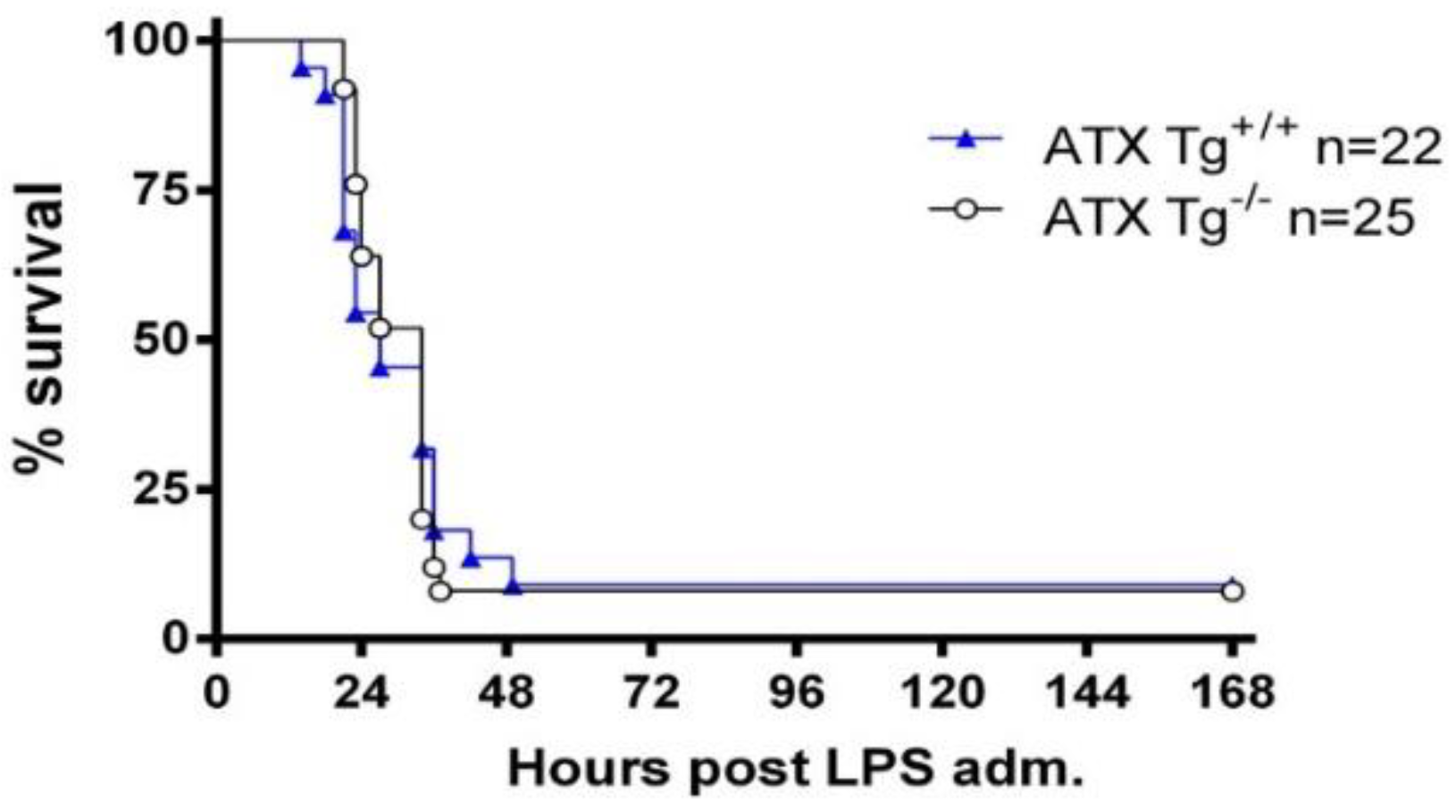
2.2. Excess Circulating ATX Levels Have No Effect on LPS-Induced Sepsis
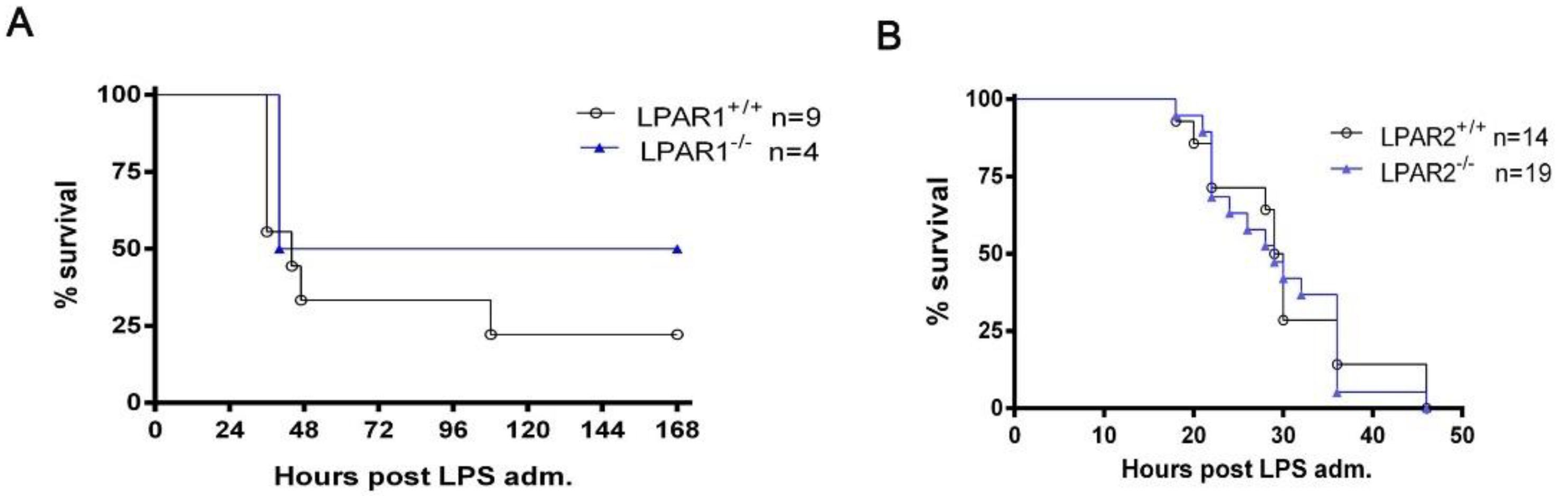
2.3. The Ubiquitous Genetic Deletion of Lpar1 and Lpar2 Has No Significant Effect on LPS-Induced Endotoxemia
2.4. Elevated ATX Levels in Critically Ill Patients with Sepsis
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Tamoxifen Treatment
4.3. Survival Study
4.4. ATX Activity Assay
4.5. Real Time RT-PCR
4.6. Human Sample Collection and Atx Measurement
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Makic, M.B.F.; Bridges, E. CE: Managing Sepsis and Septic Shock: Current Guidelines and Definitions. Am. J. Nurs. 2018, 118, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Umezu-Goto, M.; Kishi, Y.; Taira, A.; Hama, K.; Dohmae, N.; Takio, K.; Yamori, T.; Mills, G.B.; Inoue, K.; Aoki, J.; et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002, 158, 227–233. [Google Scholar] [CrossRef]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbayianni, E.; Kaffe, E.; Aidinis, V.; Kokotos, G. Autotaxin, a secreted lysophospholipase D, as a promising therapeutic target in chronic inflammation and cancer. Prog. Lipid Res. 2015, 58, 76–96. [Google Scholar] [CrossRef]
- Ninou, I.; Magkrioti, C.; Aidinis, V. Autotaxin in Pathophysiology and Pulmonary Fibrosis. Front. Med. 2018, 5, 180. [Google Scholar] [CrossRef]
- Park, G.Y.; Lee, Y.G.; Berdyshev, E.; Nyenhuis, S.; Du, J.; Fu, P.; Gorshkova, I.A.; Li, Y.; Chung, S.; Karpurapu, M.; et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am. J. Respir. Crit. Care Med. 2013, 188, 928–940. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, N.; Mouratis, M.A.; Tzouvelekis, A.; Kaffe, E.; Valavanis, C.; Vilaras, G.; Karameris, A.; Prestwich, G.D.; Bouros, D.; Aidinis, V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 566–574. [Google Scholar] [CrossRef]
- Magkrioti, C.; Galaris, A.; Kanellopoulou, P.; Stylianaki, E.A.; Kaffe, E.; Aidinis, V. Autotaxin and chronic inflammatory diseases. J. Autoimmun. 2019, 104, 102327. [Google Scholar] [CrossRef]
- Nikitopoulou, I.; Fanidis, D.; Ntatsoulis, K.; Moulos, P.; Mpekoulis, G.; Evangelidou, M.; Vassiliou, A.G.; Dimakopoulou, V.; Jahaj, E.; Tsipilis, S.; et al. Increased Autotaxin Levels in Severe COVID-19, Correlating with IL-6 Levels, Endothelial Dysfunction Biomarkers, and Impaired Functions of Dendritic Cells. Int. J. Mol. Sci. 2021, 22, 6. [Google Scholar] [CrossRef]
- Cho, W.H.; Park, T.; Park, Y.Y.; Huh, J.W.; Lim, C.M.; Koh, Y.; Song, D.K.; Hong, S.B. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Kwak, D.S.; Park, Y.Y.; Chang, Y.; Huh, J.W.; Lim, C.M.; Koh, Y.; Song, D.K.; Hong, S.B. Impact of serial measurements of lysophosphatidylcholine on 28-day mortality prediction in patients admitted to the intensive care unit with severe sepsis or septic shock. J. Crit. Care 2014, 29, 882.e5–882.e11. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.M.; Zia, R.; Napoli, S.; Wolfer, K.; Huang, X.; Morgan, P.E.; Husbyn, H.; Elgosbi, M.; Lucangeli, M.; Miquel, R.; et al. Dysregulation of the Lysophosphatidylcholine/Autotaxin/Lysophosphatidic Acid Axis in Acute-on-Chronic Liver Failure Is Associated With Mortality and Systemic Inflammation by Lysophosphatidic Acid-Dependent Monocyte Activation. Hepatology 2021, 74, 907–925. [Google Scholar] [CrossRef]
- Fotopoulou, S.; Oikonomou, N.; Grigorieva, E.; Nikitopoulou, I.; Paparountas, T.; Thanassopoulou, A.; Zhao, Z.; Xu, Y.; Kontoyiannis, D.L.; Remboutsika, E.; et al. ATX expression and LPA signalling are vital for the development of the nervous system. Dev. Biol. 2010, 339, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwenk, F.; Baron, U.; Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995, 23, 5080–5081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Meeteren, L.A.; Ruurs, P.; Stortelers, C.; Bouwman, P.; van Rooijen, M.A.; Pradere, J.P.; Pettit, T.R.; Wakelam, M.J.; Saulnier-Blache, J.S.; Mummery, C.L.; et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 2006, 26, 5015–5022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Okudaira, S.; Kishi, Y.; Ohkawa, R.; Iseki, S.; Ota, M.; Noji, S.; Yatomi, Y.; Aoki, J.; Arai, H. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 2006, 281, 25822–25830. [Google Scholar] [CrossRef] [Green Version]
- Wichterman, K.A.; Baue, A.E.; Chaudry, I.H. Sepsis and septic shock--a review of laboratory models and a proposal. J. Surg. Res. 1980, 29, 189–201. [Google Scholar] [CrossRef]
- Nikitopoulou, I.; Kampisiouli, E.; Jahaj, E.; Vassiliou, A.G.; Dimopoulou, I.; Mastora, Z.; Tsakiris, S.; Perreas, K.; Tzanela, M.; Routsi, C.; et al. Ghrelin alterations during experimental and human sepsis. Cytokine 2020, 127, 154937. [Google Scholar] [CrossRef]
- Rosier, F.; Nuñez, N.F.; Torres, M.; Loriod, B.; Rihet, P.; Pradel, L.C. Transcriptional Response in a Sepsis Mouse Model Reflects Transcriptional Response in Sepsis Patients. Int. J. Mol. Sci. 2022, 23, 821. [Google Scholar] [CrossRef]
- Katsifa, A.; Kaffe, E.; Nikolaidou-Katsaridou, N.; Economides, A.N.; Newbigging, S.; McKerlie, C.; Aidinis, V. The Bulk of Autotaxin Activity Is Dispensable for Adult Mouse Life. PLoS ONE 2015, 10, e0143083. [Google Scholar] [CrossRef] [PubMed]
- Pamuklar, Z.; Federico, L.; Liu, S.; Umezu-Goto, M.; Dong, A.; Panchatcharam, M.; Fulkerson, Z.; Berdyshev, E.; Natarajan, V.; Fang, X.; et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J. Biol. Chem. 2009, 284, 7385–7394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikitopoulou, I.; Oikonomou, N.; Karouzakis, E.; Sevastou, I.; Nikolaidou-Katsaridou, N.; Zhao, Z.; Mersinias, V.; Armaka, M.; Xu, Y.; Masu, M.; et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 2012, 209, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninou, I.; Sevastou, I.; Magkrioti, C.; Kaffe, E.; Stamatakis, G.; Thivaios, S.; Panayotou, G.; Aoki, J.; Kollias, G.; Aidinis, V. Genetic deletion of Autotaxin from CD11b+ cells decreases the severity of experimental autoimmune encephalomyelitis. PLoS ONE 2020, 15, e0226050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contos, J.J.; Fukushima, N.; Weiner, J.A.; Kaushal, D.; Chun, J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA 2000, 97, 13384–13389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulger, E.M.; Maier, R.V. Lipid mediators in the pathophysiology of critical illness. Crit. Care Med. 2000, 28, N27–N36. [Google Scholar] [CrossRef]
- Nemzek, J.A.; Hugunin, K.M.; Opp, M.R. Modeling sepsis in the laboratory: Merging sound science with animal well-being. Comp. Med. 2008, 58, 120–128. [Google Scholar]
- Korneev, K.V. Mouse Models of Sepsis and Septic Shock. Mol. Biol. 2019, 53, 704–717. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [Green Version]
- Joshi, L.; Plastira, I.; Bernhart, E.; Reicher, H.; Triebl, A.; Köfeler, H.C.; Sattler, W. Inhibition of Autotaxin and Lysophosphatidic Acid Receptor 5 Attenuates Neuroinflammation in LPS-Activated BV-2 Microglia and a Mouse Endotoxemia Model. Int. J. Mol. Sci. 2021, 22, 8519. [Google Scholar] [CrossRef]
- Kaffe, E.; Katsifa, A.; Xylourgidis, N.; Ninou, I.; Zannikou, M.; Harokopos, V.; Foka, P.; Dimitriadis, A.; Evangelou, K.; Moulas, A.N.; et al. Hepatocyte autotaxin expression promotes liver fibrosis and cancer. Hepatology 2017, 65, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Kraemer, M.; Vandra, J.; Halder, S.; Ubele, M.; Morris, A.J.; Smyth, S.S. Adipose-derived autotaxin regulates inflammation and steatosis associated with diet-induced obesity. PLoS ONE 2019, 14, e0208099. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, K.; Nzirorera, C.; Cowie, A.M.; Varghese, G.P.; Trivedi, P.; Eichmann, T.O.; Biswas, D.; Touaibia, M.; Morris, A.J.; Aidinis, V.; et al. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 2018, 59, 1805–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; He, D.; Su, Y.; Berdyshev, E.; Chun, J.; Natarajan, V.; Zhao, Y. Lysophosphatidic acid receptor 1 modulates lipopolysaccharide-induced inflammation in alveolar epithelial cells and murine lungs. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L547–L556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wei, J.; Weathington, N.; Jacko, A.M.; Huang, H.; Tsung, A.; Zhao, Y. Lysophosphatidic acid receptor 1 antagonist ki16425 blunts abdominal and systemic inflammation in a mouse model of peritoneal sepsis. Transl. Res. 2015, 166, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Sexton, T.; Chalhoub, G.; Ye, S.; Morris, W.; Annabathula, R.; Dugan, A.; Smyth, S. Autotaxin Activity Predicts 30-Day Mortality in Sepsis Patients and Correlates With Platelet Count and Vascular Dysfunction. Shock 2020, 54, 738–743. [Google Scholar] [CrossRef]
- Morris, A.J.; Panchatcharam, M.; Cheng, H.Y.; Federico, L.; Fulkerson, Z.; Selim, S.; Miriyala, S.; Escalante-Alcalde, D.; Smyth, S.S. Regulation of blood and vascular cell function by bioactive lysophospholipids. J. Thromb. Haemost. JTH 2009, 7 (Suppl. S1), 38–43. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, X.; Wang, H.; Liao, Y.; Zhou, Y.; Wang, K.; Hu, J.; Cheng, M.; Zeng, Z.; Wang, T.; et al. Autotaxin levels in serum and bronchoalveolar lavage fluid are associated with inflammatory and fibrotic biomarkers and the clinical outcome in patients with acute respiratory distress syndrome. J. Intensive Care 2021, 9, 44. [Google Scholar] [CrossRef]
- Matralis, A.N.; Afantitis, A.; Aidinis, V. Development and therapeutic potential of autotaxin small molecule inhibitors: From bench to advanced clinical trials. Med. Res. Rev. 2019, 39, 976–1013. [Google Scholar] [CrossRef]
- Ntatsoulis, K.; Karampitsakos, T.; Tsitoura, E.; Stylianaki, E.A.; Matralis, A.N.; Tzouvelekis, A.; Antoniou, K.; Aidinis, V. Commonalities Between ARDS, Pulmonary Fibrosis and COVID-19: The Potential of Autotaxin as a Therapeutic Target. Front. Immunol. 2021, 12, 687397. [Google Scholar] [CrossRef]
- Ventura, A.; Kirsch, D.G.; McLaughlin, M.E.; Tuveson, D.A.; Grimm, J.; Lintault, L.; Newman, J.; Reczek, E.E.; Weissleder, R.; Jacks, T. Restoration of p53 function leads to tumour regression in vivo. Nature 2007, 445, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Contos, J.J.; Ishii, I.; Fukushima, N.; Kingsbury, M.A.; Ye, X.; Kawamura, S.; Brown, J.H.; Chun, J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: Signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 2002, 22, 6921–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, S.; McMahon, A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Clinical Characteristics | |
|---|---|
| Number of patients (n) | 26 |
| Sex, n (%) | Male 22 (85%) Female 4 (15%) |
| Age (years, median, IQR) | 46 (24–58) |
| Comorbidities, n (%) | Hypertension 13 (50%) Diabetes 4 (15%) COPD 9 (35%) |
| Hospital stay, days (median, IQR) | 13 (7–30) |
| Hospital mortality (% n) | 15% |
| PaO2/FiO2, mmHg (mean ± SD) | 259 ± 88 |
| APACHE II score (median, IQR) | 11 (8–14) |
| SOFA score (median, IQR) | 6 (4–7) |
| CRP (mg/dL) (median, IQR) | 8 (3–24) |
| White blood cell count (per μL) (median, IQR) | 6000 (3800–7000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitopoulou, I.; Katsifa, A.; Kanellopoulou, P.; Jahaj, E.; Vassiliou, A.G.; Mastora, Z.; Dimopoulou, I.; Orfanos, S.E.; Aidinis, V.; Kotanidou, A. Autotaxin Has a Negative Role in Systemic Inflammation. Int. J. Mol. Sci. 2022, 23, 7920. https://doi.org/10.3390/ijms23147920
Nikitopoulou I, Katsifa A, Kanellopoulou P, Jahaj E, Vassiliou AG, Mastora Z, Dimopoulou I, Orfanos SE, Aidinis V, Kotanidou A. Autotaxin Has a Negative Role in Systemic Inflammation. International Journal of Molecular Sciences. 2022; 23(14):7920. https://doi.org/10.3390/ijms23147920
Chicago/Turabian StyleNikitopoulou, Ioanna, Aggeliki Katsifa, Paraskevi Kanellopoulou, Edison Jahaj, Alice G. Vassiliou, Zafeiria Mastora, Ioanna Dimopoulou, Stylianos E. Orfanos, Vassilis Aidinis, and Anastasia Kotanidou. 2022. "Autotaxin Has a Negative Role in Systemic Inflammation" International Journal of Molecular Sciences 23, no. 14: 7920. https://doi.org/10.3390/ijms23147920






