Tropism of the Novel AAVBR1 Capsid Following Subretinal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Injections
2.3. AAVs and Blocking Peptide
2.4. Imaging and Analysis
2.5. Fluorescent Immunohistochemistry: Brains, Flatmounts, and Sections
3. Results
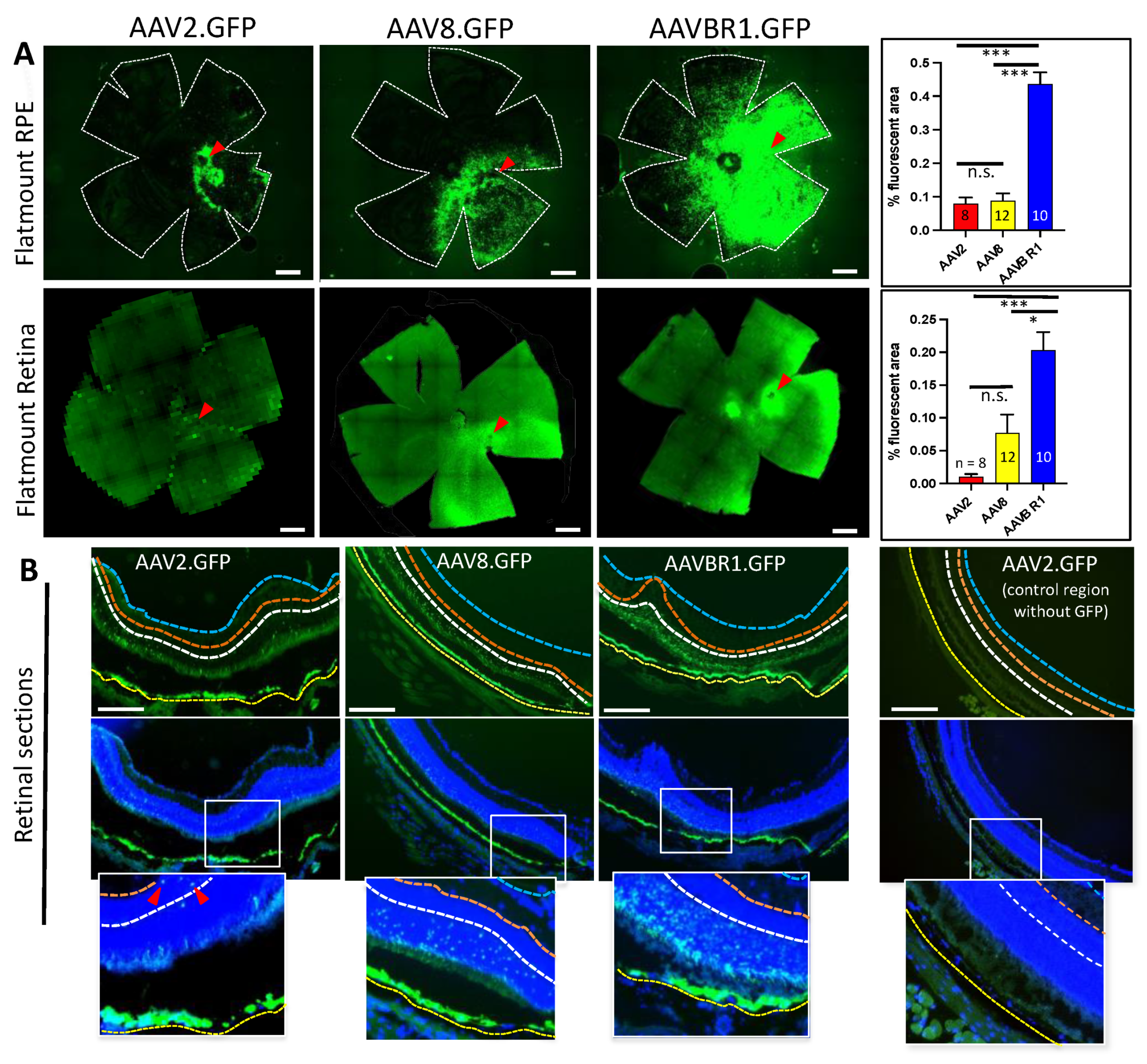
3.1. Variable Ocular Expression of AAVBR1.GFP Using Different Delivery Routes
3.2. Subretinal Injections: Time Course of Signal Expression and Transduction Efficiency
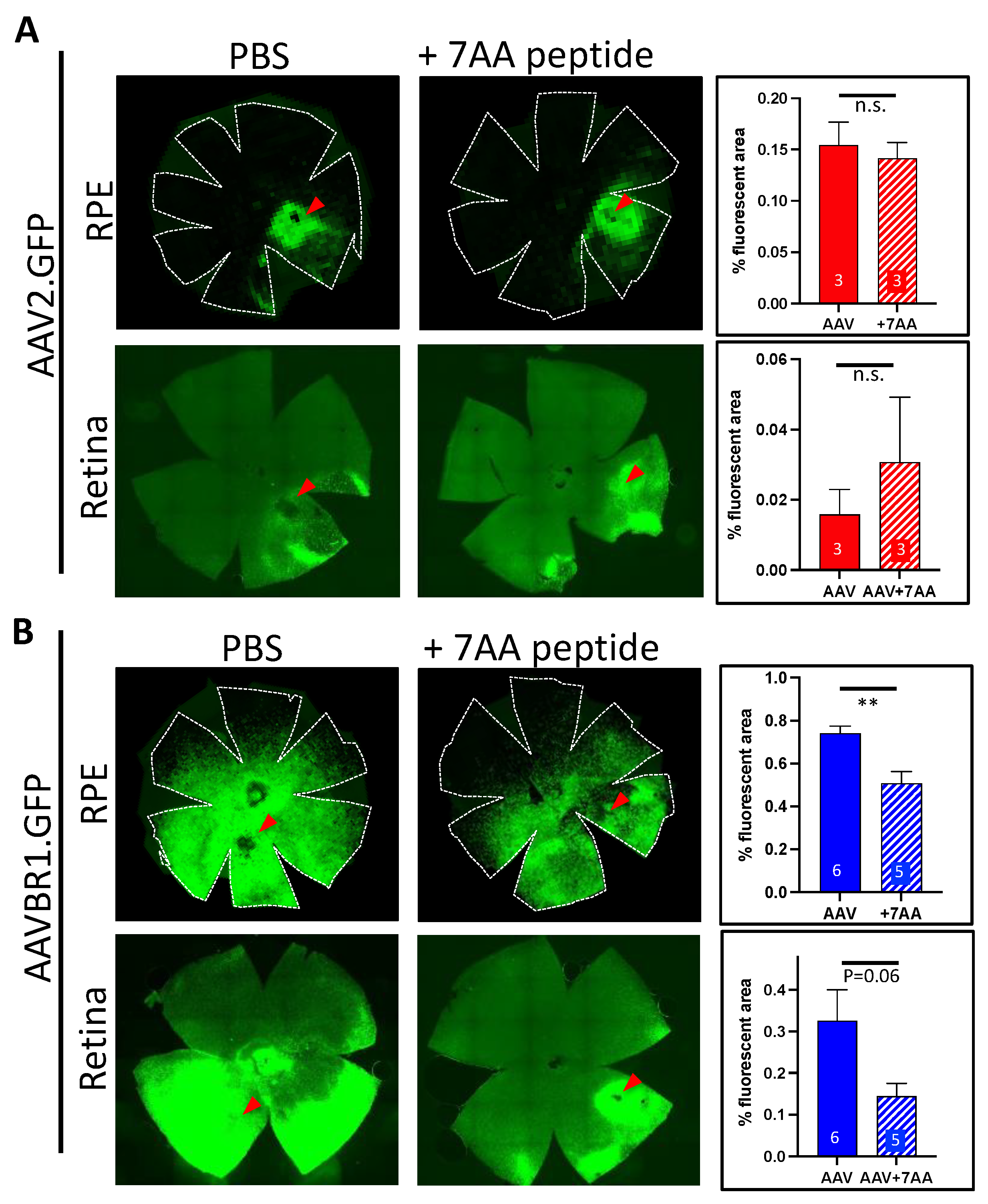
3.3. Co-Injection of the Unique AAVBR1 Peptide Motif Is Sufficient to Block AAVBR1 Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bainbridge, J.W.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of Gene Therapy on Visual Function in Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, A.R.; Mowat, F.; Bartoe, J.T.; Boye, S.L.; Hauswirth, W.; Petersen-Jones, S. Evaluation of Lateral Spread of Transgene Expression following Subretinal AAV–Mediated Gene Delivery in Dogs. PLoS ONE 2013, 8, e60218. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Yin, L.; Strazzeri, J.M.; Flannery, J.; Merigan, W.H.; Schaffer, D.V. Antibody neutralization poses a barrier to intravitreal adeno-associated viral vector gene delivery to non-human primates. Gene Ther. 2015, 22, 116–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmers, A.M.; Newmark, J.A.; Turunen, H.T.; Farivar, T.; Liu, J.; Song, C.; Ye, G.-J.; Pennock, S.; Gaskin, C.; Knop, D.R.; et al. Ocular Inflammatory Response to Intravitreal Injection of Adeno-Associated Virus Vector: Relative Contribution of Genome and Capsid. Hum. Gene Ther. 2020, 31, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Li, H.; Mingozzi, F.; Sabatino, D.E.; Hui, D.J.; Edmonson, S.A.; High, K.A. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J. Med. Virol. 2009, 81, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körbelin, J.; Dogbevia, G.; Michelfelder, S.; Ridder, D.A.; Hunger, A.; Wenzel, J.; Seismann, H.; Lampe, M.; Bannach, J.; Pasparakis, M.; et al. A brain microvasculature endothelial cell-specific viral vector with the potential to treat neurovascular and neurological diseases. EMBO Mol. Med. 2016, 8, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.; Corona, C.; Eleftheriou, C.G.; Stout, R.F., Jr.; Körbelin, J.; Sagdullaev, B.T. AAV-BR1 targets endothelial cells in the retina to reveal their morphological diversity and to deliver Cx43. J. Comp. Neurol. 2022, 530, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Khabou, H.; Cordeau, C.; Pacot, L.; Fisson, S.; Dalkara, D. Dosage Thresholds and Influence of Transgene Cassette in Adeno-Associated Virus–Related Toxicity. Hum. Gene Ther. 2018, 29, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.R.; Kompella, U.B. Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. J. Ocul. Pharmacol. Ther. 2018, 34, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Summerford, C.; Samulski, R.J. Membrane-Associated Heparan Sulfate Proteoglycan Is a Receptor for Adeno-Associated Virus Type 2 Virions. J. Virol. 1998, 72, 1438–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogbevia, G.; Grasshoff, H.; Othman, A.; Penno, A.; Schwaninger, M. Brain endothelial specific gene therapy improves experimental Sandhoff disease. J. Cereb. Blood Flow Metab. 2020, 40, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kim, S.; Huang, S.; Yoo, J.Y.; Körbelin, J.; Lee, T.J.; Kaur, B.; Dash, P.K.; Chen, P.R.; Kim, E. Selective Endothelial Hyperactivation of Oncogenic KRAS Induces Brain Arteriovenous Malformations in Mice. Ann. Neurol. 2021, 89, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.L.M.; Hede, E.; Routhe, L.J.; Körbelin, J.; Helgudottir, S.S.; Thomsen, L.B.; Schwaninger, M.; Burkhart, A.; Moos, T. A novel strategy for delivering N iemann-Pick type C2 proteins across the blood–brain barrier using the brain endothelial-specific AAV-BR1 virus. J. Neurochem. 2022, in press. [CrossRef]
- Anand, V.; Duffy, B.; Yang, Z.; Dejneka, N.S.; Maguire, A.M.; Bennett, J. A Deviant Immune Response to Viral Proteins and Transgene Product Is Generated on Subretinal Administration of Adenovirus and Adeno-associated Virus. Mol.Ther. J. Am. Soc. Gene Ther. 2002, 5, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.E.; Broderick, C.A.; Robbie, S.J.; Duran, Y.; Natkunarajah, M.; Buch, P.; Balaggan, K.S.; MacLaren, R.E.; Bainbridge, J.W.B.; Smith, A.J.; et al. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene Med. 2009, 11, 486–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Miller, R.; Han, P.-Y.; Pang, J.; Dinculescu, A.; Chiodo, V.; Hauswirth, W.W. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 2008, 14, 1760–1769. [Google Scholar] [PubMed]
- Xiong, W.; Wu, D.M.; Xue, Y.; Wang, S.K.; Chung, M.J.; Ji, X.; Rana, P.; Zhao, S.R.; Mai, S.; Cepko, C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA 2019, 116, 5785–5794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palfi, A.; Chadderton, N.; Millington-Ward, S.; Post, I.; Humphries, P.; Kenna, P.F.; Farrar, G.J. AAV-PHP.eB transduces both the inner and outer retina with high efficacy in mice. Mol. Ther. Methods Clin. Dev. 2022, 25, 236–249. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carroll, L.; Uehara, H.; Zhang, X.; Ambati, B. Tropism of the Novel AAVBR1 Capsid Following Subretinal Delivery. Int. J. Mol. Sci. 2022, 23, 7738. https://doi.org/10.3390/ijms23147738
Carroll L, Uehara H, Zhang X, Ambati B. Tropism of the Novel AAVBR1 Capsid Following Subretinal Delivery. International Journal of Molecular Sciences. 2022; 23(14):7738. https://doi.org/10.3390/ijms23147738
Chicago/Turabian StyleCarroll, Lara, Hironori Uehara, Xiaohui Zhang, and Balamurali Ambati. 2022. "Tropism of the Novel AAVBR1 Capsid Following Subretinal Delivery" International Journal of Molecular Sciences 23, no. 14: 7738. https://doi.org/10.3390/ijms23147738





