Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces
Abstract
:1. Introduction
2. Common Lithographic Methods Used for Biopolymer Patterning
3. Description of Cell Proliferation and Differentiation Processes
3.1. Basics of Cell Proliferation
3.2. Basics of Cell Differentiation
4. Microscopic Techniques for Cell Proliferation and Differentiation Assessment and Observation
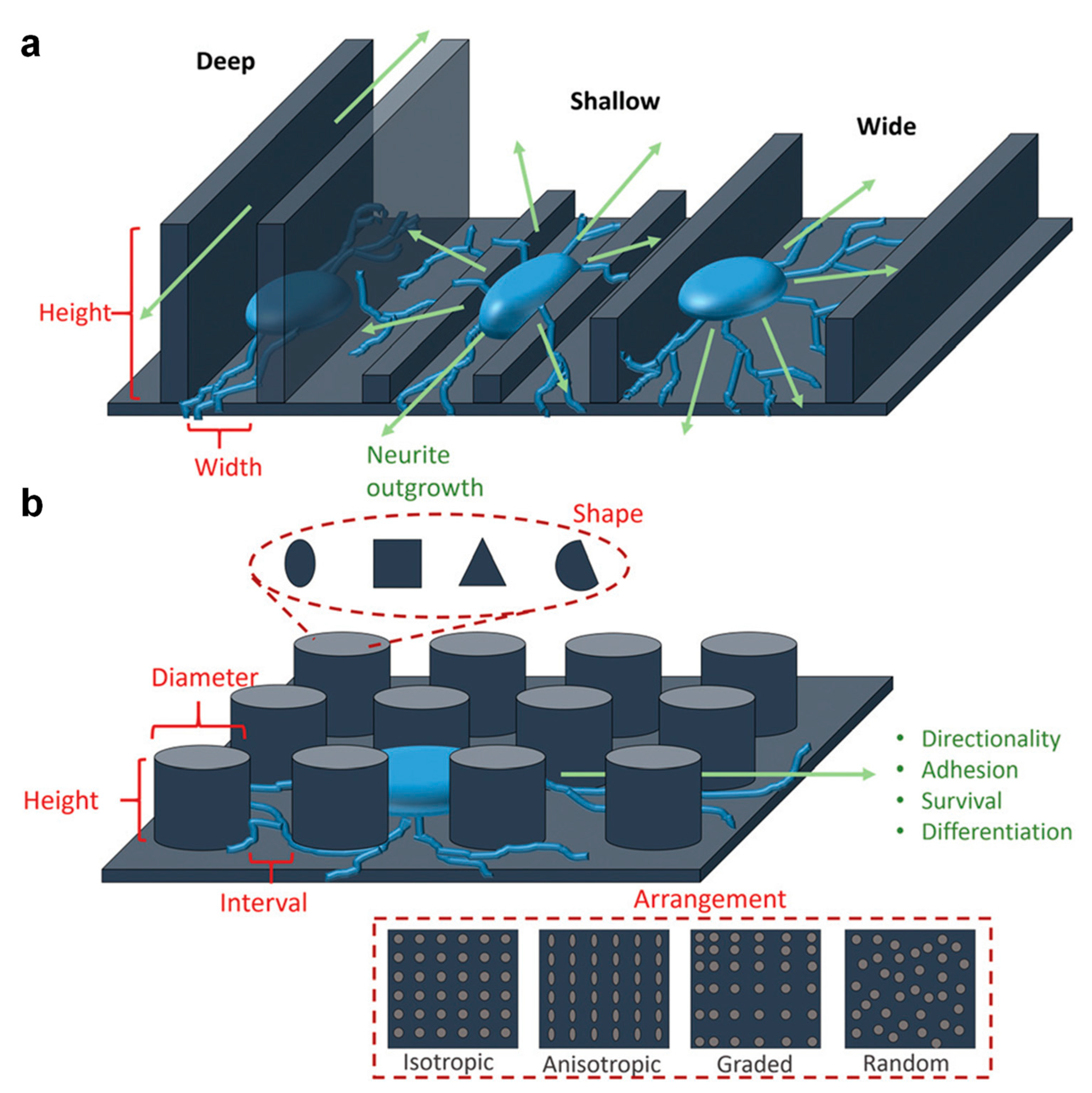
5. Insight into the Role of the Surface Topography on Cell Proliferation and Differentiation
5.1. The Impact of the Surface Topography on Cell Proliferation
5.2. The Impact of the Surface Topography on Cell Differentiation
| Surface Patterns | Material | Cell Type | Results | Ref. |
|---|---|---|---|---|
| Proliferation | ||||
| Filaments | PLLA/laminin | Rat Schwann cells and neurons from DRG | Neuron’s neurites grown on the coated filaments were longitudinally oriented and were longer than those uncoated | [130] |
| Ridge Depth: 30 µm Top width: 10 µm Base width: 2 µm | Collagen | Human corneal keratocytes and retinal pigment epithelial cells (D407 line) | Patterns changed the cytoskeletal arrangement of keratocytes, f-actin filaments being aligned longitudinally; D407 cells grow better on flat surfaces | [134] |
| Lines Width: 10–50 µm | PMMA/laminin | Rat Schwann cells | The lines oriented the cells longitudinally (smaller lines increased the orientation degree); laminin-patterned areas increased the cells adhesion, thus induced proliferation | [139] |
| Grooves Width: 10 µm | PDLA/laminin | Rat Schwann cells and neurons from sciatic DRG | Grooves provided physical guidance and laminin assured stronger adhesion that promotes proliferation | [140] |
| Filaments Diameter: 5 µm; 30 µm; 100 µm; 200 µm; 500 µm | PAN-PVC/laminin PAN-PVC/fibronectin | Rat Schwann cells and neurons from DRG | Alignment and outgrowth of neurites were most prominent on filament bundles with individual filament diameters between 5 and 30 µm for laminin and 5 µm for fibronectin | [141] |
| Grooves Width: 10/20 µm Spacing: 10/20 µm Depth: 0.5/1.5/3 µm | Silicon/laminin and sillicon/collagen type I | Rat Schwann cells | 20/20/1.5 µm grooves had the biggest impact (cells alignment). Laminin coated grooves increased the alignment and adhesion of 60% of the cells; the collagen type I coating increased the alignment and adhesion of the 51% of the cells compared to uncoated substrates | [142] |
| Anisotropic (gratings) and isotropic (dots, grids) patterns | PDMS/polylysine and laminin coating | Hippocampal neurons | Gratings promoted directional axonal growth and most enhanced axonal outgrowth | [144] |
| Grooves Width: 2 µm (P2); 10 (P10); 100 µm (P100) | PDMS/biocellulose coating | Fibroblasts | P2 and P10 grooves showed reduced migration of cells; grooves with size closer to the cell size had stronger alignment effects | [146] |
| Pores Diameter: 85–325 µm | Collagen-glycosaminoglycan | MC3T3-E1 pre-osteoblast cell line | The highest cell proliferation rate was on largest pore size | [150] |
| Proliferation and differentiation | ||||
| Pits Diameter: 6–20 µm Spacing: 6–23 µm | PDMS/biocellulose coating | Human dermal fibroblasts and macrophages | The most enhanced reduction of adhesion rate was given by pits with diameter of 5 µm and 10 µm distance between pits. Also, pits with same sizes showed the higher reduction of cell differentiation | [148] |
| Differentiation | ||||
| Aligned fibers | Collagen I and heparin | Human MSCs; C2C12 myoblasts cell line | The alignment of the collagen fibers and the addition of heparin didn’t have any effect on the adipogenic differentiation of MSCs; instead, the aligned fibers promoted skeletal muscle morphogenesis | [158] |
| Grooves Width: 10/10 µm; 20/20 µm; 30/30 µm; 50/50 µm | Chitosan | Schwann cells from lumbar dorsal root and sciatic nerves of rats | Schwann cells on 30/30 µm patterns kept orientationally growth and increased proliferation compared to cells seeded on the other patterns size | [61] |
| Spots Diameter: 500 µm | Fibronectin and collagen I (mixture) coating (+ growth factors) | Mouse embryonic stem cells | Stem cells cultured on substrate spots having hepatocyte growth factor exhibited hepatic differentiation and loss of pluripotency; co-culture with non-parenchymal liver cells enhanced the differentiation rate | [171] |
| Micropits Area: 3 × 3 µm2 Height: 2 or 4 µm | Fibronectin coating | C3H10T1/2 mouse MSCs line | 4 µm height micropits induced acceleration of osteogenic differentiation | [161] |
| Gold nanowires (AuNWs) based structures | Fibrin hydrogel/ AuNWs | Human amniotic MSCs | AuNWs in stiff substrate promoted osteogenic differentiation and AuNWs in soft substrate promoted chondrogenic differentiation | [162] |
| Anisotropically aligned fibers Diameter: ~891 nm | Chitosan | Human-induced pluripotent stem cells | Tenogenic differentiation through activating mechanic-signal pathway | [163] |
| Biomimetic geometry | Fibronectin coating | Human MSCs | Adipocyte mimetic geometries showed increased MSCs adipogenesis properties | [165] |
| Grooves: Width: 10/20 µm Spacing: 20 µm Depth: 3 µm | PDMS/fibronectin and gelatin coating | Human MSCs | 20 µm width grooves accelerated osteogenic differentiation | [169] |
| Aligned/fibrous scaffolds | Silk fibroin | MC3T3-E1 pre-osteoblasts | Aligned scaffold promoted cell proliferation and osteogenic differentiation | [170] |
6. Outlook and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saiz, N.; Mora-Bitria, L.; Rahman, S.; George, H.; Herder, J.P.; Garcia-Ojalvo, J.; Hadjantonakis, A.-K. Growth-Factor-Mediated Coupling between Lineage Size and Cell Fate Choice Underlies Robustness of Mammalian Development. Elife 2020, 9, e56079. [Google Scholar] [CrossRef] [PubMed]
- de Souza Santos, R. Sex and Media: Considerations for Cell Culture Studies. ALTEX 2018, 35, 435–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Cavanagh, B.L.; Ahearne, M. Effect of Substrate Topography on the Regulation of Human Corneal Stromal Cells. Colloids Surf. B 2020, 190, 110971. [Google Scholar] [CrossRef] [PubMed]
- Rusen, L.; Cazan, M.; Mustaciosu, C.; Filipescu, M.; Sandel, S.; Zamfirescu, M.; Dinca, V.; Dinescu, M. Tailored Topography Control of Biopolymer Surfaces by Ultrafast Lasers for Cell–Substrate Studies. Appl. Surf. Sci. 2014, 302, 256–261. [Google Scholar] [CrossRef]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical Aspects of Cell Culture Substrates: Topography, Roughness, and Elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [Green Version]
- Arango, M.-T.; Quintero-Ronderos, P.; Castiblanco, J.; Montoya-Ortíz, G. Cell Culture and Cell Analysis. In Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013; ISBN 9789587383768. [Google Scholar]
- Malm, M.; Saghaleyni, R.; Lundqvist, M.; Giudici, M.; Chotteau, V.; Field, R.; Varley, P.G.; Hatton, D.; Grassi, L.; Svensson, T.; et al. Evolution from Adherent to Suspension: Systems Biology of HEK293 Cell Line Development. Sci. Rep. 2020, 10, 18996. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Nara, S.; Chameettachal, S.; Ghosh, S. Precise Patterning of Biopolymers and Cells by Direct Write Technique. Mater. Technol. 2014, 29, B10–B14. [Google Scholar] [CrossRef]
- Castillejo, M.; Rebollar, E.; Oujja, M.; Sanz, M.; Selimis, A.; Sigletou, M.; Psycharakis, S.; Ranella, A.; Fotakis, C. Fabrication of Porous Biopolymer Substrates for Cell Growth by UV Laser: The Role of Pulse Duration. Appl. Surf. Sci. 2012, 258, 8919–8927. [Google Scholar] [CrossRef] [Green Version]
- Christopherson, G.T.; Song, H.; Mao, H.-Q. The Influence of Fiber Diameter of Electrospun Substrates on Neural Stem Cell Differentiation and Proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef]
- Sevcik, E.N.; Szymanski, J.M.; Jallerat, Q.; Feinberg, A.W. Patterning on Topography for Generation of Cell Culture Substrates with Independent Nanoscale Control of Chemical and Topographical Extracellular Matrix Cues. Curr. Protoc. Cell Biol. 2017, 75, 10.23.1–10.23.25. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From Nano to Micro: Topographical Scale and Its Impact on Cell Adhesion, Morphology and Contact Guidance. J. Phys. Condens. Matter. 2016, 28, 183001. [Google Scholar] [CrossRef]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Kurland, N.E.; Jiang, C.; Kundu, S.C.; Zhang, N.; Yadavalli, V.K. Fabrication of Precise Shape-Defined Particles of Silk Proteins Using Photolithography. Eur. Polym. J. 2016, 85, 421–430. [Google Scholar] [CrossRef]
- Zhu, S.; Zeng, W.; Meng, Z.; Luo, W.; Ma, L.; Li, Y.; Lin, C.; Huang, Q.; Lin, Y.; Liu, X.Y. Using Wool Keratin as a Basic Resist Material to Fabricate Precise Protein Patterns. Adv. Mater. 2019, 31, 1900870. [Google Scholar] [CrossRef]
- Jiang, J.; Qin, N.; Tao, T.H. Nanomanufacturing of Biopolymers Using Electron and Ion Beams. J. Micromech. Microeng. 2020, 30, 033001. [Google Scholar] [CrossRef]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef]
- Makita, R.; Akasaka, T.; Tamagawa, S.; Yoshida, Y.; Miyata, S.; Miyaji, H.; Sugaya, T. Preparation of Micro/Nanopatterned Gelatins Crosslinked with Genipin for Biocompatible Dental Implants. Beilstein J. Nanotechnol. 2018, 9, 1735–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kumar, M.; Calo, A.; Albisetti, E.; Zheng, X.; Manning, K.B.; Elacqua, E.; Weck, M.; Ulijn, R.V.; Riedo, E. Sub-10 Nm Resolution Patterning of Pockets for Enzyme Immobilization with Independent Density and Quasi-3D Topography Control. ACS Appl. Mater. Interfaces 2019, 11, 41780–41790. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Dundar, F.; Carter, K.R.; Watkins, J.J. Fabrication of Patterned Cellulose Film via Solvent-Assisted Soft Nanoimprint Lithography at a Submicron Scale. Cellulose 2018, 25, 5185–5194. [Google Scholar] [CrossRef]
- Ganesh Kumar, B.; Melikov, R.; Mohammadi Aria, M.; Ural Yalcin, A.; Begar, E.; Sadeghi, S.; Guven, K.; Nizamoglu, S. Silk-Based Aqueous Microcontact Printing. ACS Biomater. Sci. Eng. 2018, 4, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.K. Folding DNA to Create Nanoscale Shapes and Patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Xia, J.; Luo, S.; Zhang, P.; Xiao, Z.; Liu, T.; Guan, J. Versatile Surface Micropatterning and Functionalization Enabled by Microcontact Printing of Poly(4-Aminostyrene). Langmuir 2014, 30, 13483–13490. [Google Scholar] [CrossRef]
- Espinha, A.; Dore, C.; Matricardi, C.; Alonso, M.I.; Goñi, A.R.; Mihi, A. Hydroxypropyl Cellulose Photonic Architectures by Soft Nanoimprinting Lithography. Nat. Photonics 2018, 12, 343–348. [Google Scholar] [CrossRef]
- Heedy, S.; Pineda, J.J.; Meli, V.S.; Wang, S.-W.; Yee, A.F. Nanopillar Templating Augments the Stiffness and Strength in Biopolymer Films. ACS Nano 2022, 16, 3311–3322. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Pan, Z.; Liu, A.; Kaplan, D.L.; Rosenblatt, M.I. Human Corneal Limbal-Epithelial Cell Response to Varying Silk Film Geometric Topography In Vitro. Acta Biomater. 2012, 8, 3732–3743. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518. [Google Scholar] [CrossRef]
- Qian, T.; Wang, Y. Micro/Nano-Fabrication Technologies for Cell Biology. Med. Biol. Eng. Comput. 2010, 48, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harawaza, K.; Cousins, B.; Roach, P.; Fernandez, A. Modification of the Surface Nanotopography of Implant Devices: A Translational Perspective. Mater. Today Bio 2021, 12, 100152. [Google Scholar] [CrossRef] [PubMed]
- Fedele, C.; Mäntylä, E.; Belardi, B.; Hamkins-Indik, T.; Cavalli, S.; Netti, P.A.; Fletcher, D.A.; Nymark, S.; Priimagi, A.; Ihalainen, T.O. Azobenzene-Based Sinusoidal Surface Topography Drives Focal Adhesion Confinement and Guides Collective Migration of Epithelial Cells. Sci. Rep. 2020, 10, 15329. [Google Scholar] [CrossRef] [PubMed]
- De Masi, A.; Tonazzini, I.; Masciullo, C.; Mezzena, R.; Chiellini, F.; Puppi, D.; Cecchini, M. Chitosan Films for Regenerative Medicine: Fabrication Methods and Mechanical Characterization of Nanostructured Chitosan Films. Biophys. Rev. 2019, 11, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Scaccini, L.; Mezzena, R.; De Masi, A.; Gagliardi, M.; Gambarotta, G.; Cecchini, M.; Tonazzini, I. Chitosan Micro-Grooved Membranes with Increased Asymmetry for the Improvement of the Schwann Cell Response in Nerve Regeneration. Int. J. Mol. Sci. 2021, 22, 7901. [Google Scholar] [CrossRef] [PubMed]
- Yulianto, E.; Chatterjee, S.; Purlys, V.; Mizeikis, V. Imaging of Latent Three-Dimensional Exposure Patterns Created by Direct Laser Writing in Photoresists. Appl. Surf. Sci. 2019, 479, 822–827. [Google Scholar] [CrossRef]
- He, W.; Poker, D.B.; Gonsalves, K.E.; Batina, N. Micro/Nano Machining of Polymeric Substrates by Ion Beam Techniques. Microelectron Eng. 2003, 65, 153–161. [Google Scholar] [CrossRef]
- Hwang, I.-T.; Oh, M.-S.; Jung, C.-H.; Choi, J.-H. Direct Patterning of Poly(Acrylic Acid) on Polymer Surfaces by Ion Beam Lithography for the Controlled Adhesion of Mammalian Cells. Biotechnol. Lett. 2014, 36, 2135–2142. [Google Scholar] [CrossRef]
- He, C.; Feng, Z.; Shan, S.; Wang, M.; Chen, X.; Zou, G. Highly Enantioselective Photo-Polymerization Enhanced by Chiral Nanoparticles and in Situ Photopatterning of Chirality. Nat. Commun. 2020, 11, 1188. [Google Scholar] [CrossRef]
- Wu, X.; Teng, F.; Libera, M. Functional Changes during Electron-Beam Lithography of Biotinylated Poly(Ethylene Glycol) Thin Films. ACS Macro Lett. 2019, 8, 1252–1256. [Google Scholar] [CrossRef]
- Shaali, M.; Lara-Avila, S.; Dommersnes, P.; Ainla, A.; Kubatkin, S.; Jesorka, A. Nanopatterning of Mobile Lipid Monolayers on Electron-Beam-Sculpted Teflon AF Surfaces. ACS Nano 2015, 9, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Oshima, A.; Oyama, T.G.; Ito, K.; Sugahara, K.; Kashiwakura, M.; Kozawa, T.; Tagawa, S. Organic Solvent-Free Sugar-Based Transparency Nanopatterning Material Derived from Biomass for Eco-Friendly Optical Biochips Using Green Lithography. In Biophotonics: Photonic Solutions for Better Health Care IV; SPIE: Bellingham, WA, USA, 2014; p. 912917. [Google Scholar] [CrossRef]
- Darko, C.; Botiz, I.; Reiter, G.; Breiby, D.W.; Andreasen, J.W.; Roth, S.V.; Smilgies, D.-M.; Metwalli, E.; Papadakis, C.M. Crystallization in Diblock Copolymer Thin Films at Different Degrees of Supercooling. Phys. Rev. E 2009, 79, 041802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botiz, I.; Freyberg, P.; Leordean, C.; Gabudean, A.-M.; Astilean, S.; Yang, A.C.-M.; Stingelin, N. Emission Properties of MEH-PPV in Thin Films Simultaneously Illuminated and Annealed at Different Temperatures. Synth. Met. 2015, 199, 33–36. [Google Scholar] [CrossRef]
- Botiz, I.; Grozev, N.; Schlaad, H.; Reiter, G. The Influence of Protic Non-Solvents Present in the Environment on Structure Formation of Poly(γ-Benzyl-l-Glutamate) in Organic Solvents. Soft Matter 2008, 4, 993. [Google Scholar] [CrossRef]
- Botiz, I.; Astilean, S.; Stingelin, N. Altering the Emission Properties of Conjugated Polymers: Emission Properties of Conjugated Polymers. Polym. Int. 2016, 65, 157–163. [Google Scholar] [CrossRef]
- Leordean, C.; Marta, B.; Gabudean, A.-M.; Focsan, M.; Botiz, I.; Astilean, S. Fabrication of Highly Active and Cost Effective SERS Plasmonic Substrates by Electrophoretic Deposition of Gold Nanoparticles on a DVD Template. Appl. Surf. Sci. 2015, 349, 190–195. [Google Scholar] [CrossRef]
- Hawkes, W.; Huang, D.; Reynolds, P.; Hammond, L.; Ward, M.; Gadegaard, N.; Marshall, J.F.; Iskratsch, T.; Palma, M. Probing the Nanoscale Organisation and Multivalency of Cell Surface Receptors: DNA Origami Nanoarrays for Cellular Studies with Single-Molecule Control. Faraday Discuss. 2019, 219, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Dague, E.; Jauvert, E.; Laplatine, L.; Viallet, B.; Thibault, C.; Ressier, L. Assembly of Live Micro-Organisms on Microstructured PDMS Stamps by Convective/Capillary Deposition for AFM Bio-Experiments. Nanotechnology 2011, 22, 395102. [Google Scholar] [CrossRef]
- Zhu, S.; Tang, Y.; Lin, C.; Liu, X.Y.; Lin, Y. Recent Advances in Patterning Natural Polymers: From Nanofabrication Techniques to Applications. Small Methods 2021, 5, 2001060. [Google Scholar] [CrossRef]
- Humenik, M.; Winkler, A.; Scheibel, T. Patterning of Protein-based Materials. Biopolymers 2021, 112, e23412. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Z.; Zhang, S.; Shi, Z.; Tabarini, J.; Lee, W.; Zhang, Y.; Gilbert Corder, S.N.; Li, X.; Dong, F.; et al. Precise Protein Photolithography (P3): High Performance Biopatterning Using Silk Fibroin Light Chain as the Resist. Adv. Sci. 2017, 4, 1700191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurland, N.E.; Dey, T.; Wang, C.; Kundu, S.C.; Yadavalli, V.K. Silk Protein Lithography as a Route to Fabricate Sericin Microarchitectures. Adv. Mater. 2014, 26, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, S.; Kirchner, R.; Lohse, O.; Guzenko, V.A.; Voigt, A.; Harder, I.; Kolander, A.; Schift, H.; Grützner, G. mr-PosEBR: A Novel Positive Tone Resist for High Resolution Electron Beam Lithography and 3D Surface Patterning. In Advances in Patterning Materials and Processes XXXIII; SPIE: Bellingham, WA, USA, 2016; p. 977925. [Google Scholar] [CrossRef] [Green Version]
- Wieberger, F.; Kolb, T.; Neuber, C.; Ober, C.K.; Schmidt, H.-W. Nanopatterning with Tailored Molecules. In Advances in Patterning Materials and Processes XXXI; SPIE: Bellingham, WA, USA, 2014; p. 90510G. [Google Scholar] [CrossRef]
- Jiang, J.; Li, X.; Mak, W.C.; Trau, D. Integrated Direct DNA/Protein Patterning and Microfabrication by Focused Ion Beam Milling. Adv. Mater. 2008, 20, 1636–1643. [Google Scholar] [CrossRef]
- Sanchez-deAlcazar, D.; Romera, D.; Castro-Smirnov, J.; Sousaraei, A.; Casado, S.; Espasa, A.; Morant-Miñana, M.C.; Hernandez, J.J.; Rodríguez, I.; Costa, R.D.; et al. Engineered Protein-Based Functional Nanopatterned Materials for Bio-Optical Devices. Nanoscale Adv. 2019, 1, 3980–3991. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Haehnle, B.; Van der Laan, X.; Kuehne, A.J.C.; Botiz, I.; Stavrinou, P.N.; Stingelin, N. Understanding Hierarchical Spheres-in-Grating Assembly for Bio-Inspired Colouration. Mater. Horiz. 2021, 8, 2230–2237. [Google Scholar] [CrossRef]
- Jeong, H.-H.; Lee, J.-H.; Lee, C.-S.; Jang, H.; Yang, Y.-H.; Kim, Y.-H.; Huh, K.M. Fabrication of Selective Anti-Biofouling Surface for Micro/Nanopatterning of Proteins. Macromol. Res. 2010, 18, 868–875. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zhang, L.; Yang, J.; Cui, W.; Yang, Y.; Zhang, H. Anisotropic Ridge/Groove Microstructure for Regulating Morphology and Biological Function of Schwann Cells. Appl. Mater. Today 2020, 18, 100468. [Google Scholar] [CrossRef]
- MacNearney, D.; Mak, B.; Ongo, G.; Kennedy, T.E.; Juncker, D. Nanocontact Printing of Proteins on Physiologically Soft Substrates to Study Cell Haptotaxis. Langmuir 2016, 32, 13525–13533. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Jackman, J.A.; Xu, X.; Weiss, P.S.; Cho, N.-J. Micropatterned Viral Membrane Clusters for Antiviral Drug Evaluation. ACS Appl. Mater. Interfaces 2019, 11, 13984–13990. [Google Scholar] [CrossRef]
- Delamarche, E.; Pereiro, I.; Kashyap, A.; Kaigala, G.V. Biopatterning: The Art of Patterning Biomolecules on Surfaces. Langmuir 2021, 37, 9637–9651. [Google Scholar] [CrossRef]
- Liu, X.; Kumar, M.; Calo’, A.; Albisetti, E.; Zheng, X.; Manning, K.B.; Elacqua, E.; Weck, M.; Ulijn, R.V.; Riedo, E. High-Throughput Protein Nanopatterning. Faraday Discuss. 2019, 219, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Lum, W.; Gautam, D.; Chen, J.; Sagle, L.B. Single Molecule Protein Patterning Using Hole Mask Colloidal Lithography. Nanoscale 2019, 11, 16228–16234. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Park, M.; Ding, J.; Hu, H.; Zhang, Z. Sub-10 Nm Patterning with DNA Nanostructures: A Short Perspective. Nanotechnology 2017, 28, 442501. [Google Scholar] [CrossRef] [Green Version]
- Howorka, S. DNA Nanoarchitectonics: Assembled DNA at Interfaces. Langmuir 2013, 29, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shen, J.; Zhao, Z.; Arellano, N.; Rettner, C.; Tang, J.; Cao, T.; Zhou, Z.; Ta, T.; Streit, J.K.; et al. Precise Pitch-Scaling of Carbon Nanotube Arrays within Three-Dimensional DNA Nanotrenches. Science 2020, 368, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Kershner, R.J.; Bozano, L.D.; Micheel, C.M.; Hung, A.M.; Fornof, A.R.; Cha, J.N.; Rettner, C.T.; Bersani, M.; Frommer, J.; Rothemund, P.W.K.; et al. Placement and Orientation of Individual DNA Shapes on Lithographically Patterned Surfaces. Nat. Nanotechnol. 2009, 4, 557–561. [Google Scholar] [CrossRef] [Green Version]
- Weiger, T.M.; Hermann, A. Cell Proliferation, Potassium Channels, Polyamines and Their Interactions: A Mini Review. Amino Acids 2014, 46, 681–688. [Google Scholar] [CrossRef]
- Blackiston, D.J.; McLaughlin, K.A.; Levin, M. Bioelectric Controls of Cell Proliferation: Ion Channels, Membrane Voltage and the Cell Cycle. Cell Cycle 2009, 8, 3527–3536. [Google Scholar] [CrossRef] [Green Version]
- Hallstrom, T.C.; Nevins, J.R. Balancing the Decision of Cell Proliferation and Cell Fate. Cell Cycle 2009, 8, 532–535. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Kakui, Y.; Toya, M. Tell the Difference Between Mitosis and Meiosis: Interplay Between Chromosomes, Cytoskeleton, and Cell Cycle Regulation. Front. Cell Dev. Biol. 2021, 9, 660322. [Google Scholar] [CrossRef]
- Fischer, M.; Dang, C.V.; DeCaprio, J.A. Control of Cell Division. In Hematology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–185. ISBN 978-0-323-35762-3. [Google Scholar]
- Bagga, S.; Bouchard, M.J. Cell Cycle Regulation During Viral Infection. In Cell Cycle Control; Methods in Molecular Biology; Noguchi, E., Gadaleta, M.C., Eds.; Springer: New York, NY, USA, 2014; Volume 1170, pp. 165–227. ISBN 978-1-4939-0887-5. [Google Scholar]
- Coller, H.A. Regulation of Cell Cycle Entry and Exit: A Single Cell Perspective. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 317–344. ISBN 978-0-470-65071-4. [Google Scholar]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The Cell Cycle in Stem Cell Proliferation, Pluripotency and Differentiation. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- John, W.; Baynes, M.; Dominiczak, H. Medical Biochemistry, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 0-7020-7299-0. [Google Scholar]
- Lents, N.H.; Baldassare, J.J. Cyclins and Cyclin-Dependent Kinases. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 423–431. ISBN 978-0-12-394796-3. [Google Scholar]
- Yang, H.W.; Cappell, S.D.; Jaimovich, A.; Liu, C.; Chung, M.; Daigh, L.H.; Pack, L.R.; Fan, Y.; Regot, S.; Covert, M.; et al. Stress-Mediated Exit to Quiescence Restricted by Increasing Persistence in CDK4/6 Activation. Elife 2020, 9, e44571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntosh, J.R. Mitosis. Cold Spring Harb. Perspect. Biol. 2016, 8, a023218. [Google Scholar] [CrossRef] [Green Version]
- Ha, G.-H.; Breuer, E.-K.Y. Mitotic Kinases and P53 Signaling. Biochem. Res. Int. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, M.; Aoki, K.; Ebisuya, M.; Matsumura, S.; Yamamoto, T.; Matsuda, M.; Toyoshima, F.; Nishida, E. Revolving Movement of a Dynamic Cluster of Actin Filaments during Mitosis. J. Cell Biol. 2010, 191, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbury, C.L. Anaphase A: Disassembling Microtubules Move Chromosomes toward Spindle Poles. Biology 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Weaver, B.A.A.; Cleveland, D.W. Decoding the Links between Mitosis, Cancer, and Chemotherapy: The Mitotic Checkpoint, Adaptation, and Cell Death. Cancer Cell 2005, 8, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, A.; Ahmadi, H.; Ghasrodashti, Z.P.; Tanide, N.; Shahriarirad, R.; Erfani, A.; Ranjbar, K.; Ashkani-Esfahani, S. Therapeutic Effects of Stem Cells in Different Body Systems, a Novel Method That Is yet to Gain Trust: A Comprehensive Review. Bosn. J. Basic Med. Sci. 2021, 21, 672. [Google Scholar] [CrossRef]
- Yang, B.; Qiu, Y.; Zhou, N.; Ouyang, H.; Ding, J.; Cheng, B.; Sun, J. Application of Stem Cells in Oral Disease Therapy: Progresses and Perspectives. Front. Physiol. 2017, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Li, S.; Le, W. Nanomaterials Modulate Stem Cell Differentiation: Biological Interaction and Underlying Mechanisms. J. Nanobiotechnol. 2017, 15, 75. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, H.; Sahebkar, A.; Sichani, L.S.; Moridikia, A.; Nazari, S.; Sadri Nahand, J.; Salehi, H.; Stenvang, J.; Masoudifar, A.; Mirzaei, H.R.; et al. Therapeutic Application of Multipotent Stem Cells. J. Cell Physiol. 2018, 233, 2815–2823. [Google Scholar] [CrossRef]
- Carpenedo, R.L.; McDevitt, T.C. Stem Cells. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 487–495. ISBN 978-0-12-374626-9. [Google Scholar]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2016, 2016, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Raza, Y.; Salman, H.; Luberto, C. Sphingolipids in Hematopoiesis: Exploring Their Role in Lineage Commitment. Cells 2021, 10, 2507. [Google Scholar] [CrossRef]
- Hanna, H.; Mir, L.M.; Andre, F.M. In Vitro Osteoblastic Differentiation of Mesenchymal Stem Cells Generates Cell Layers with Distinct Properties. Stem Cell Res. Ther. 2018, 9, 203. [Google Scholar] [CrossRef]
- van der Sanden, B.; Dhobb, M.; Berger, F.; Wion, D. Optimizing Stem Cell Culture. J. Cell. Biochem. 2010, 111, 801–807. [Google Scholar] [CrossRef]
- Prokhorovich, M.A.; Lagar’kova, M.A.; Shilov, A.G.; Karamysheva, T.V.; Kiselyov, S.L.; Rubtsov, N.B. Cultures of HESM Human Embryonic Stem Cells: Chromosomal Aberrations and Karyotype Stability. Bull. Exp. Biol. Med. 2007, 144, 126–129. [Google Scholar] [CrossRef]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rao, B.; Zandstra, P. Culture Development for Human Embryonic Stem Cell Propagation: Molecular Aspects and Challenges. Curr. Opin. Biotechnol. 2005, 16, 568–576. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Cell Culture Medium Composition and Translational Adult Bone Marrow-Derived Stem Cell Research. Stem Cells 2006, 24, 1409–1410. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-Cell Culture Media: History, Characteristics, and Current Issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaljaničová, I.; Slepička, P.; Heitz, J.; Barb, R.A.; Sajdl, P.; Švorčík, V. Comparison of KrF and ArF Excimer Laser Treatment of Biopolymer Surface. Appl. Surf. Sci. 2015, 339, 144–150. [Google Scholar] [CrossRef]
- Khare, T.; Oak, U.; Shriram, V.; Verma, S.K.; Kumar, V. Biologically Synthesized Nanomaterials and Their Antimicrobial Potentials. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 263–289. ISBN 978-0-12-821320-9. [Google Scholar]
- Benninger, R.K.P.; Piston, D.W. Two-Photon Excitation Microscopy for the Study of Living Cells and Tissues. Curr. Protoc. Cell Biol. 2013, 4, 4.11.1–4.11.24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorn, K. A Quick Guide to Light Microscopy in Cell Biology. Mol. Biol. Cell. 2016, 27, 219–222. [Google Scholar] [CrossRef]
- Daskalova, A.; Nathala, C.S.R.; Kavatzikidou, P.; Ranella, A.; Szoszkiewicz, R.; Husinsky, W.; Fotakis, C. FS Laser Processing of Bio-Polymer Thin Films for Studying Cell-to-Substrate Specific Response. Appl. Surf. Sci. 2016, 382, 178–191. [Google Scholar] [CrossRef]
- Inoué, S. Foundations of Confocal Scanned Imaging in Light Microscopy. In Handbook Of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: Boston, MA, USA, 2006; pp. 1–19. ISBN 978-0-387-45524-2. [Google Scholar]
- Ragazzi, M.; Piana, S.; Longo, C.; Castagnetti, F.; Foroni, M.; Ferrari, G.; Gardini, G.; Pellacani, G. Fluorescence Confocal Microscopy for Pathologists. Mod. Pathol. 2014, 27, 460–471. [Google Scholar] [CrossRef]
- Slepička, P.; Michaljaničová, I.; Rimpelová, S.; Švorčík, V. Surface Roughness in Action–Cells in Opposition. Mater. Sci. Eng. C 2017, 76, 818–826. [Google Scholar] [CrossRef]
- Ye, C.; Nikolov, S.V.; Calabrese, R.; Dindar, A.; Alexeev, A.; Kippelen, B.; Kaplan, D.L.; Tsukruk, V.V. Self-(Un)Rolling Biopolymer Microstructures: Rings, Tubules, and Helical Tubules from the Same Material. Angew. Chem. Int. Ed. 2015, 54, 8490–8493. [Google Scholar] [CrossRef]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Dalvi, Y.B.; Rehman, S.R.U.; Varghese, R.; Unni, R.N.; Yalcin, H.C.; Alfkey, R.; Thomas, S.; Al Moustafa, A.-E. Growth Factor Loaded in Situ Photocrosslinkable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Gelatin Methacryloyl Hybrid Patch for Diabetic Wound Healing. Mater. Sci. Eng. C 2021, 118, 111519. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Moore, K.M.; Ainslie, K.M.; Yadavalli, V.K. Flexible, Microstructured Surfaces Using Chitin-Derived Biopolymers. J. Mater. Chem. B 2019, 7, 5328–5335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estevam-Alves, R.; Ferreira, P.H.D.; Coatrini, A.C.; Oliveira, O.N.; Fontana, C.R.; Mendonca, C.R. Femtosecond Laser Patterning of the Biopolymer Chitosan for Biofilm Formation. Int. J. Mol. Sci. 2016, 17, 1243. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.I.; Vasconcelos, N.L.; Oliveira, S.M.; Ruiz-Molina, D.; Mano, J.F. High-Throughput Topographic, Mechanical, and Biological Screening of Multilayer Films Containing Mussel-Inspired Biopolymers. Adv. Funct. Mater. 2016, 26, 2745–2755. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Ning, L.; Chen, X. Bioprinting of Vascularized Tissue Scaffolds: Influence of Biopolymer, Cells, Growth Factors, and Gene Delivery. J. Healthc Eng. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Sahu, V.; Marichi, R.B.; Singh, G.; Sharma, R.K. Multifunctional, Self-Activating Oxygen-Rich Holey Carbon Monolith Derived from Agarose Biopolymer. ACS Sustain. Chem. Eng. 2017, 5, 8747–8755. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K.; Wu, S. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation To Promote Bacteria-Accompanied Wound Healing. ACS Nano 2018, 12, 1747–1759. [Google Scholar] [CrossRef]
- Cheng, L.; Yao, B.; Hu, T.; Cui, X.; Shu, X.; Tang, S.; Wang, R.; Wang, Y.; Liu, Y.; Song, W.; et al. Properties of an Alginate-Gelatin-Based Bioink and Its Potential Impact on Cell Migration, Proliferation, and Differentiation. Int. J. Biol. Macromol. 2019, 135, 1107–1113. [Google Scholar] [CrossRef]
- Neznalová, K.; Fajstavr, D.; Rimpelová, S.; Kasálková, N.S.; Kolská, Z.; Švorčík, V.; Slepička, P. Honeycomb-Patterned Poly(L-Lactic) Acid on Plasma-Activated FEP as Cell Culture Scaffold. Polym. Degrad. Stab. 2020, 181, 109370. [Google Scholar] [CrossRef]
- Michaljaničová, I.; Slepička, P.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Regular Pattern Formation on Surface of Aromatic Polymers and Its Cytocompatibility. Appl. Surf. Sci. 2016, 370, 131–141. [Google Scholar] [CrossRef]
- Fajstavrová, K.; Rimpelová, S.; Fajstavr, D.; Švorčík, V.; Slepička, P. PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth. Polymers 2020, 12, 2436. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Ermis, M.; Demirci, U.; Hasirci, N.; Hasirci, V. Square Prism Micropillars Improve Osteogenicity of Poly(Methyl Methacrylate) Surfaces. J. Mater. Sci Mater. Med. 2018, 29, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, M.; Zhang, Q.; Yong, L.; Zhang, T.; Tian, T.; Ma, Q.; Lin, S.; Zhu, B.; Cai, X. Effect of Substrate Stiffness on Proliferation and Differentiation of Periodontal Ligament Stem Cells. Cell Prolif. 2018, 51, e12478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A Comparative Study of Proliferation and Osteogenic Differentiation of Adipose-Derived Stem Cells on Akermanite and β-TCP Ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef]
- Jin, R.; Song, G.; Chai, J.; Gou, X.; Yuan, G.; Chen, Z. Effects of Concentrated Growth Factor on Proliferation, Migration, and Differentiation of Human Dental Pulp Stem Cells in Vitro. J. Tissue Eng. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D Gelatin-Chitosan Hybrid Hydrogels Combined with Human Platelet Lysate Highly Support Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. J. Tissue Eng. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural Tissue Engineering: The Influence of Scaffold Surface Topography and Extracellular Matrix Microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef]
- Rangappa, N.; Romero, A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Laminin-Coated Poly(L-Lactide) Filaments Induce Robust Neurite Growth While Providing Directional Orientation. J. Biomed. Mater. Res. 2000, 51, 625–634. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Simitzi, C.; Efstathopoulos, P.; Kourgiantaki, A.; Ranella, A.; Charalampopoulos, I.; Fotakis, C.; Athanassakis, I.; Stratakis, E.; Gravanis, A. Laser Fabricated Discontinuous Anisotropic Microconical Substrates as a New Model Scaffold to Control the Directionality of Neuronal Network Outgrowth. Biomaterials 2015, 67, 115–128. [Google Scholar] [CrossRef]
- Antmen, E.; Ermis, M.; Demirci, U.; Hasirci, V. Engineered Natural and Synthetic Polymer Surfaces Induce Nuclear Deformation in Osteosarcoma Cells. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 366–376. [Google Scholar] [CrossRef]
- Vrana, N.E.; Elsheikh, A.; Builles, N.; Damour, O.; Hasirci, V. Effect of Human Corneal Keratocytes and Retinal Pigment Epithelial Cells on the Mechanical Properties of Micropatterned Collagen Films. Biomaterials 2007, 28, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Park, S.-H.; Marchant, J.; Omenetto, F.; Kaplan, D.L. Response of Human Corneal Fibroblasts on Silk Film Surface Patterns. Macromol. Biosci. 2010, 10, 664–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Chen, G.; Liu, J.; Xia, Y.; Chen, H.; Tang, H.; Zhang, F.; Gu, N. Effect of Surface Topography and Bioactive Properties on Early Adhesion and Growth Behavior of Mouse Preosteoblast MC3T3-E1 Cells. ACS Appl. Mater. Interfaces 2014, 6, 17134–17143. [Google Scholar] [CrossRef] [PubMed]
- Nagata, I.; Kawana, A.; Nakatsuji, N. Perpendicular Contact Guidance of CNS Neuroblasts on Artificial Microstructures. Development 1993, 117, 401–408. [Google Scholar] [CrossRef]
- Simitzi, C.; Ranella, A.; Stratakis, E. Controlling the Morphology and Outgrowth of Nerve and Neuroglial Cells: The Effect of Surface Topography. Acta. Biomater. 2017, 51, 21–52. [Google Scholar] [CrossRef] [Green Version]
- Schmalenberg, K.E.; Uhrich, K.E. Micropatterned Polymer Substrates Control Alignment of Proliferating Schwann Cells to Direct Neuronal Regeneration. Biomaterials 2005, 26, 1423–1430. [Google Scholar] [CrossRef]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Micropatterned Schwann Cell–Seeded Biodegradable Polymer Substrates Significantly Enhance Neurite Alignment and Outgrowth. Tissue Eng. 2001, 7, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Tresco, P.A. Effect of Filament Diameter and Extracellular Matrix Molecule Precoating on Neurite Outgrowth and Schwann Cell Behavior on Multifilament Entubulation Bridging Devicein Vitro. J. Biomed. Mater. Res. A 2006, 76A, 626–637. [Google Scholar] [CrossRef]
- Hsu, S.; Chen, C.-Y.; Lu, P.S.; Lai, C.-S.; Chen, C.-J. Oriented Schwann Cell Growth on Microgrooved Surfaces. Biotechnol. Bioeng. 2005, 92, 579–588. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; Pêgo, A.P.; Lakke, E.A.J.F.; Deenen, M.; Marani, E.; Thomeer, R.T.W.M. Adhesion and Proliferation of Human Schwann Cells on Adhesive Coatings. Biomaterials 2004, 25, 2741–2751. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, Q.Y.; Jadhav, A.D.; Narang, A.; Qian, W.X.; Shi, P.; Pang, S.W. Large-Scale Topographical Screen for Investigation of Physical Neural-Guidance Cues. Sci. Rep. 2015, 5, 8644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Bottan, S.; Robotti, F.; Jayathissa, P.; Hegglin, A.; Bahamonde, N.; Heredia-Guerrero, J.A.; Bayer, I.S.; Scarpellini, A.; Merker, H.; Lindenblatt, N.; et al. Surface-Structured Bacterial Cellulose with Guided Assembly-Based Biolithography (GAB). ACS Nano 2015, 9, 206–219. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Robotti, F.; Bottan, S.; Fraschetti, F.; Mallone, A.; Pellegrini, G.; Lindenblatt, N.; Starck, C.; Falk, V.; Poulikakos, D.; Ferrari, A. A Micron-Scale Surface Topography Design Reducing Cell Adhesion to Implanted Materials. Sci. Rep. 2018, 8, 10887. [Google Scholar] [CrossRef] [PubMed]
- Ber, S.; Torun Köse, G.; Hasırcı, V. Bone Tissue Engineering on Patterned Collagen Films: An in Vitro Study. Biomaterials 2005, 26, 1977–1986. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast Adhesion on Biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The Interaction of Schwann Cells with Chitosan Membranes and Fibers in Vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zhao, W.; Zhang, L.; Wang, C.; Jiang, M.; Gu, X.; Yang, Y. Porous Chitosan Scaffolds with Surface Micropatterning and Inner Porosity and Their Effects on Schwann Cells. Biomaterials 2014, 35, 8503–8513. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, S.; Gliemann, H.; López-García, M.; Petershans, A.; Auernheimer, J.; Schimmel, T.; Bruns, M.; Schambony, A.; Kessler, H.; Wedlich, D. Isothiocyanate-Functionalized RGD Peptides for Tailoring Cell-Adhesive Surface Patterns. Biomaterials 2008, 29, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- binte, M.; Yusoff, N.Z.; Riau, A.K.; Yam, G.H.F.; binte Halim, N.S.H.; Mehta, J.S. Isolation and Propagation of Human Corneal Stromal Keratocytes for Tissue Engineering and Cell Therapy. Cells 2022, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; Tang, C.; Fei, Y.; Wu, H.; Ruan, D.; Paul, M.E.; Chen, X.; Yin, Z.; Heng, B.C.; et al. The Relationship between Substrate Topography and Stem Cell Differentiation in the Musculoskeletal System. Cell. Mol. Life Sci. 2019, 76, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Lanfer, B.; Seib, F.P.; Freudenberg, U.; Stamov, D.; Bley, T.; Bornhäuser, M.; Werner, C. The Growth and Differentiation of Mesenchymal Stem and Progenitor Cells Cultured on Aligned Collagen Matrices. Biomaterials 2009, 30, 5950–5958. [Google Scholar] [CrossRef]
- Tay, C.Y.; Yu, H.; Pal, M.; Leong, W.S.; Tan, N.S.; Ng, K.W.; Leong, D.T.; Tan, L.P. Micropatterned Matrix Directs Differentiation of Human Mesenchymal Stem Cells towards Myocardial Lineage. Exp. Cell Res. 2010, 316, 1159–1168. [Google Scholar] [CrossRef]
- Younesi, M.; Islam, A.; Kishore, V.; Anderson, J.M.; Akkus, O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Adv. Funct. Mater. 2014, 24, 5762–5770. [Google Scholar] [CrossRef] [Green Version]
- Seo, C.H.; Jeong, H.; Feng, Y.; Montagne, K.; Ushida, T.; Suzuki, Y.; Furukawa, K.S. Micropit Surfaces Designed for Accelerating Osteogenic Differentiation of Murine Mesenchymal Stem Cells via Enhancing Focal Adhesion and Actin Polymerization. Biomaterials 2014, 35, 2245–2252. [Google Scholar] [CrossRef]
- Hashemzadeh, H.; Allahverdi, A.; Ghorbani, M.; Soleymani, H.; Kocsis, Á.; Fischer, M.B.; Ertl, P.; Naderi-Manesh, H. Gold Nanowires/Fibrin Nanostructure as Microfluidics Platforms for Enhancing Stem Cell Differentiation: Bio-AFM Study. Micromachines 2019, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yuan, H.; Liu, H.; Chen, X.; Lu, P.; Zhu, T.; Yang, L.; Yin, Z.; Heng, B.C.; Zhang, Y.; et al. Well-Aligned Chitosan-Based Ultrafine Fibers Committed Teno-Lineage Differentiation of Human Induced Pluripotent Stem Cells for Achilles Tendon Regeneration. Biomaterials 2015, 53, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Minelli, C.; Gentleman, E.; LaPointe, V.; Patankar, S.N.; Kallivretaki, M.; Chen, X.; Roberts, C.J.; Stevens, M.M. Substrate Stiffness Affects Early Differentiation Events in Embryonic Stem Cells. Eur. Cells Mater. 2009, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Slater, J.H.; Culver, J.C.; Dickinson, M.E.; West, J.L. Biomimetic Surface Patterning Promotes Mesenchymal Stem Cell Differentiation. ACS Appl. Mater. Interfaces 2016, 8, 21883–21892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metavarayuth, K.; Sitasuwan, P.; Zhao, X.; Lin, Y.; Wang, Q. Influence of Surface Topographical Cues on the Differentiation of Mesenchymal Stem Cells in Vitro. ACS Biomater. Sci. Eng. 2016, 2, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Yang, M.; Alom Ruiz, S.; Pirone, D.; Tan, J.; Chen, C.S. Activation of ROCK by RhoA Is Regulated by Cell Adhesion, Shape, and Cytoskeletal Tension. Exp. Cell Res. 2007, 313, 3616–3623. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-K.; Yu, X.; Cohen, D.M.; Wozniak, M.A.; Yang, M.T.; Gao, L.; Eyckmans, J.; Chen, C.S. Bone Morphogenetic Protein-2-Induced Signaling and Osteogenesis Is Regulated by Cell Shape, RhoA/ROCK, and Cytoskeletal Tension. Stem Cells Dev. 2012, 21, 1176–1186. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.R.; Kang, Y.G.; Shin, J.W.; Shin, J.-W. Simultaneous Engagement of Mechanical Stretching and Surface Pattern Promotes Cardiomyogenic Differentiation of Human Mesenchymal Stem Cells. J. Biosci. Bioeng. 2017, 123, 252–258. [Google Scholar] [CrossRef]
- Ding, H.; Zhong, J.; Xu, F.; Song, F.; Yin, M.; Wu, Y.; Hu, Q.; Wang, J. Establishment of 3D Culture and Induction of Osteogenic Differentiation of Pre-Osteoblasts Using Wet-Collected Aligned Scaffolds. Mater. Sci. Eng. C 2017, 71, 222–230. [Google Scholar] [CrossRef]
- Tuleuova, N.; Lee, J.Y.; Lee, J.; Ramanculov, E.; Zern, M.A.; Revzin, A. Using Growth Factor Arrays and Micropatterned Co-Cultures to Induce Hepatic Differentiation of Embryonic Stem Cells. Biomaterials 2010, 31, 9221–9231. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, M.; Jäger, H. Current Status in the Utilization of Biobased Polymers for 3D Printing Process: A Systematic Review of the Materials, Processes, and Challenges. ACS Appl. Bio. Mater. 2021, 4, 325–369. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Moura, C.; Ascenso, R.M.T.; Amado, S.; Alves, N.; Pascoal-Faria, P. Comprehensive Review on Full Bone Regeneration through 3D Printing Approaches. In Design and Manufacturing; Yasa, E., Mhadhbi, M., Santecchia, E., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-865-5. [Google Scholar]









| Lithography | Patterned Material | Resulting Pattern | Pattern Dimension | Ref. |
|---|---|---|---|---|
| DLW | chitosan, starch | pores | μm size | [13] |
| UV light | silk protein | non-spherical particles | several μm | [18] |
| UV light | wool keratin protein | lines circular patterns crosses triangles | 2 μm/width 3 μm/diameter 3 μm/width tens of μm | [19] |
| EBL | sugar-based polymer | moth-eye patterns | 120 nm/period | [43] |
| EBL | biotinylated PEG | pads | ~10 μm | [5] |
| IBL | DNA oligonucleotides neutravidin anti-mouse IgG | line assays complex stripes-based patterns | 1–2 μm/width down to 100 nm/width | [57] |
| NIL | chitosan | lines circular pillars | 10 μm/width 500 nm/diameter | [61] [29] |
| NIL | proteins | lines | 700 nm/period | [58] |
| NIL | gelatins/genipin | grooves holes pillars | 500 nm/width 500 nm/diameter 100 nm/diameter | [22] |
| NIL | cellulose | holes lines square pillars rhombus pillars holes | 400 nm/diameter 140 nm/width 1 μm/diameter 600 nm/width 600 nm/diameter | [28] [24] |
| μCP | protein/Sylgard 527 | arrays of nanodots | 200 nm × 200 nm | [62] |
| μCP | biomolecules/poly(4-aminostyrene) | stripes pads | ~2 μm/width ~7 μm/diameter | [27] |
| μCP | silk | lines | hundreds of μm/width | [25] |
| μCP | neutravidin/biotin | arrays of nanodots | ~62 nm/diameter | [63] |
| μCP | amyloid | spider web arrays | hundreds of μm/width | [61] |
| TCSPL | enzyme | rectangles squares lines dots | 4.5 μm × 1.5 μm 100 nm ×100 nm 8–9 nm/width 8 nm/diameter | [23] |
| PL | streptavidin | patches | 15 nm/diameter | [66] |
| DNSA | DNA | squares disks five-point stars rectangles triangles | ~100 nm/diameter ~100 nm/diameter ~100 nm/diameter ~100 nm/diameter ~100 nm/diameter | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudureanu, R.; Handrea-Dragan, I.M.; Boca, S.; Botiz, I. Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces. Int. J. Mol. Sci. 2022, 23, 7731. https://doi.org/10.3390/ijms23147731
Tudureanu R, Handrea-Dragan IM, Boca S, Botiz I. Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces. International Journal of Molecular Sciences. 2022; 23(14):7731. https://doi.org/10.3390/ijms23147731
Chicago/Turabian StyleTudureanu, Raluca, Iuliana M. Handrea-Dragan, Sanda Boca, and Ioan Botiz. 2022. "Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces" International Journal of Molecular Sciences 23, no. 14: 7731. https://doi.org/10.3390/ijms23147731







