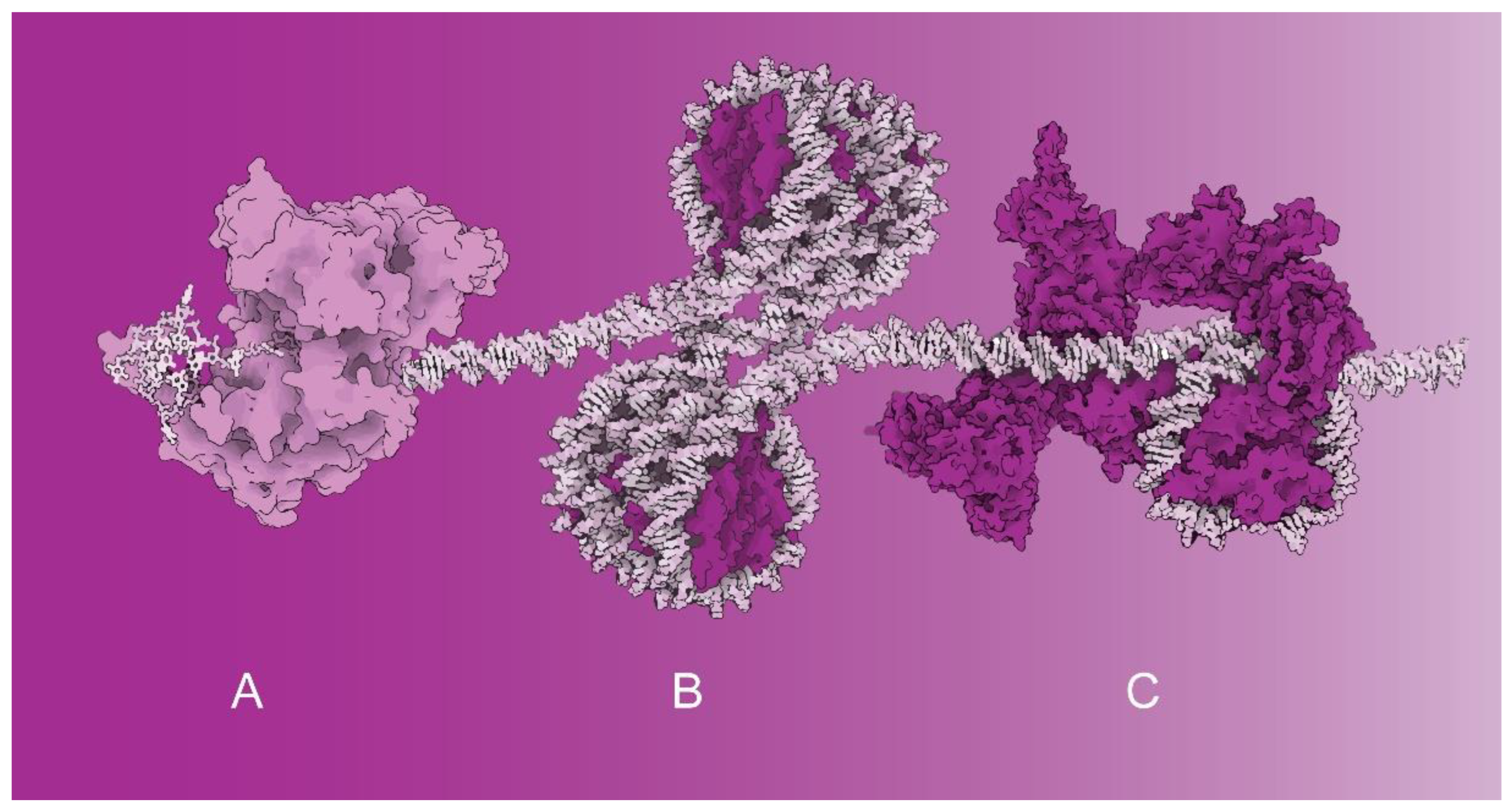
Chromatin Molecular Complexes—Functional Organization, Protection and Regulation of the Genome
1. G-Quadruplexes as Epigenetic Regulators of Transcription
2. Histone Variant H1.4 Phosphorylation—Role in Transcription Activation
3. Chromatin Remodeling in Transcription Regulation
4. Chromatin Molecular Complexes Reviewed—Structure and Compaction Perspective
Funding
Conflicts of Interest
References
- Chen, M.C.; Tippana, R.; Demeshkina, N.A.; Murat, P.; Balasubramanian, S.; Myong, S.; Ferré-D’Amaré, A.R. Structural basis of G-quadruplex unfolding by the DEAH/RHA helicase DHX36. Nature 2018, 558, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.L.; Balasubramanian, S. Prevalence of quadruplexes in the human genome. Nucleic Acids Res. 2005, 33, 2908–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasello, G.; Armenia, I.; Molla, G. The Protein Imager: A full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 2020, 36, 2909–2911. [Google Scholar] [CrossRef] [PubMed]
- Komůrková, D.; Svobodová Kovaříková, A.; Bártová, E. G-Quadruplex Structures Colocalize with Transcription Factories and Nuclear Speckles Surrounded by Acetylated and Dimethylated Histones H3. Int. J. Mol. Sci. 2021, 22, 1995. [Google Scholar] [CrossRef] [PubMed]
- Lago, S.; Nadai, M.; Cernilogar, F.M.; Kazerani, M.; Domíniguez Moreno, H.; Schotta, G.; Richter, S.N. Promoter G-quadruplexes and transcription factors cooperate to shape the cell type-specific transcriptome. Nat. Commun. 2021, 12, 3885. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, M.; Engeholm, M.; Dienemann, C.; Dodonova, S.; Cramer, P. Histone H1 binding to nucleosome arrays depends on linker DNA length and trajectory. Nat. Struct. Mol. Biol. 2022, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Seward, C.H.; Stubbs, L.; Mizzen, C.A. Site-Specific Phosphorylation of Histone H1.4 Is Associated with Transcription Activation. Int. J. Mol. Sci. 2020, 21, 8861. [Google Scholar] [CrossRef] [PubMed]
- Shidlovskii, Y.V.; Bylino, O.V.; Shaposhnikov, A.V.; Kachaev, Z.M.; Lebedeva, L.A.; Kolesnik, V.V.; Amendola, D.; De Simone, G.; Formicola, N.; Schedl, P.; et al. Subunits of the PBAP Chromatin Remodeler Are Capable of Mediating Enhancer-Driven Transcription in Drosophila. Int. J. Mol. Sci. 2021, 22, 2856. [Google Scholar] [CrossRef] [PubMed]
- Valletta, M.; Russo, R.; Baglivo, I.; Russo, V.; Ragucci, S.; Sandomenico, A.; Iaccarino, E.; Ruvo, M.; De Feis, I.; Angelini, C.; et al. Exploring the Interaction between the SWI/SNF Chromatin Remodeling Complex and the Zinc Finger Factor CTCF. Int. J. Mol. Sci. 2020, 21, 8950. [Google Scholar] [CrossRef]
- Kadoch, C.; Hargreaves, D.C.; Hodges, C.; Elias, L.; Ho, L.; Ranish, J.; Crabtree, G.R. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat. Genet. 2013, 45, 592. [Google Scholar] [CrossRef]
- Xiao, L.; Parolia, A.; Qiao, Y.; Bawa, P.; Eyunni, S.; Mannan, R.; Carson, S.E.; Chang, Y.; Wang, X.; Zhang, Y.; et al. Targeting SWI/SNF ATPases in enhancer-addicted prostate cancer. Nature 2022, 601, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Morrison, O.; Thakur, J. Molecular Complexes at Euchromatin, Heterochromatin and Centromeric Chromatin. Int. J. Mol. Sci. 2021, 22, 6922. [Google Scholar] [CrossRef] [PubMed]
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofr, C. Chromatin Molecular Complexes—Functional Organization, Protection and Regulation of the Genome. Int. J. Mol. Sci. 2022, 23, 7516. https://doi.org/10.3390/ijms23147516
Hofr C. Chromatin Molecular Complexes—Functional Organization, Protection and Regulation of the Genome. International Journal of Molecular Sciences. 2022; 23(14):7516. https://doi.org/10.3390/ijms23147516
Chicago/Turabian StyleHofr, Ctirad. 2022. "Chromatin Molecular Complexes—Functional Organization, Protection and Regulation of the Genome" International Journal of Molecular Sciences 23, no. 14: 7516. https://doi.org/10.3390/ijms23147516




