Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan
Abstract
:1. Introduction
2. Results
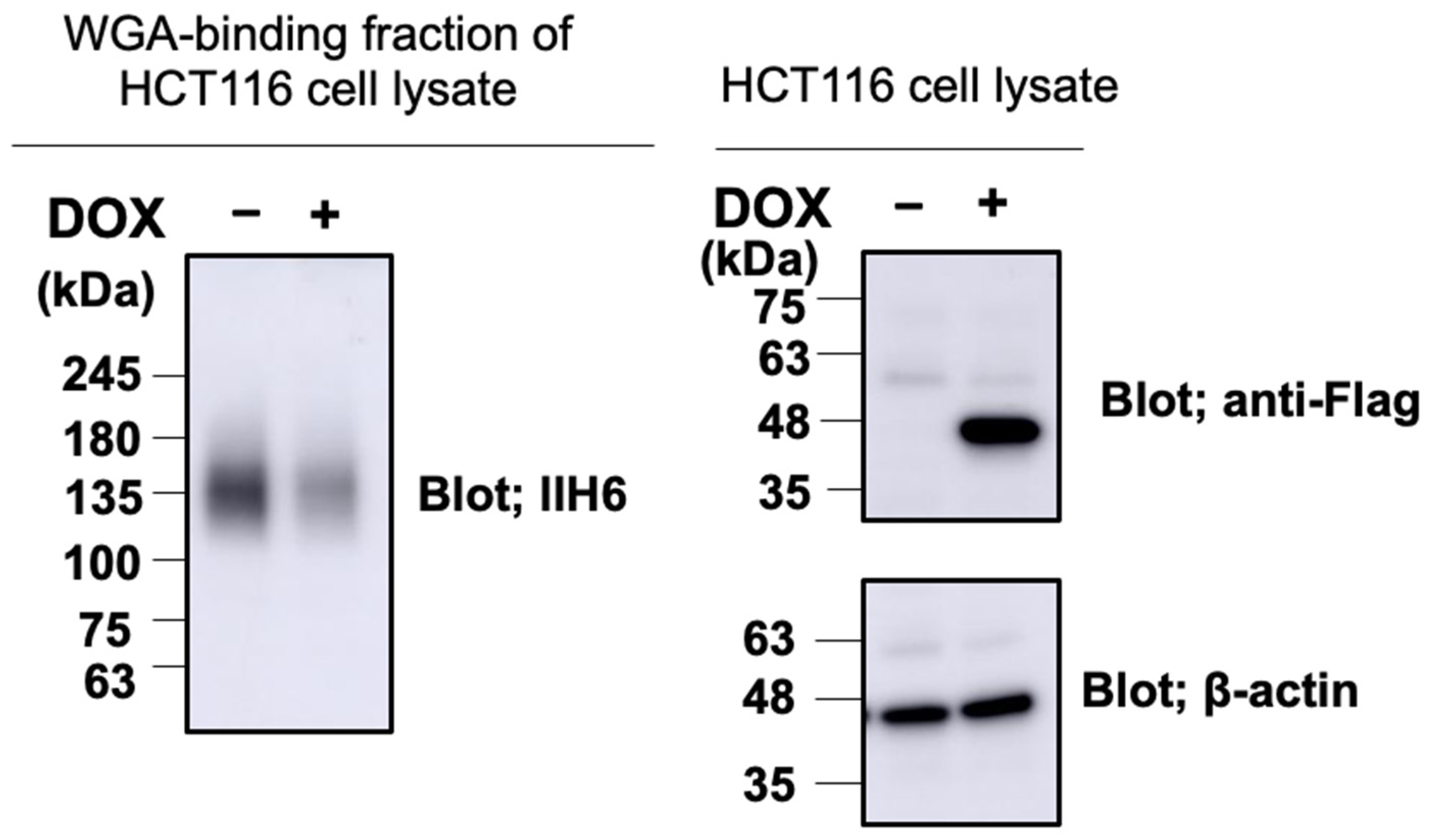
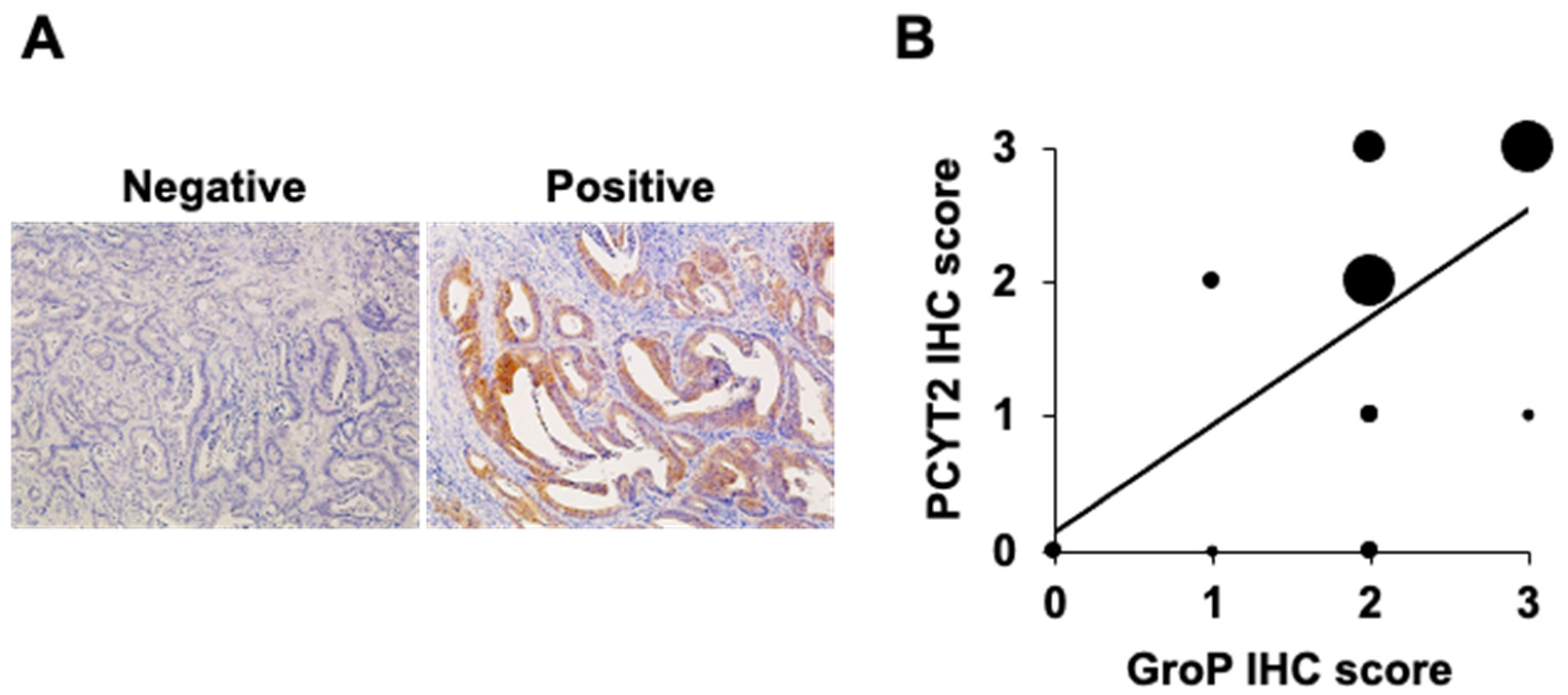
2.1. GroP Modification Is Enhanced in Cancer Tissues
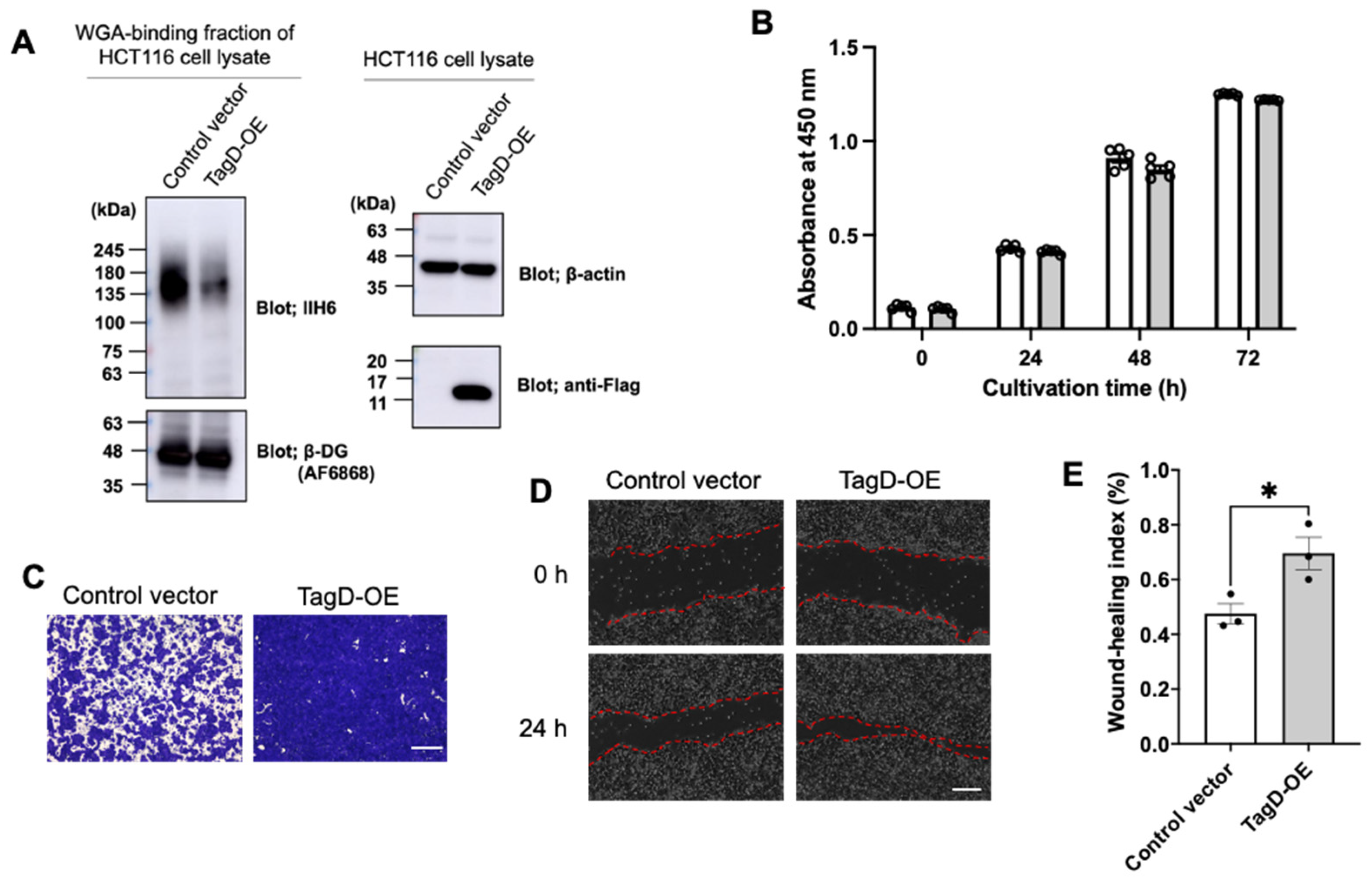
2.2. GroP Modification Promotes Cancer Migration
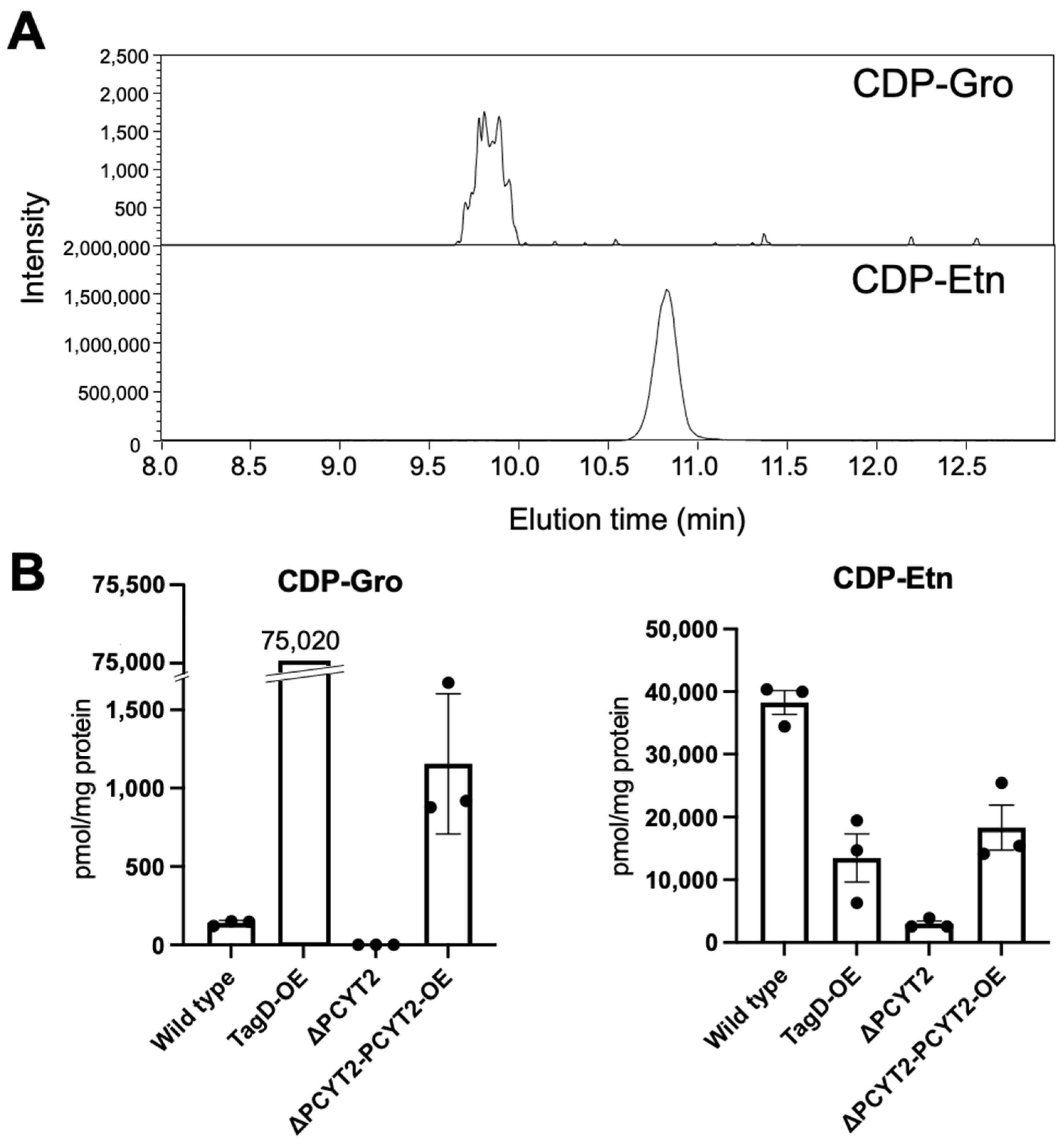
2.3. Subsubsection PCYT2 Synthesizes CDP-Gro
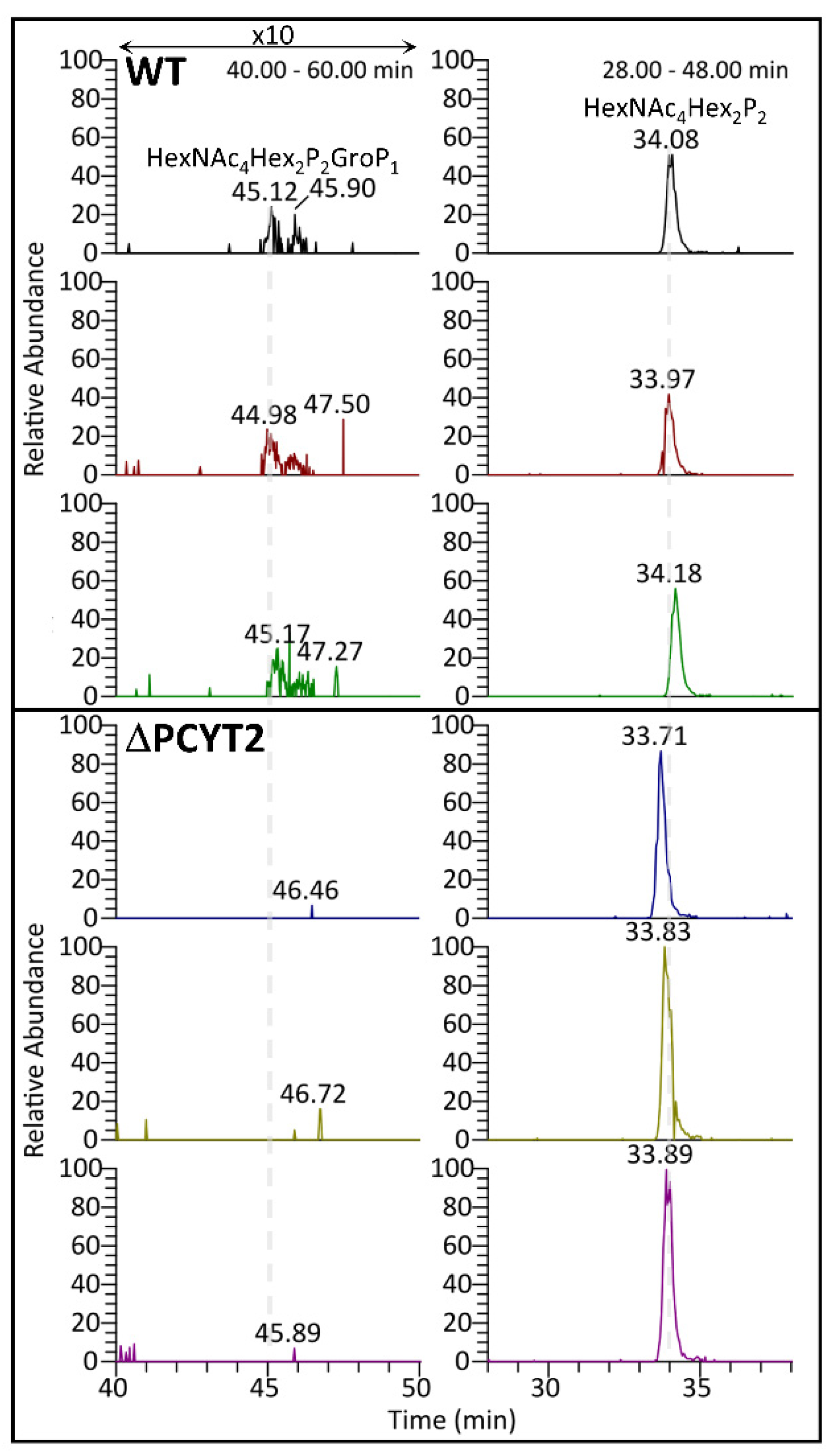
2.4. GroP Modification of α-DG Critically Depends on PCYT2
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Patients
4.3. Immunohistochemistry
4.4. cDNA Construction
4.5. Cell Culture and Transfection
4.6. Western Blot
4.7. Cell Proliferation Assay
4.8. Cell Migration Assay
4.9. Protein Expression and Purification
4.10. HPLC Analysis of In Vitro Enzymatic Reactions
4.11. CRISPR/Cas9 Genome Editing
4.12. Stable Cell Line Generation
4.13. LC-MS/MS Analysis of Nucleotide Derivatives
4.14. LC-MS/MS Analysis of Glycopeptides
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, D.A.; Cooper, C.R.; Sikes, R.A. Changes in extracellular matrix (ECM) and ECM-associated proteins in the metastatic progression of prostate cancer. Reprod. Biol. Endocrinol. 2004, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, M.; Sciandra, F.; Brancaccio, A. Role of gelatinases in pathological and physiological processes involving the dystrophin-glycoprotein complex. Matrix Biol. 2015, 44–46, 130–137. [Google Scholar] [CrossRef]
- Ervasti, J.M.; Ohlendieck, K.; Kahl, S.D.; Gaver, M.G.; Campbell, K.P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990, 345, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Kanagawa, M.; Kunz, S.; Yoshida-Moriguchi, T.; Satz, J.S.; Kobayashi, Y.M.; Zhu, Z.; Burden, S.J.; Oldstone, M.B.A.; Campbell, K.P. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 17426–17431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida-Moriguchi, T.; Campbell, K.P. Matriglycan: A novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology 2015, 25, 702–713. [Google Scholar] [CrossRef] [Green Version]
- Manya, H.; Endo, T. Glycosylation with ribitol-phosphate in mammals: New insights into the O-mannosyl glycan. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 2462–2472. [Google Scholar] [CrossRef]
- Inamori, K.; Yoshida-Moriguchi, T.; Hara, Y.; Anderson, M.E.; Yu, L.; Campbell, K.P. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science 2012, 335, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Stevens, E.; Carss, K.J.; Cirak, S.; Foley, A.R.; Torelli, S.; Willer, T.; Tambunan, D.E.; Yau, S.; Brodd, L.; Sewry, C.A.; et al. Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 2013, 92, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Nakagawa, N.; Saito, T.; Kiyonari, H.; Abe, T.; Toda, T.; Wu, S.W.; Khoo, K.H.; Oka, S.; Kato, K. AGO61-dependent GlcNAc modification primes the formation of functional glycans on α-dystroglycan. Sci. Rep. 2013, 3, 3288. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, S.; Balasubramanian, A.; Pond, H.L.; Rozkalne, A.; Pantaleoni, C.; Saredi, S.; Gupta, V.A.; Sunu, C.M.; Yu, T.W.; Kang, P.B.; et al. POMK mutations disrupt muscle development leading to a spectrum of neuromuscular presentations. Hum. Mol. Genet. 2014, 23, 5781–5792. [Google Scholar] [CrossRef] [Green Version]
- Yoshida-Moriguchi, T.; Willer, T.; Anderson, M.E.; Venzke, D.; Whyte, T.; Muntoni, F.; Lee, H.; Nelson, S.F.; Yu, L.; Campbell, K.P. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science 2013, 341, 896–899. [Google Scholar] [CrossRef] [Green Version]
- Willer, T.; Inamori, K.-I.; Venzke, D.; Harvey, C.; Morgensen, G.; Hara, Y.; Beltrán Valero de Bernabé, D.; Yu, L.; Wright, K.M.; Campbell, K.P. The glucuronyltransferase B4GAT1 is required for initiation of LARGE-mediated α-dystroglycan functional glycosylation. eLife 2014, 3, 1–24. [Google Scholar] [CrossRef]
- Praissman, J.L.; Live, D.H.; Wang, S.; Ramiah, A.; Chinoy, Z.S.; Boons, G.-J.; Moremen, K.W.; Wells, L. B4GAT1 is the priming enzyme for the LARGE-dependent functional glycosylation of α-dystroglycan. eLife 2014, 3, 1–18. [Google Scholar] [CrossRef]
- Kanagawa, M.; Kobayashi, K.; Tajiri, M.; Manya, H.; Kuga, A.; Yamaguchi, Y.; Akasaka-Manya, K.; Furukawa, J.; Mizuno, M.; Kawakami, H.; et al. Identification of a Post-translational Modification with Ribitol-Phosphate and Its Defect in Muscular Dystrophy. Cell Rep. 2016, 14, 2209–2223. [Google Scholar] [CrossRef] [Green Version]
- Manya, H.; Yamaguchi, Y.; Kanagawa, M.; Kobayashi, K.; Tajiri, M.; Akasaka-Manya, K.; Kawakami, H.; Mizuno, M.; Wada, Y.; Toda, T.; et al. The muscular dystrophy gene TMEM5 encodes a ribitol ô1,4-Xylosyltransferase required for the functional glycosylation of dystroglycan. J. Biol. Chem. 2016, 291, 24618–24627. [Google Scholar] [CrossRef] [Green Version]
- Willer, T.; Lee, H.; Lommel, M.; Yoshida-Moriguchi, T.; De Bernabe, D.B.V.; Venzke, D.; Cirak, S.; Schachter, H.; Vajsar, J.; Voit, T.; et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat. Genet. 2012, 44, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Riemersma, M.; Froese, D.S.; Van Tol, W.; Engelke, U.F.; Kopec, J.; Van Scherpenzeel, M.; Ashikov, A.; Krojer, T.; Von Delft, F.; Tessari, M.; et al. Human ISPD Is a Cytidyltransferase Required for Dystroglycan O-Mannosylation. Chem. Biol. 2015, 22, 1643–1652. [Google Scholar] [CrossRef]
- Gerin, I.; Ury, B.; Breloy, I.; Bouchet-Seraphin, C.; Bolsée, J.; Halbout, M.; Graff, J.; Vertommen, D.; Muccioli, G.G.; Seta, N.; et al. ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto α-dystroglycan. Nat. Commun. 2016, 7, 11534. [Google Scholar] [CrossRef] [Green Version]
- Yagi, H.; Kuo, C.-W.; Obayashi, T.; Ninagawa, S.; Khoo, K.-H.; Kato, K. Direct mapping of additional modifications on phosphorylated O-glycans of α-dystroglycan by mass spectrometry analysis in conjunction with knocking out of causative genes for dystroglycanopathy. Mol. Cell. Proteom. 2016, 15, 3424–3434. [Google Scholar] [CrossRef] [Green Version]
- Imae, R.; Manya, H.; Tsumoto, H.; Osumi, K.; Tanaka, T.; Mizuno, M.; Kanagawa, M.; Kobayashi, K.; Toda, T.; Endo, T. CDP-glycerol inhibits the synthesis of the functional O-mannosyl glycan of α-dystroglycan. J. Biol. Chem. 2018, 293, 12186–12198. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Yin, Y.; Zhang, Y.; Lu, L.; Li, Y.; Jiang, Y. Genomic characterization of ribitol teichoic acid synthesis in Staphylococcus aureus: Genes, genomic organization and gene duplication. BMC Genom. 2006, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Formstone, A.; Carballido-López, R.; Noirot, P.; Errington, J.; Scheffers, D.-J. Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis. J. Bacteriol. 2008, 190, 1812–1821. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, F.; Umezawa, F.; Sensui, T.; Anzo, M.; Abo, H.; Kuo, C.W.; Khoo, K.H.; Kato, K.; Yagi, H.; Kawashima, H. Establishment of a novel monoclonal antibody against truncated glycoforms of α-dystroglycan lacking matriglycans. Biochem. Biophys. Res. Commun. 2021, 579, 8–14. [Google Scholar] [CrossRef]
- Jing, J.; Lien, C.F.; Sharma, S.; Rice, J.; Brennan, P.A.; Górecki, D.C. Aberrant expression, processing and degradation of dystroglycan in squamous cell carcinomas. Eur. J. Cancer 2004, 40, 2143–2151. [Google Scholar] [CrossRef]
- Sgambato, A.; Migaldi, M.; Montanari, M.; Camerini, A.; Brancaccio, A.; Rossi, G.; Cangiano, R.; Losasso, C.; Capelli, G.; Trentini, G.P.; et al. Dystroglycan expression is frequently reduced in human breast and colon cancers and is associated with tumor progression. Am. J. Pathol. 2003, 162, 849–860. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.J.; Tucker, J.D.; Branch, E.K.; Guo, F.; Blaeser, A.R.; Lu, Q.L. Ribitol enhances matriglycan of α-dystroglycan in breast cancer cells without affecting cell growth. Sci. Rep. 2020, 10, 4935. [Google Scholar] [CrossRef]
- Muschler, J.; Levy, D.; Boudreau, R.; Henry, M.; Campbell, K.; Bissell, M.J. A role for dystroglycan in epithelial polarization: Loss of function in breast tumor cells. Cancer Res. 2002, 62, 7102–7109. [Google Scholar]
- Imperiali, M.; Thoma, C.; Pavoni, E.; Brancaccio, A.; Callewaert, N.; Oxenius, A. O Mannosylation of alpha -Dystroglycan Is Essential for Lymphocytic Choriomeningitis Virus Receptor Function. J. Virol. 2005, 79, 14297–14308. [Google Scholar] [CrossRef] [Green Version]
- Sgambato, A.; Camerini, A.; Amoroso, D.; Genovese, G.; De Luca, F.; Cecchi, M.; Migaldi, M.; Rettino, A.; Valsuani, C.; Tartarelli, G.; et al. Expression of dystroglycan correlates with tumor grade and predicts survival in renal cell carcinoma. Cancer Biol. Ther. 2007, 6, 1840–1846. [Google Scholar] [CrossRef] [Green Version]
- Coco, C.; Zannoni, G.F.; Caredda, E.; Sioletic, S.; Boninsegna, A.; Migaldi, M.; Rizzo, G.; Bonetti, L.R.; Genovese, G.; Stigliano, E.; et al. Increased expression of CD133 and reduced dystroglycan expression are strong predictors of poor outcome in colon cancer patients. J. Exp. Clin. Cancer Res. 2012, 31, 71. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Xia, X.Y.; Zhu, F.; Shen, H.; Song, K.; Shang, Z.J. Correlation of deregulated like-acetylglucosaminyl transferase and aberrant α-dystroglycan expression with human tongue cancer metastasis. J. Oral Maxillofac. Surg. 2014, 72, 1106–1118. [Google Scholar] [CrossRef]
- Sgambato, A.; Camerini, A.; Faraglia, B.; Pavoni, E.; Montanari, M.; Spada, D.; Losasso, C.; Brancaccio, A.; Cittadini, A. Increased expression of dystroglycan inhibits the growth and tumorigenicity of human mammary epithelial cells. Cancer Biol. Ther. 2004, 3, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Kobayashi, M.; Hatakeyama, S.; Angata, K.; Gullberg, D.; Nakayama, J.; Fukuda, M.N.; Fukuda, M. Tumor suppressor function of laminin-binding α-dystroglycan requires a distinct β3-N-acetylglucosaminyltransferase. Proc. Natl. Acad. Sci. USA 2009, 106, 12109–12114. [Google Scholar] [CrossRef] [Green Version]
- Bleijerveld, O.B.; Klein, W.; Vaandrager, A.B.; Helms, J.B.; Houweling, M. Control of the CDPethanolamine pathway in mammalian cells: Effect of CTP:phosphoethanolamine cytidylyltransferase overexpression and the amount of intracellular diacylglycerol. Biochem. J. 2004, 379, 711–719. [Google Scholar] [CrossRef]
- Pavlovic, Z.; Bakovic, M. Regulation of Phosphatidylethanolamine Homeostasis—The Critical Role of CTP: Phosphoethanolamine Cytidylyltransferase (Pcyt2). Int. J. Mol. Sci. 2013, 14, 2529–2550. [Google Scholar] [CrossRef] [Green Version]
- Kleene, R.; Schachner, M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004, 5, 195–208. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Oswald, D.M.; Oliva, K.D.; Kreisman, L.S.C.; Cobb, B.A. The Glycoscience of Immunity. Trends Immunol. 2018, 39, 523–535. [Google Scholar] [CrossRef]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Krautter, F.; Iqbal, A.J. Glycans and Glycan-Binding Proteins as Regulators and Potential Targets in Leukocyte Recruitment. Front. Cell Dev. Biol. 2021, 9, 122. [Google Scholar] [CrossRef]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef] [Green Version]
- Fullerton, M.D.; Hakimuddin, F.; Bakovic, M. Developmental and Metabolic Effects of Disruption of the Mouse CTP: Phosphoethanolamine Cytidylyltransferase Gene (Pcyt2). Mol. Cell. Biol. 2007, 27, 3327–3336. [Google Scholar] [CrossRef] [Green Version]
- Liou, L.Y.; Walsh, K.B.; Vartanian, A.R.; Beltran-Valero de Bernabe, D.; Welch, M.; Campbell, K.P.; Oldstone, M.B.A.; Kunz, S. Functional glycosylation of dystroglycan is crucial for thymocyte development in the mouse. PLoS ONE 2010, 5, e9915. [Google Scholar] [CrossRef] [Green Version]
- Imae, R.; Manya, H.; Tsumoto, H.; Miura, Y.; Endo, T. PCYT2 synthesizes CDP-glycerol in mammals and reduced PCYT2 enhances the expression of functionally glycosylated α-dystroglycan. J. Biochem. 2021, 170, 183–194. [Google Scholar] [CrossRef]
- Ito, J.; Herter, T.; Baidoo, E.E.K.; Lao, J.; Vega-Sánchez, M.E.; Michelle Smith-Moritz, A.; Adams, P.D.; Keasling, J.D.; Usadel, B.; Petzold, C.J.; et al. Analysis of plant nucleotide sugars by hydrophilic interaction liquid chromatography and tandem mass spectrometry. Anal. Biochem. 2014, 448, 14–22. [Google Scholar] [CrossRef]
- Harada, Y.; Nakajima, K.; Li, S.; Suzuki, T.; Taniguchi, N. Protocol for analyzing the biosynthesis and degradation of N-glycan precursors in mammalian cells. STAR Protoc. 2021, 2, 100316. [Google Scholar] [CrossRef]


| Gene | Protein | Identity | Query Cover |
|---|---|---|---|
| CDS1 | Phosphatidate cytidylyltransferase 1 | 57% | 10% |
| CDS2 | Phosphatidate cytidylyltransferase 2 | 67% | 8% |
| PCYT1A | Choline-phosphate cytidylyltransferase A | 35% | 93% |
| PCYT1B | Choline-phosphate cytidylyltransferase B | 36% | 93% |
| PCYT2 | Ethanolamine-phosphate cytidylyltransferase | 30% | 89% |
| CDP-Etn | CDP-Gro | |||
|---|---|---|---|---|
| PCYT2α | PCYT2β | PCYT2α | PCYT2β | |
| Vmax (mM/h) | 33.7 | 37.3 | 22.7 | 28.9 |
| Km (mM) | 181.2 | 171.2 | 1614 | 2899 |
| Catalytic efficiency (kcat/Km) (mM−1h−1) | 0.3530 | 0.3974 | 0.0089 | 0.0060 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umezawa, F.; Natsume, M.; Fukusada, S.; Nakajima, K.; Yamasaki, F.; Kawashima, H.; Kuo, C.-W.; Khoo, K.-H.; Shimura, T.; Yagi, H.; et al. Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan. Int. J. Mol. Sci. 2022, 23, 6662. https://doi.org/10.3390/ijms23126662
Umezawa F, Natsume M, Fukusada S, Nakajima K, Yamasaki F, Kawashima H, Kuo C-W, Khoo K-H, Shimura T, Yagi H, et al. Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan. International Journal of Molecular Sciences. 2022; 23(12):6662. https://doi.org/10.3390/ijms23126662
Chicago/Turabian StyleUmezawa, Fumiko, Makoto Natsume, Shigeki Fukusada, Kazuki Nakajima, Fumiya Yamasaki, Hiroto Kawashima, Chu-Wei Kuo, Kay-Hooi Khoo, Takaya Shimura, Hirokazu Yagi, and et al. 2022. "Cancer Malignancy Is Correlated with Upregulation of PCYT2-Mediated Glycerol Phosphate Modification of α-Dystroglycan" International Journal of Molecular Sciences 23, no. 12: 6662. https://doi.org/10.3390/ijms23126662






