Effect of Light and Dark on the Phenolic Compound Accumulation in Tartary Buckwheat Hairy Roots Overexpressing ZmLC
Abstract
:1. Introduction
2. Results
2.1. Hairy Root Induction
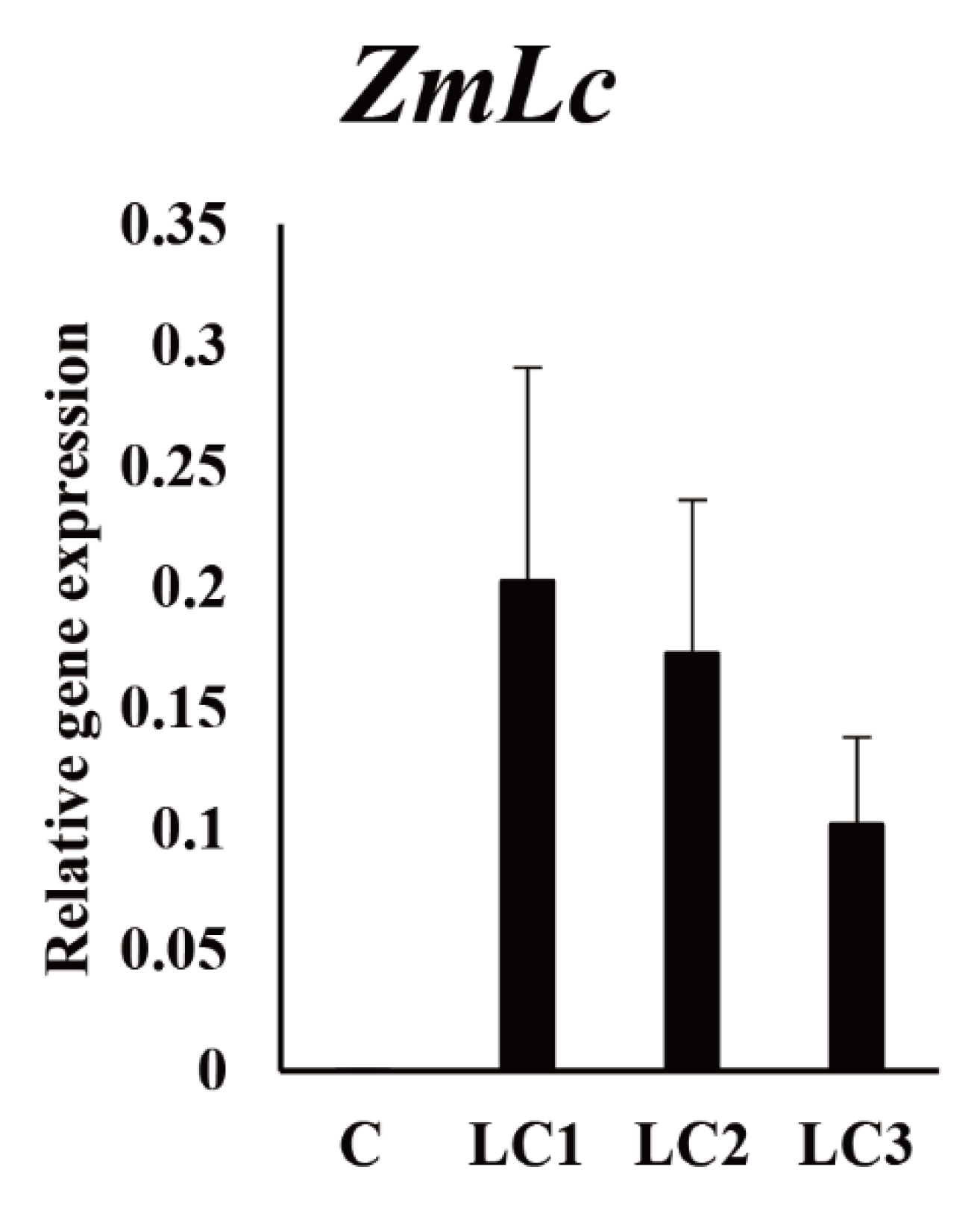
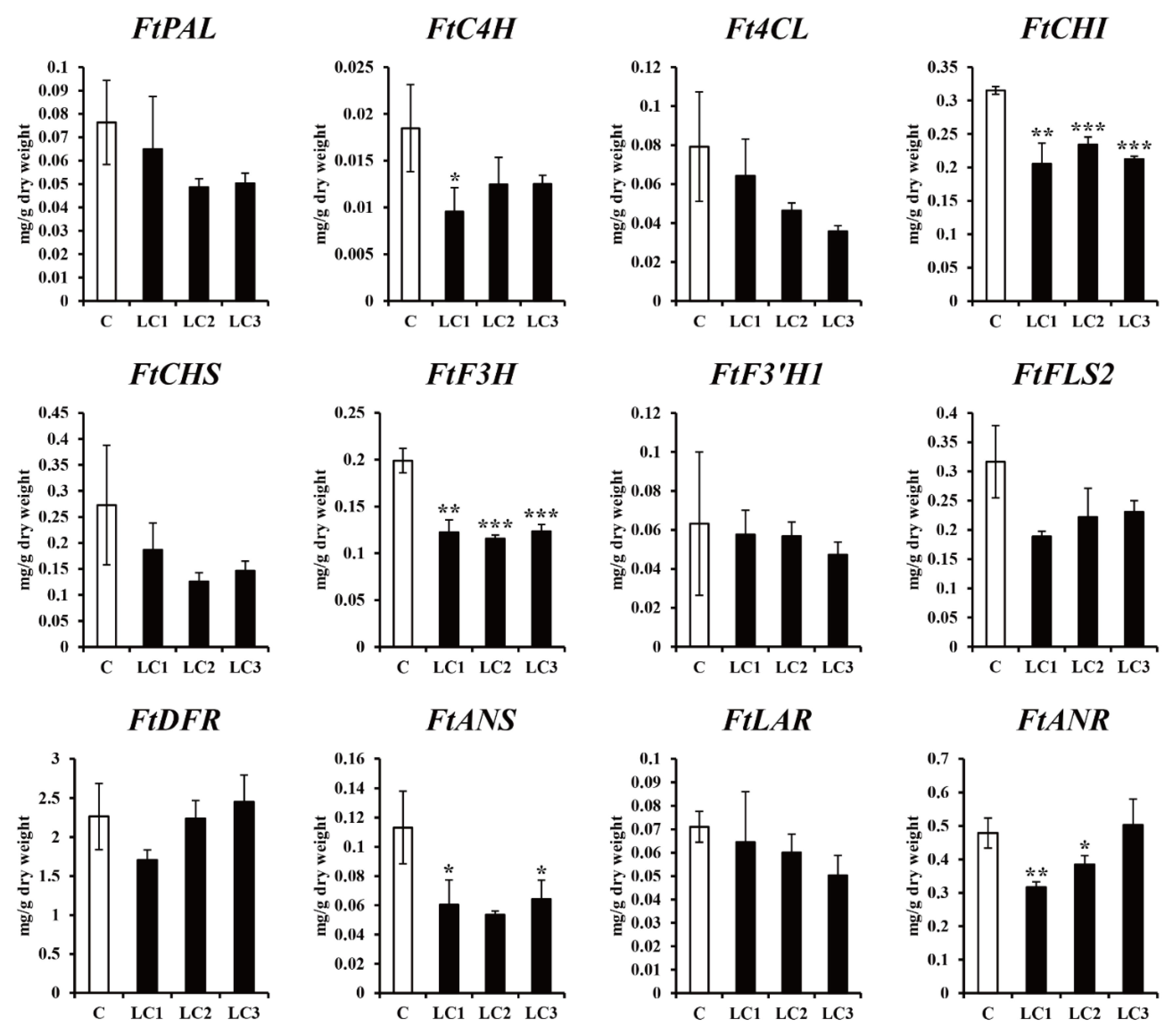
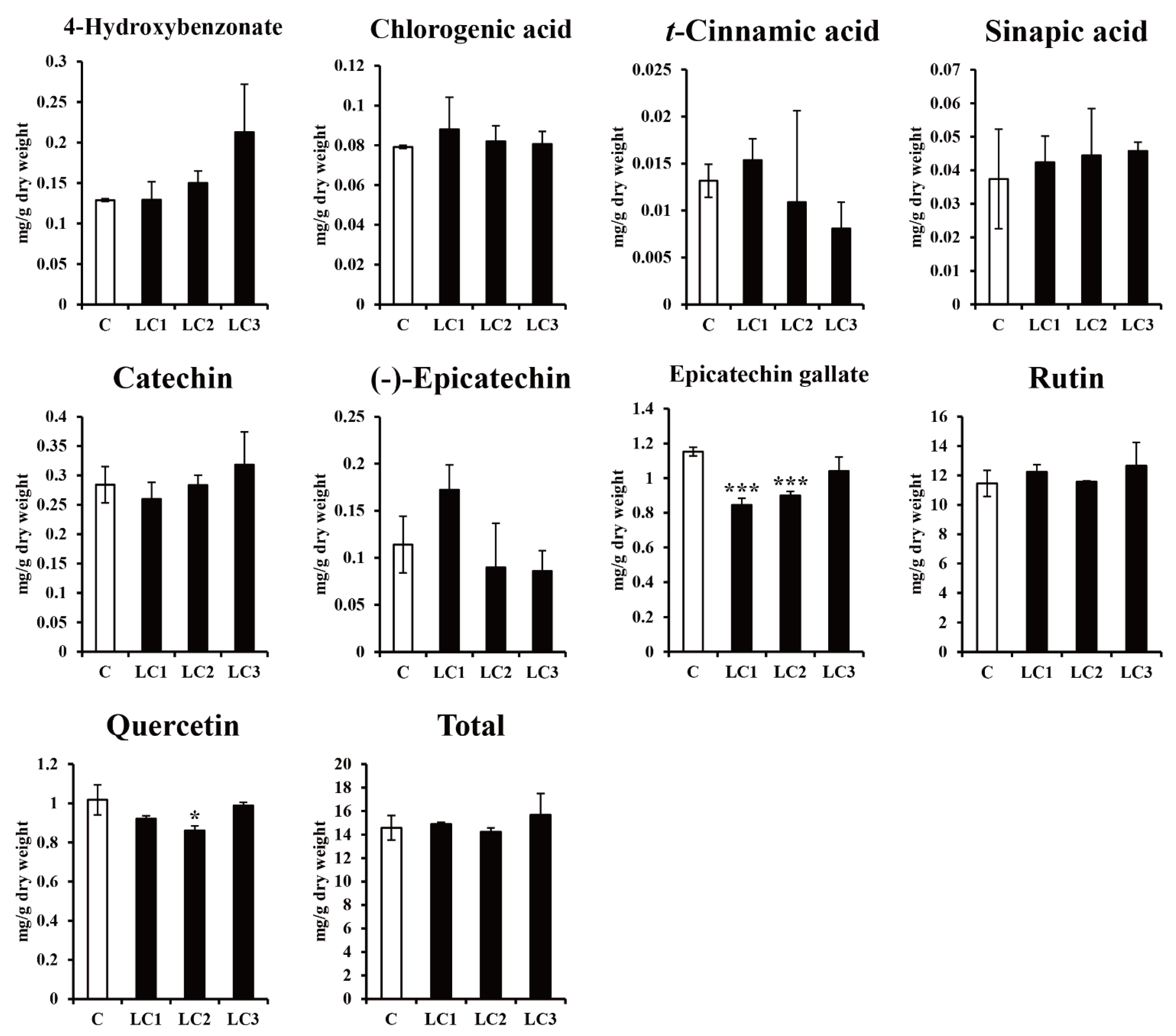
2.2. Effect of Dark Treatment on the Production of Phenolic Compounds in ZmLC- and GUS-Transgenic Hairy Root Lines of Tartary Buckwheat
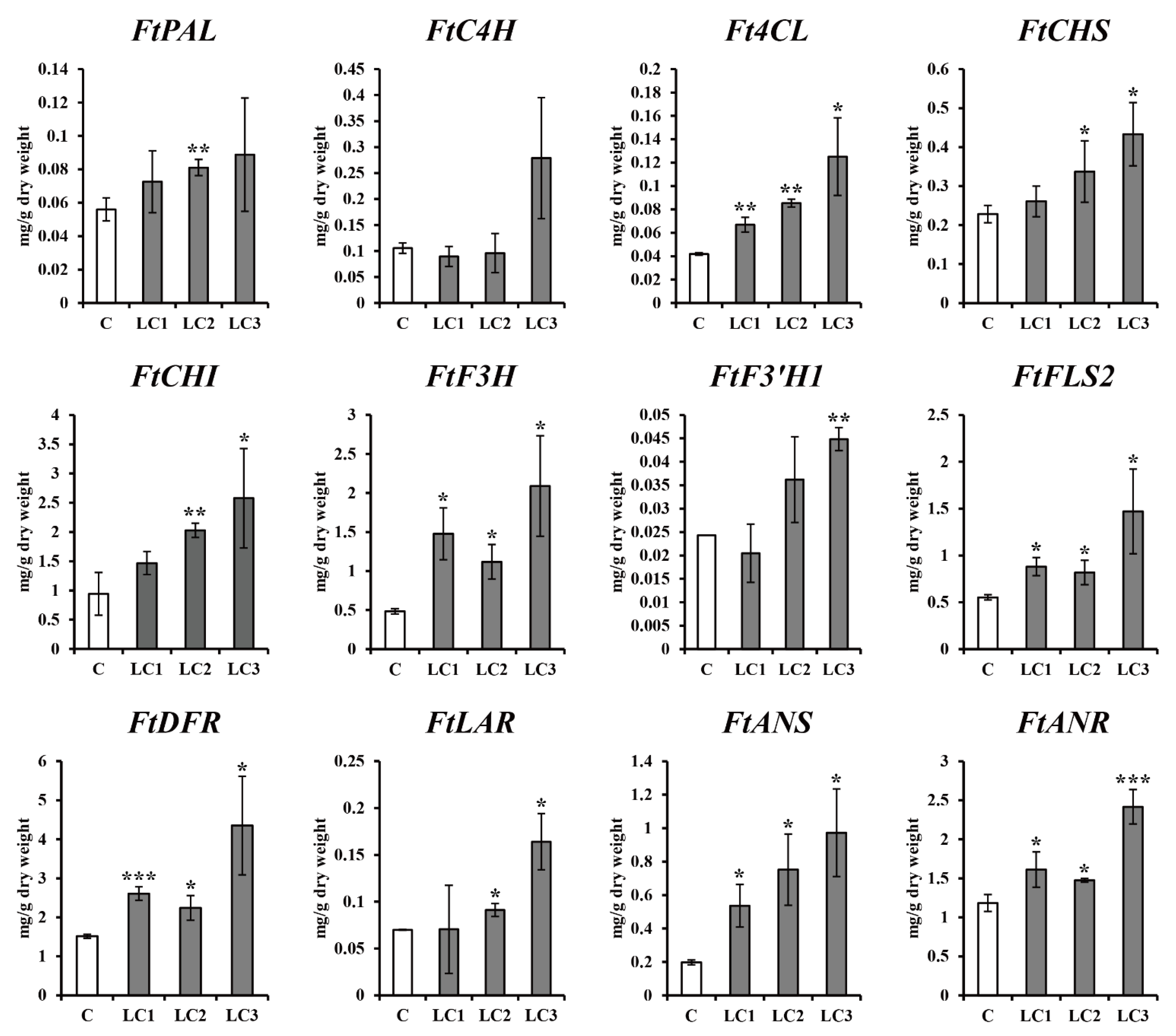
2.3. Effect of Light Treatment on the Production of Phenolic Compound in ZmLC- and GUS-Transgenic Hairy Root Lines of Tartary Buckwheat
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials
4.3. Hairy Root Induction
4.4. Extraction of Genomic DNA and Polymerase Chain Reaction (PCR) Analysis
4.5. Extraction of Total RNA and cDNA Synthesis
4.6. Gene Expression Analysis
4.7. HPLC Analysis of Phenolic Compounds
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Kawa, J.M.; Taylor, C.G.; Przybylski, R. Buckwheat concentrate reduces serum glucose in streptozotocin-diabetic rats. J. Agric. Food Chem. 2003, 51, 7287–7291. [Google Scholar] [CrossRef]
- Chan, P.-K. Inhibition of tumor growth in vitro by the extract of fagopyrum cymosum (fago-c). Life Sci. 2003, 72, 1851–1858. [Google Scholar] [CrossRef]
- Ujita, M.; Nagayama, H.; Kanie, S.; Koike, S.; Ikeyama, Y.; Ozaki, T.; Okumura, H. Carbohydrate binding specificity of recombinant human macrophage β-glucan receptor dectin-1. Biosci. Biotechnol. Biochem. 2009, 73, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.B.; Du, M. Hairy root and its application in plant genetic engineering. J. Integr. Plant Biol. 2006, 48, 121–127. [Google Scholar] [CrossRef]
- Shanks, J.V.; Morgan, J. Plant ‘hairy root’culture. Curr. Opin. Biotechnol. 1999, 10, 151–155. [Google Scholar] [CrossRef]
- Kim, Y.J.; Wyslouzil, B.E.; Weathers, P.J. Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell. Dev. Biol. Plant 2002, 38, 1–10. [Google Scholar] [CrossRef]
- Makhzoum, A.B.; Sharma, P.; Bernards, M.A.; Trémouillaux-Guiller, J. Hairy roots: An ideal platform for transgenic plant production and other promising applications. In Phytochemicals, Plant Growth, and the Environment; David, R.G., Ed.; Springer: New York, NY, USA, 2013; Volume 42, pp. 95–142. [Google Scholar]
- Ludwig, S.R.; Habera, L.F.; Dellaporta, S.L.; Wessler, S.R. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proc. Natl. Acad. Sci. USA 1989, 86, 7092–7096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.-A.; Zhao, T.; Wang, N.; Zheng, S.-S. Ectopic expression of Lc differentially regulated anthocyanin biosynthesis in the floral parts of tobacco (Nicotiana tobacum L.) plants. Bot. Stud. 2016, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Li, H.H.; Flachowsky, H.; Fischer, T.C.; Hanke, M.-V.; Forkmann, G.; Treutter, D.; Schwab, W.; Hoffmann, T.; Szankowski, I. Maize Lc transcription factor enhances biosynthesis of anthocyanins, distinct proanthocyanidins and phenylpropanoids in apple (Malus domestica Borkh.). Planta 2007, 226, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Xu, H.; Yeo, H.J.; Park, Y.E.; Hwang, G.-S.; Park, N.I.; Park, S.U. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab. Eng. 2021, 64, 64–73. [Google Scholar] [CrossRef]
- Boase, M.R.; Bradley, J.M.; Borst, N.K. Genetic transformation mediated by Agrobacterium tumefaciens of florists’ chrysanthemum (Dendranthema xgrandiflorum) cultivar ‘Peach Margaret’. In Vitro Cell. Dev. Biol. Plant 1998, 34, 46–51. [Google Scholar] [CrossRef]
- Bradley, J.M.; Deroles, S.C.; Boase, M.R.; Bloor, S.; Swinny, E.; Davies, K.M. Variation in the ability of the maize Lc regulatory gene to upregulate flavonoid biosynthesis in heterologous systems. Plant Sci. 1999, 140, 31–39. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, Y.-M.; Lee, J.-Y.; Kim, S.J.; Cho, K.-C.; Chandrasekhar, T.; Song, P.-S.; Woo, Y.-M.; Kim, J.-I. Production of purple-colored creeping bentgrass using maize transcription factor genes Pl and Lc through Agrobacterium-mediated transformation. Plant Cell Rep. 2009, 28, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Procissi, A.; Dolfini, S.; Ronchi, A.; Tonelli, C. Light-dependent spatial and temporal expression of pigment regulatory genes in developing maize seeds. Plant Cell 1997, 9, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, H.; Yu, M.; Auser, P.; Blahut-Beatty, L.; McKersie, B.; Bowley, S.; Westcott, N.; Coulman, B.; Lloyd, A.; Gruber, M.Y. Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol. 2003, 132, 1448–1463. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Fan, B.; Wang, Y.; Yang, W. Anthocyanin accumulation enhanced in Lc-transgenic cotton under light and increased resistance to bollworm. Plant Biotechnol. Rep. 2016, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thwe, A.; Valan Arasu, M.; Li, X.; Park, C.H.; Kim, S.J.; Al-Dhabi, N.A.; Park, S.U. Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum Gaertn). Front. Microbiol. 2016, 7, 318. [Google Scholar] [CrossRef]
- Li, X.; Sathasivam, R.; Park, N.I.; Wu, Q.; Park, S.U. Enhancement of phenylpropanoid accumulation in tartary buckwheat hairy roots by overexpression of MYB transcription factors. Ind. Crops Prod. 2020, 156, 112887. [Google Scholar] [CrossRef]
- Kim, Y.K.; Li, X.; Xu, H.; Park, N.I.; Uddin, M.R.; Pyon, J.Y.; Park, S.U. Production of phenolic compounds in hairy root culture of tartary buckwheat (Fagopyrum tataricum Gaertn). J. Crop Sci. Biotech. 2009, 12, 53–57. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, Y.J.; Li, X.; Kim, Y.B.; Park, N.-I.; Kim, H.H.; Kim, S.-J.; Park, S.U. Accumulation of phenylpropanoids and correlated gene expression in hairy roots of tartary buckwheat under light and dark conditions. Appl. Biochem. Biotechnol. 2014, 174, 2537–2547. [Google Scholar] [CrossRef]
- Kim, N.S.; Kwon, S.-J.; Cuong, D.M.; Jeon, J.; Park, J.S.; Park, S.U. Accumulation of phenylpropanoids in tartary buckwheat (Fagopyrum tataricum) under salt stress. Agronomy 2019, 9, 739. [Google Scholar] [CrossRef] [Green Version]
- Bradley, J.M.; Davies, K.M.; Deroles, S.C.; Bloor, S.J.; Lewis, D.H. The maize Lc regulatory gene up-regulates the flavonoid biosynthetic pathway of Petunia. Plant J. 1998, 13, 381–392. [Google Scholar] [CrossRef]
- Bovy, A.; de Vos, R.; Kemper, M.; Schijlen, E.; Pertejo, M.A.; Muir, S.; Collins, G.; Robinson, S.; Verhoeyen, M.; Hughes, S. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes LC and C1. Plant Cell 2002, 14, 2509–2526. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tian, C.; Xia, Y.; Mutanda, I.; Wang, K.; Wang, Y. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab. Eng. 2019, 52, 124–133. [Google Scholar] [CrossRef]
- Solopova, A.; van Tilburg, A.Y.; Foito, A.; Allwood, J.W.; Stewart, D.; Kulakauskas, S.; Kuipers, O.P. Engineering Lactococcus lactis for the production of unusual anthocyanins using tea as substrate. Metab. Eng. 2019, 54, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Sáez-Sáez, J.; Wang, G.; Marella, E.R.; Sudarsan, S.; Pastor, M.C.; Borodina, I. Engineering the oleaginous yeast Yarrowia lipolytica for high-level resveratrol production. Metab. Eng. 2020, 62, 51–61. [Google Scholar] [CrossRef]
- Park, C.H.; Zhao, S.; Yeo, H.J.; Park, Y.E.; Baska, T.B.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Comparison of Different Strains of Agrobacterium rhizogenes for Hairy Root Induction and Betulin and Betulinic Acid Production in Morus alba. Nat. Prod. Commun. 2017, 12, 1934578X1701200403. [Google Scholar] [CrossRef] [Green Version]
- Jacob, A.; Malpathak, N. Green hairy root cultures of Solanum khasianum Clarke—A new route to in vitro solasodine production. Curr. Sci. 2004, 1442–1447. [Google Scholar]
- Marsh, Z.; Yang, T.; Nopo-Olazabal, L.; Wu, S.; Ingle, T.; Joshee, N.; Medina-Bolivar, F. Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora. Phytochem 2014, 107, 50–60. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea Purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]
- Liu, C.-Z.; Guo, C.; Wang, Y.-C.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia Annu. Process Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Cuong, D.M.; Park, C.H.; Bong, S.J.; Kim, N.S.; Kim, J.K.; Park, S.U. Enhancement of glucosinolate production in watercress (Nasturtium officinale) hairy roots by overexpressing cabbage transcription factors. J. Agric. Food Chem. 2019, 67, 4860–4867. [Google Scholar] [CrossRef] [PubMed]


| Line | Fresh Weight (g) |
|---|---|
| C 1 | 1.52 ± 0.10 |
| LC1 2 | 1.83 ± 0.45 |
| LC2 | 1.69 ± 0.18 |
| LC3 | 2.04 ± 0.21 |
| Line | Fresh Weight (g) |
|---|---|
| C 1 | 1.83 ± 0.86 |
| LC1 2 | 3.25 ± 1.25 |
| LC2 | 2.45 ± 0.95 |
| LC3 | 2.11 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Park, Y.E.; Yeo, H.J.; Park, N.I.; Park, S.U. Effect of Light and Dark on the Phenolic Compound Accumulation in Tartary Buckwheat Hairy Roots Overexpressing ZmLC. Int. J. Mol. Sci. 2021, 22, 4702. https://doi.org/10.3390/ijms22094702
Park CH, Park YE, Yeo HJ, Park NI, Park SU. Effect of Light and Dark on the Phenolic Compound Accumulation in Tartary Buckwheat Hairy Roots Overexpressing ZmLC. International Journal of Molecular Sciences. 2021; 22(9):4702. https://doi.org/10.3390/ijms22094702
Chicago/Turabian StylePark, Chang Ha, Ye Eun Park, Hyeon Ji Yeo, Nam Il Park, and Sang Un Park. 2021. "Effect of Light and Dark on the Phenolic Compound Accumulation in Tartary Buckwheat Hairy Roots Overexpressing ZmLC" International Journal of Molecular Sciences 22, no. 9: 4702. https://doi.org/10.3390/ijms22094702






