The Emerging Role of Macrophages in Immune System Dysfunction under Real and Simulated Microgravity Conditions
Abstract
:1. Introduction
2. Comparison of Facilities Used for Microgravity Research
2.1. Parabolic Flight
2.2. 2-D Clinostat
2.3. Random Positioning Machine
2.4. Rotating Wall Vessel
| Item | Modeled Microgravity | Real Microgravity | Cell | Levels | Reference |
|---|---|---|---|---|---|
| IL-1 | RWV | U937 (Human) | Increased | [37] | |
| During spaceflight | B6MP102 cells (Murine) | Increased | [38] | ||
| Spacelab | PBMC (Human) | Decreased | [5] | ||
| Postflight | PBMC (Monkey) | Decreased | [39] | ||
| IL-2 | Postflight | Whole blood-T cell | Decreased | [40] | |
| RWV | U937 (Human) | Increased | [37] | ||
| IL-6 | During spaceflight | PBMC (Human) | Decreased | [41] | |
| Postflight | Blood-monocytes (Human) | Decreased | [42] | ||
| RCCS | Macrophages (Murine) | Increased | [16] | ||
| IFN-α | During spaceflight | Lymphocytes (Human) | Increased | [43] | |
| During spaceflight | Spleen cells (Murine) | Increased | [44] | ||
| IFN-β | During spaceflight | Lymph node T cells (Murine) | Increased | [43] | |
| IFN-γ | During spaceflight | peripheral blood lymphocytes (Human) | Increased | [44] | |
| Postflight | Splenocytes (Rat) | Decreased | [45] | ||
| TNF-α | During spaceflight | Peripheral blood(Human) | Decreased | [41] | |
| During spaceflight | B6MP102 cells (Murine) | Increased | [38] | ||
| Postflight | Whole blood (Human) | Decreased | [46] | ||
| RCCS | Macrophages (Murine) | Decreased | [17] |
| Cell | Modeled Microgravity | Real Microgravity | Cell Location | Alterations | References |
|---|---|---|---|---|---|
| Lymphocyte | RWV | Lymph nodes (Mouse) | Abrogated antigen-specific function | [47] | |
| Spacelab | Blood (Human) | Inhibited response to mitogen Con A | [48] | ||
| RWV | Peripheral blood (Human) | Inhibited locomotion, blunted ability to respond to PHA | [35] | ||
| RWV | PBMC (Human) | Suppression of PHA activation | [36] | ||
| Postflight | PBMC (Human) | Reduction of activity | [49] | ||
| Natural killer cell | Postflight | PBMC (Human) | Suppressed cytotoxic | [49] | |
| Postflight | Peripheral blood (Human) | Lower cell counts | [50] | ||
| Spaceflight | Spleen (Rat) | Inhibited cytotoxicity | [51] | ||
| Neutrophil | Postflight | Blood (Human) | Increased number | [45,52] | |
| Postflight | Peripheral blood (Human) | Increased number | [52,53] | ||
| Postflight | Circulating leukocyte subsets (Human) | Increased number | [54] | ||
| Postflight | Blood (Human) | Increased number, lower phagocytosis, and oxidative burst capacities | [49,55] | ||
| Monocyte/ macrophage | SLS-1 | Blood (Human) | Increased number | [56] | |
| Parabolic flight | BMDM (Mouse) | Enhanced proliferation, inhibited differentiation | [27] | ||
| Postflight | Blood (Human) | Monocytopenia | [57] | ||
| Postflight | Spleen (Rat) | Decreased number | [58] | ||
| Postflight | Peripheral blood (Human) | Increased number | [53] | ||
| Postflight | Peripheral blood leucocytes (Human) | Increased number | [59] | ||
| RCCS | Spleen (Mouse) | Decreased number | [60] | ||
| Postflight | PBMC (Human) | Reduction in phagocytosis | [49,61] |
| Devices | Principle | Application | Characteristic | Shortcoming | References |
|---|---|---|---|---|---|
| RPM | Randomizing the gravity vector direction and the gravity vector is averaged to nearly zero over time | Osteoblasts; T lymphocytes; adherent cells | Two axes with different speeds and directions | Cell behavior affected by the shear forces and other forces; no gas change | [29,62] |
| 2-D Clinostat | Plants; small organism; unicellular; slow responsive living objects | One axis with fast and constant rotation | Vibration and centrifugal forces may lead to artifacts; no gas change | [63,64,65,66] | |
| RWV (RCCS) | Suspended and anchorage-dependent cells; cell differentiation | Co-culture multiple cell types in a 3D spheroid morphology with low shear force | Lack of measurability; limited transfer of matter; additional environmental conditions such as the mixture | [67,68,69] | |
| Parabolic Flight | Centrifugal forces counteract the gravity vector | Fast events, such as signal transduction, hormone secretion, binding of ligands to cell membranes | By controlling acceleration, creating a centrifugal force; about 25 s microgravity time | External conditions are not easy to control; high cost; short time of microgravity simulation | [25] |
3. Macrophages under Microgravity Conditions
3.1. TNF-α
3.1.1. TNF-α Expression under Simulated Microgravity Conditions
3.1.2. TNF-α Expression in Real Microgravity
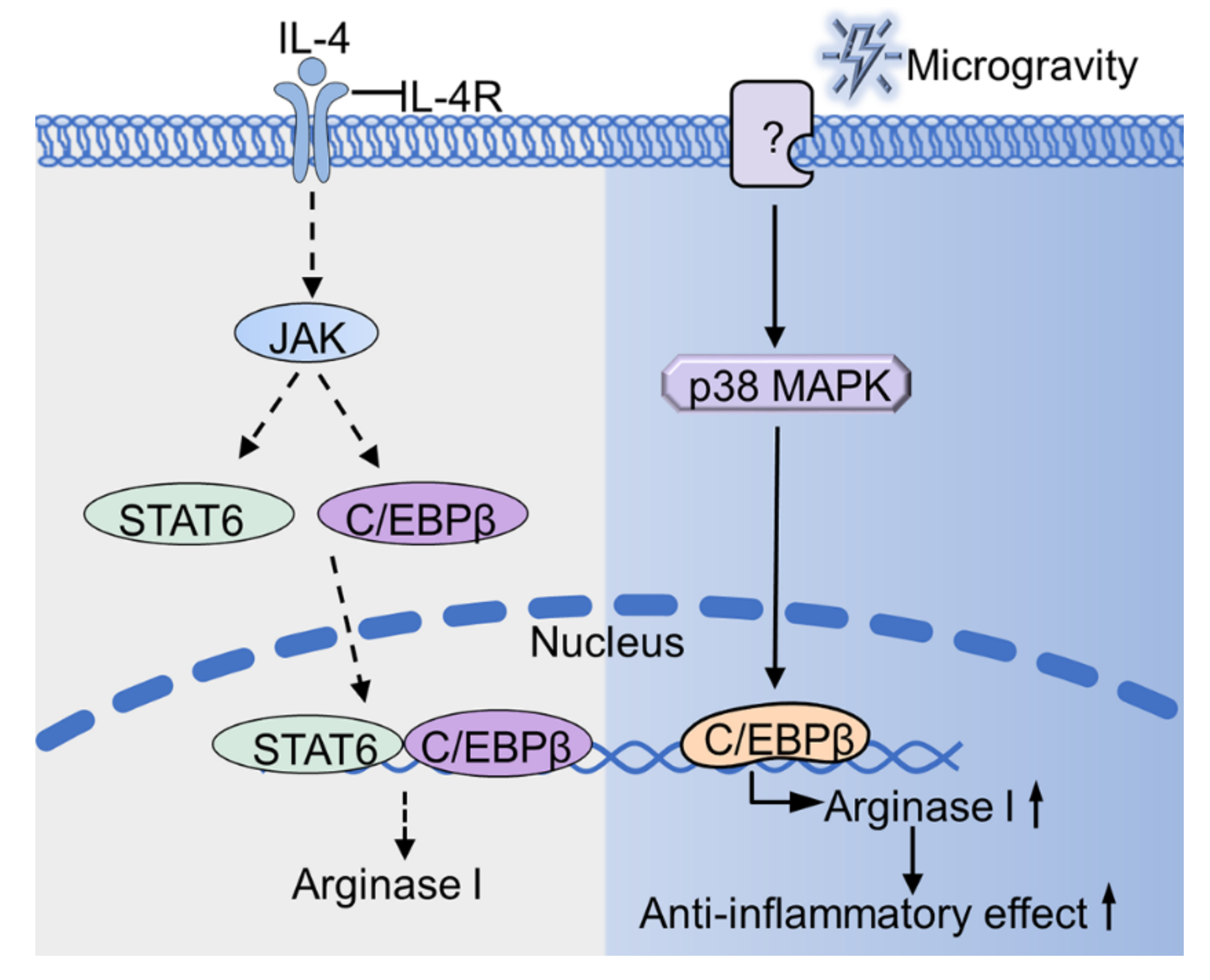
3.2. Arginase I
3.2.1. Arginase I Expression under Simulated Microgravity Conditions
- STAT6
- C/EBPβ
3.2.2. Arginase I Expression in Real Microgravity
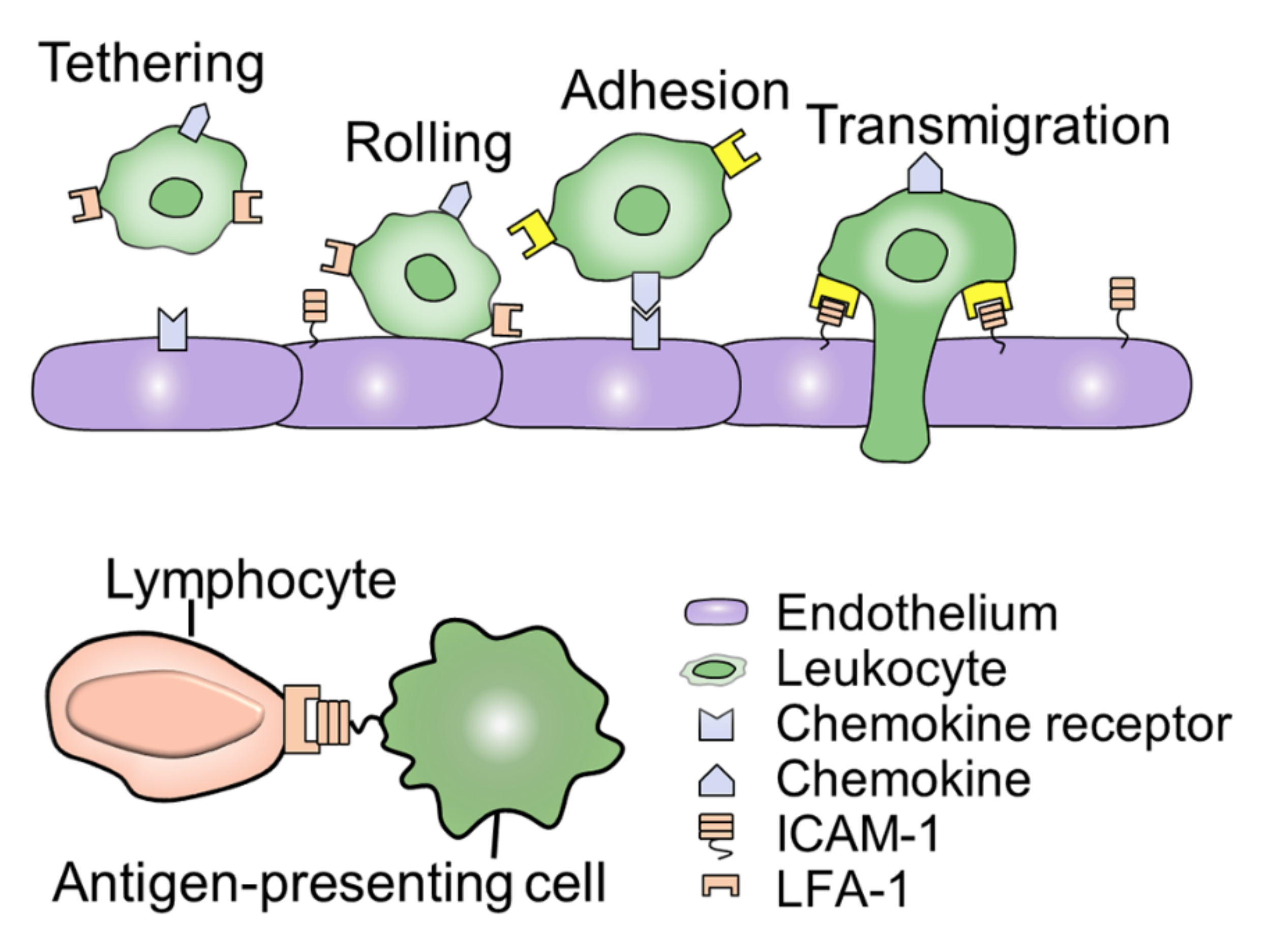
3.3. ICAM-1
3.3.1. ICAM-1 Expression under Simulated Microgravity Conditions
3.3.2. ICAM-1 Expression in Real Microgravity
4. Discussion
4.1. Molecules in Macrophages Sensitive to Real and Simulated Microgravity
4.2. The Impact of Real and Simulated Microgravity on Immune Cells
4.3. The Problems of Simulation of Microgravity in Comparison to Real Microgravity
4.4. Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-D clinostat | Two-dimensional clinostat |
| AP-1 | Activator protein 1 |
| BMDM | Bone marrow-derived macrophage |
| C/EBPβ | CCAAT-enhancer-binding proteins β |
| Con A | Concanavalin A |
| HSE | Heat shock element |
| HSF-1 | Heat shock factors 1 |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IL-4R | Interleukin-4 receptor |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible nitric oxide synthase |
| IkB | Inhibitor of nuclear factor kappa-B |
| JAK | Janus kinase |
| LFA -1 | Lymphocyte function-associated antigen 1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| NF-kB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| PBMC | Peripheral blood mononuclear cells |
| PHA | Phytohemagglutinin |
| RCCS | Rotary cell culture system |
| RPM | Random positioning machine; |
| RWV | Rotating wall vessel |
| STAT | Signal transducer and activator of transcription |
| TLR4 | Toll-like receptors 4 |
| TNF-α | Tumor necrosis factor α |
| YM1 | Chitinase-like protein |
References
- Akiyama, T.; Horie, K.; Hinoi, E.; Hiraiwa, M.; Kato, A.; Maekawa, Y.; Takahashi, A.; Furukawa, S. How does spaceflight affect the acquired immune system? NPJ Microgravity 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Choukèr, A.; Simpson, R.J.; Mehta, S.; Marshall, G.; Smith, S.M.; Zwart, S.R.; Heer, M.; Ponomarev, S.; Whitmire, A.; et al. Immune System Dysregulation During Spaceflight: Potential Countermeasures for Deep Space Exploration Missions. Front. Immunol. 2018, 9, 1437. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, W.R.; Zieglschmid, J.F. Clinical aspects of crew health. In Biomedical Results of Apollo; Johnson, R.S., Dietlein, L.F., Berry, C.A., Eds.; NASA SP-368; U.S. Government Printing Office: Washington, DC, USA, 1975; pp. 43–81. [Google Scholar]
- Cogoli, A. The effect of hypogravity and hypergravity on cells of the immune system. J. Leukoc. Biol. 1993, 54, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. CMAJ 2009, 180, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA twins study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [PubMed]
- Stowe, R.P.; Mehta, S.K.; Ferrando, A.A.; Feeback, D.L.; Pierson, D.L. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat. Space Environ. Med. 2001, 72, 884–891. [Google Scholar]
- Pierson, D.L.; Stowe, R.P.; Phillips, T.M.; Lugg, D.J.; Mehta, S.K. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav. Immun. 2005, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Crucian, B.; Pierson, D.L.; Sams, C.; Stowe, R.P. Monitoring immune system function and reactivation of latent viruses in the Artificial Gravity Pilot Study. J. Gravit. Physiol. 2007, 14, 21–25. [Google Scholar]
- Hsieh, C.L.; Chao, P.D.L.; Fang, S.H. Morin sulphates/glucuronides enhance macrophage function in microgravity culture system. Eur. J. Clin. Investig. 2005, 35, 591–596. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. To infinity … and beyond! Human spaceflight and life science. FASEB J. 2011, 25, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Todd, P. Gravity-dependent phenomena at the scale of the single cell. Gravit. Space Biol. 1989, 2, 95–113. [Google Scholar]
- Adrian, A.; Schoppmann, K.; Sromicki, J.; Brungs, S.; Von Der Wiesche, M.; Hock, B.; Kolanus, W.; Hemmersbach, R.; Ullrich, O. The oxidative burst reaction in mammalian cells depends on gravity. Cell Commun. Signal. 2013, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Luo, H.; Zhu, L.; Yang, F.; Chu, Z.; Tian, H.; Feng, M.; Zhao, Y.; Shang, P. Microgravity inhibition of lipopolysaccharide-induced tumor necrosis factor-α expression in macrophage cells. Inflamm. Res. 2014, 63, 91–98. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Luo, H.; Zhu, L.; Zhao, Y.; Tian, H.; Wang, R.; Shang, P.; Zhao, Y. Microgravity activates p38 MAPK-C/EBPβ pathway to regulate the expression of arginase and inflammatory cytokines in macrophages. Inflamm. Res. 2015, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; De Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brungs, S.; Egli, M.; Wuest, S.L.; Christianen, P.C.M.; Van Loon, J.J.W.A.; Anh, T.J.N.; Hemmersbach, R. Facilities for simulation of microgravity in the ESA ground-based facility programme. Microgravity Sci. Technol. 2016, 28, 191–203. [Google Scholar] [CrossRef]
- Pletser, V. European aircraft parabolic flights for microgravity research, applications and exploration: A review. Reach 2016, 1, 11–19. [Google Scholar] [CrossRef]
- Cogoli, A.; Cogoli-Greuter, M. Chapter 2 Activation and Proliferation of Lymphocytes and other Mammalian Cells in Microgravity. Adv. Space Biol. Med. 1997, 6, 33–79. [Google Scholar]
- Yi, B.; Matzel, S.; Feuerecker, M.; Hörl, M.; Ladinig, C.; Abeln, V.; Choukèr, A.; Schneider, S. The impact of chronic stress burden of 520-d isolation and confinement on the physiological response to subsequent acute stress challenge. Behav. Brain Res. 2015, 281, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.W.; Gerren, R.A.; Chapes, S.K. The Effect of Space and Parabolic Flight on Macrophage Hematopoiesis and Function. Exp. Cell Res. 1995, 216, 160–168. [Google Scholar] [CrossRef]
- Brungs, S.; Hauslage, J.; Hemmersbach, R. Validation of random positioning versus clinorotation using a macrophage model system. Microgravity Sci. Technol. 2019, 31, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Versari, S.; Maier, J.A.M.; Bradamante1, S. Cell behavior in simulated microgravity: A comparison of results obtained with RWV and RPM. Gravit. Space Biol. 2005, 18, 89–90. [Google Scholar]
- Krüger, M.; Pietsch, J.; Bauer, J.; Kopp, S.; Carvalho, D.T.O.; Baatout, S.; Moreels, M.; Melnik, D.; Wehland, M.; Egli, M.; et al. Growth of endothelial cells in space and in simulated microgravity—A comparison on the secretory level. Cell. Physiol. Biochem. 2019, 52, 1039–1060. [Google Scholar] [PubMed] [Green Version]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, J.J.W.A. Some history and use of the random positioning machine, RPM, in gravity related research. Adv. Space Res. 2007, 39, 1161–1165. [Google Scholar] [CrossRef]
- Moes, M.; Boonstra, J.; Regan-Klapisz, E. Novel role of cPLA(2)alpha in membrane and actin dynamics. Cell. Mol. Life Sci. 2010, 67, 1547–1557. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, N.; Zhang, C.; Sun, S.; Gao, Y.; Long, M. Effects of Simulated Microgravity on Functions of Neutrophil-like HL-60 Cells. Microgravity Sci. Technol. 2015, 27, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Pellis, N.R.; Goodwin, T.J.; Risin, D.; Mcintyre, B.W.; Pizzini, R.P.; Cooper, D.; Baker, T.L.; Spaulding, G.F. Changes in gravity inhibit lymphocyte locomotion through type I collagen. In Vitro Cell. Dev. Biol.-Anim. 1997, 33, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Pellis, N.R. Suppressed PHA activation of T lymphocytes in simulated microgravity is restored by direct activation of protein kinase C. J. Leukoc. Biol. 1998, 63, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M. Impact of simulated microgravity on cell cycle control and cytokine release by U937 cells. Int. J. Immunopathol. Pharmacol. 2006, 19, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Chapes, S.K.; Morrison, D.R.; Guikema, J.A.; Lewis, M.L.; Spooner, B.S. Production and action of cytokines in space. Adv. Space Res. 1994, 14, 5–9. [Google Scholar] [CrossRef]
- Sonnenfeld, G.; Davis, S.; Taylor, G.R.; Mandel, A.D.; Konstantinova, I.V.; Lesnyak, A.; Fuchs, B.B.; Peres, C.; Tkackzuk, J.; Schmitt, D.A. Effect of Space Flight on Cytokine Production and Other Immunologic Parameters of Rhesus Monkeys. J. Interferon Cytokine Res. 2009, 16, 409–415. [Google Scholar] [CrossRef]
- Crucian, B.E.; Cubbage, M.L.; Sams, C.F. Altered Cytokine Production by Specific Human Peripheral Blood Cell Subsets Immediately Following Space Flight. J. Interferon Cytokine Res. 2002, 20, 547–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef]
- Kaur, I.; Simons, E.R.; Kapadia, A.S.; Ott, C.M.; Pierson, D.L. Effect of spaceflight on ability of monocytes to respond to endotoxins of gram-negative bacteria. Clin. Vaccine Immunol. CVI 2008, 15, 1523–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tálas, M.; Bátkai, L.; Stöger, I.; Nagy, L.; Hiros, L.; Konstantinova, I.; Rykova, M.; Mozgovaya, I.; Guseva, O.; Kozharinov, V. Results of space experiment program “Interferon”. I. Production of interferon in vitro by human lymphocytes aboard space laboratory Solyut-6 (“Interferon I”) and influence of space flight on lymphocyte functions of cosmonauts (“Interferon III”). Acta Microbiol. Hung. 1983, 30, 53–61. [Google Scholar]
- Chapes, S.K.; Morrison, D.R.; Guikema, J.A.; Lewis, M.L.; Spooner, B.S. Cytokine secretion by immune cells in space. J. Leukoc. Biol. 1992, 52, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Walther, I.; Li, C.-F.; Boonyaratanakornkit, J.; Galleri, G.; Meloni, M.A.; Pippia, P.; Cogoli, A.; Hughes-Fulford, M. The Rel/NF-κB pathway and transcription of immediate early genes in T cell activation are inhibited by microgravity. J. Leukoc. Biol. 2012, 92, 1133–1145. [Google Scholar] [CrossRef] [Green Version]
- Crucian, B.; Stowe, R.; Quiriarte, H.; Pierson, D.; Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat. Space Environ. Med. 2011, 82, 857–862. [Google Scholar] [CrossRef]
- Sastry, K.J.; Nehete, P.N.; Savary, C.A. Impairment of Antigen-Specific Cellular Immune Responses Under Simulated Microgravity Conditions. In Vitro Cell. Dev. Biol.-Anim. 2001, 37, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cogoli, A.; Tschopp, A.; Fuchs-Bislin, P. Cell sensitivity to gravity. Science 1984, 225, 228–230. [Google Scholar] [CrossRef]
- Rykova, M.P.; Antropova, E.N.; Larina, I.M.; Morukov, B.V. Humoral and cellular immunity in cosmonauts after the ISS missions. Acta Astronaut. 2008, 63, 697–705. [Google Scholar] [CrossRef]
- Konstantinova, I.V.; Rykova, M.; Meshkov, D.; Peres, C.; Husson, D.; Schmitt, D.A. Natural killer cells after altaïr mission. Acta Astronaut. 1995, 36, 713–718. [Google Scholar] [CrossRef]
- Lewis, M.L.; Reynolds, J.L.; Cubano, L.A.; Hatton, J.P.; Lawless, B.D.; Piepmeier, E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat). FASEB J. 1998, 12, 1007–1018. [Google Scholar] [CrossRef]
- Taylor, G.R.; Dardano, J.R. Human cellular immune responsiveness following space flight. Aviat. Space Environ. Med. 1984, 18, 74–80. [Google Scholar]
- Mills, P.J.; Meck, J.V.; Waters, W.W.; D’Aunno, D.; Ziegler, M.G. Peripheral leukocyte subpopulations and catecholamine levels in astronauts as a function of mission duration. Psychosom. Med. 2001, 63, 886–890. [Google Scholar] [CrossRef]
- Stowe, R.P.; Sams, C.F.; Mehta, S.K.; Kaur, I.; Jones, M.L.; Feeback, D.L.; Pierson, D.L. Leukocyte subsets and neutrophil function after short-term spaceflight. J. Leukoc. Biol. 1999, 65, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Simons, E.R.; Castro, V.; Pierson, D.L. Changes in Neutrophil Functions in Astronauts Source. Brain Behav. Immun. 2004, 18, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.R. Cellular changes in microgravity and the design of space radiation experiments. Adv. Space Res. 1994, 14, 1005–1019. [Google Scholar] [CrossRef]
- Chapes, S.K.; Ortega, T.M. Understanding macrophage differentiation during space flight: The importance of ground-based experiments before space flight. Recent Pat Space Technol. 2013, 3, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baqai, F.P.; Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Stodieck, L.S.; Ferguson, V.; Chapes, S.K.; Pecaut, M.J. Effects of spaceflight on innate immune function and antioxidant gene expression. J. Appl. Physiol. 2009, 106, 1935–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meehan, R.T.; Neale, L.S.; Kraus, E.T.; Stuart, C.A.; Smith, M.L.; Cintron, N.M.; Sams, C.F. Alteration in human mononuclear leucocytes following space flight. Immunology 1992, 76, 491–497. [Google Scholar]
- Chen, H.; Luo, H.; Liu, J.; Wang, P.; Dong, D.; Shang, P.; Zhao, Y. The Distinctive Sensitivity to Microgravity of Immune Cell Subpopulations. Microgravity Sci. Technol. 2015, 27, 427–436. [Google Scholar] [CrossRef]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in monocyte functions of astronauts. Brain Behav. Immun. 2005, 19, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Huijser, R.H. Desktop RPM: New small size microgravity simulator for the bioscience laboratory. Fokker Space 2000, 1, 1–5. [Google Scholar]
- Albrecht-Buehler, G. The simulation of microgravity conditions on the ground. Gravit. Space Res. 1992, 5, 3–10. [Google Scholar]
- Unsworth, B.R.; Lelkes, P.I. Growing tissues in microgravity. Nat. Med. 1998, 4, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Nagaya, T.; Koga, K.; Kambe, F.; Nomura, Y.; Seo, H. Rotation in clinostat results in apoptosis of osteoblastic ROS 17/2.8 cells. J. Gravit. Physiol. 2000, 7, P71-2. [Google Scholar]
- Klaus, D.M. Clinostats and bioreactors. Gravitational and space biology bulletin: Publication of the American Society for Gravitational and Space Biology. Gravit. Space Res. 2001, 14, 55–64. [Google Scholar]
- Mitteregger, R.; Vogt, G.; Rossmanith, E.; Falkenhagen, D. Rotary Cell Culture System (RCCS): A new Method for Cultivating Hepatocytes on Microcarriers. Int. J. Artif. Organs 1999, 22, 816–822. [Google Scholar] [CrossRef]
- Zayzafoon, M.; Meyers, V.E.; McDonald, J.M. Microgravity: The immune response and bone. Immunol. Rev. 2005, 208, 267–280. [Google Scholar] [CrossRef]
- Hwang, S.-a.; Pan, C.; Boyd, S.; Pellis, N.R.; Actor, J.K. Modeled Microgravity Conditions Suppress Innate Macrophage and Lymphocytic Responses to Common Mitogens and Mycobacterium tuberculosis Infection. Gravit. Space Biol. 2012, 26, 25–33. [Google Scholar]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445. [Google Scholar] [CrossRef]
- Ortega, M.T.; Lu, N.; Chapes, S.K. Evaluation of in vitro macrophage differentiation during space flight. Adv. Space Res. 2012, 49, 1441–1455. [Google Scholar] [CrossRef] [Green Version]
- Meloni, M.A.; Galleri, G.; Pippia, P.; Cogoli-Greuter, M. Cytoskeleton changes and impaired motility of monocytes at modelled low gravity. Protoplasma 2006, 229, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes-Fulford, M.; Chang, T.; Li, C.-F. Effect of gravity on monocyte differentiation. In Life in Space for Life on Earth; ESA-SP: Angers, France, 2008; pp. 22–27. [Google Scholar]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune system dysregulation following short- vs long-duration spaceflight. Aviat. Space Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Patarroyo, M.; Makgoba, M. Leucocyte adhesion to cells in immune and inflammatory responses. Lancet 1989, 334, 1139–1142. [Google Scholar] [CrossRef]
- Beutler, B.; Milsark, I.W.; Cerami, A.C.; Alerts, E. Passive Immunization Against Cachectin/Tumor Necrosis Factor Protects Mice from Lethal Effect of Endotoxin. Science 1985, 229, 869–871. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The impact of spaceflight and simulated microgravity on cell adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef]
- Barnes, P.J.; Karin, M. Nuclear Factor-κB—A Pivotal Transcription Factor in Chronic Inflammatory Diseases. N. Engl. J. Med. 2002, 336, 1066–1071. [Google Scholar] [CrossRef]
- Brungs, S.; Kolanus, W.; Hemmersbach, R. Syk phosphorylation—A gravisensitive step in macrophage signalling. Cell Commun. Signal. 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Qin, J. Modulation of Toll-interleukin 1 receptor mediated signaling. J. Mol. Med. 2005, 83, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Anckar, J.; Sistonen, L. Regulation of HSF 1 Function in the Heat Stress Response: Implications in Aging and Disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Ensor, J.E.; Crawford, E.K.; Hasday, J.D. Warming macrophages to febrile range destabilizes tumor necrosis factor-alpha mRNA without inducing heat shock. Am. J. Physiol.-Cell Physiol. 2017, 269, 1140–1146. [Google Scholar] [CrossRef]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M.; Sistonen, L.; Morimoto, R.I. Stress-Inducible Regulation of Heat Shock Factor 1 by the Deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Singh, I.S.; He, J.-R.; Hester, L.; Fenton, M.J.; Hasday, J.D. Bacterial endotoxin modifies heat shock factor-1 activity in RAW 264.7 cells: Implications for TNF-α regulation during exposure to febrile range temperatures. J. Endotoxin Res. 2004, 10, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Garren, S.B.; Garris, C.S.; Kohler, R.H.; Oh, J.; Pittet, M.J.; Weissleder, R. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics 2018, 8, 5842–5854. [Google Scholar] [CrossRef]
- Bansal, V.; Ochoa, J.B. Arginine availability, arginase, and the immune response. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 223–228. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, E.; Dy, M. The role of arginase in the immune response. Immunol. Today 1985, 6, 136–140. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, K.E.; Shandilya, H.; Kepka-Lenhart, D.; Poljakovic, M.; Ghosh, A.; Morris, S.M. Shaping the Murine Macrophage Phenotype: IL-4 and Cyclic AMP Synergistically Activate the Arginase I Promoter. J. Immunol. 2013, 191, 2290–2298. [Google Scholar] [CrossRef]
- Katz, S.; Kowenz-Leutz, E.; Müller, C.; Meese, K.; Ness, S.A.; Leutz, A. The NF-M transcription factor is related to C/EBP beta and plays a role in signal transduction, differentiation and leukemogenesis of avian myelomonocytic cells. EMBO J. 1993, 12, 1321–1332. [Google Scholar] [CrossRef]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell. Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Graves, J.D.; Draves, K.E.; Craxton, A.; Saklatvala, J.; Krebs, E.G.; Clark, E.A. Involvement of stress-activated protein kinase and p38 mitogen-activated protein kinase in mIgM-induced apoptosis of human B lymphocytes. Proc. Natl. Acad. Sci. USA 2002, 93, 13814–13818. [Google Scholar] [CrossRef] [Green Version]
- Sahin, E.; Haubenwallner, S.; Kuttke, M.; Kollmann, I.; Halfmann, A.; Dohnal, A.M.; Chen, L.; Cheng, P.; Hoesel, B.; Einwallner, E.; et al. Macrophage PTEN Regulates Expression and Secretion of Arginase I Modulating Innate and Adaptive Immune Responses. J. Immunol. 2014, 193, 5350. [Google Scholar] [CrossRef]
- Springer, T.A. Adhesion Receptors of the Immune System. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Singer, K.H.; Denning, S.M.; Whichard, L.P.; Haynes, B.F. Thymocyte LFA-1 and thymic epithelial cell ICAM-1 molecules mediate binding of activated human thymocytes to thymic epithelial cells. J. Immunol. 1990, 144, 2931–2939. [Google Scholar]
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Gölz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in Cells of the Monocyte/Macrophage System in Microgravity. BioMed Res. Int. 2015, 2015, 538786. [Google Scholar] [CrossRef]
- Zhang, Y.; Sang, C.; Paulsen, K.; Arenz, A.; Zhao, Z.; Jia, X.; Ullrich, O.; Zhuang, F. ICAM-1 expression and organization in human endothelial cells is sensitive to gravity. Acta Astronaut. 2010, 67, 1073–1080. [Google Scholar] [CrossRef]
- Miodrag, Č.; Drabek, D. Expression and function of intercellular adhesion molecule 1 (ICAM-1) on rat thymic macrophages in culture. Immunol. Lett. 1991, 28, 251–257. [Google Scholar] [CrossRef]
- Schatten, H.; Lewis, M.L.; Chakrabarti, A. Spaceflight and clinorotation cause cytoskeleton and mitochondria changes and increases in apoptosis in cultured cells. Acta Astronaut. 2001, 49, 399–418. [Google Scholar] [CrossRef]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2003, 50, 183–191. [Google Scholar] [CrossRef]
- Paulsen, K.; Tauber, S.; Goelz, N.; Simmet, D.M.; Engeli, S.; Birlem, M.; Dumrese, C.; Karer, A.; Hunziker, S.; Biskup, J.; et al. Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronaut. 2014, 94, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, B.B.; Medvedev, A.E. Countermeasures for ameliorating in-flight immune dysfunction. J. Leukoc. Biol. 1993, 54, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pippia, P.; Sciola, L.; Cogoli-Greuter, M.; Meloni, M.A.; Spano, A.; Cogoli, A. Activation signals of T lymphocytes in microgravity. J. Biotechnol. 1996, 47, 215–222. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Sonnenfeld, G. Immune Responses in Space Flight. Int. J. Sports Med. 1998, 19, 195–204. [Google Scholar] [CrossRef]
- Herranz, R.; Valbuena, M.A.; Manzano, A.; Kamal, K.Y.; Medina, F.J. Use of microgravity simulators for plant biological studies. Methods Mol. Biol. 2015, 1309, 239–254. [Google Scholar] [PubMed]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef] [Green Version]
- Liemersdorf, C.; Lichterfeld, Y.; Hemmersbach, R.; Hauslage, J. The MAPHEUS module CellFix for studying the influence of altered gravity on the physiology of single cells. Rev. Sci. Instrum. 2020, 91, 014101. [Google Scholar] [CrossRef] [PubMed]
- Hauslage, J.; Görög, M.; Krause, L.; Schüler, O.; Schäfer, M.; Witten, A.; Kesseler, L.; Böhmer, M.; Hemmersbach, R. ARABIDOMICS-A new experimental platform for molecular analyses of plants in drop towers, on parabolic flights, and sounding rockets. Rev. Sci. Instrum. 2020, 91, 034504. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Ille, F.; Wuest, S.; Haack, C.; Koller, A.; Giger-Lange, C.; Zocchi, M.; Egli, M.; Castiglioni, S.; Maier, J.A. Scalable Microgravity Simulator Used for Long-Term Musculoskeletal Cells and Tissue Engineering. Int. J. Mol. Sci. 2020, 21, 8908. [Google Scholar] [CrossRef] [PubMed]
- Rutter, L.; Barker, R.; Bezdan, D.; Cope, H.; Costes, S.V.; Degoricija, L.; Fisch, K.M.; Gabitto, M.I.; Gebre, S.; Giacomello, S.; et al. A New Era for Space Life Science: International Standards for Space Omics Processing. Patterns 2020, 1, 100148. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Kuang, Y.; Zuo, Z. The Emerging Role of Macrophages in Immune System Dysfunction under Real and Simulated Microgravity Conditions. Int. J. Mol. Sci. 2021, 22, 2333. https://doi.org/10.3390/ijms22052333
Sun Y, Kuang Y, Zuo Z. The Emerging Role of Macrophages in Immune System Dysfunction under Real and Simulated Microgravity Conditions. International Journal of Molecular Sciences. 2021; 22(5):2333. https://doi.org/10.3390/ijms22052333
Chicago/Turabian StyleSun, Yulong, Yuanyuan Kuang, and Zhuo Zuo. 2021. "The Emerging Role of Macrophages in Immune System Dysfunction under Real and Simulated Microgravity Conditions" International Journal of Molecular Sciences 22, no. 5: 2333. https://doi.org/10.3390/ijms22052333






