Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes
Abstract
:1. Introduction
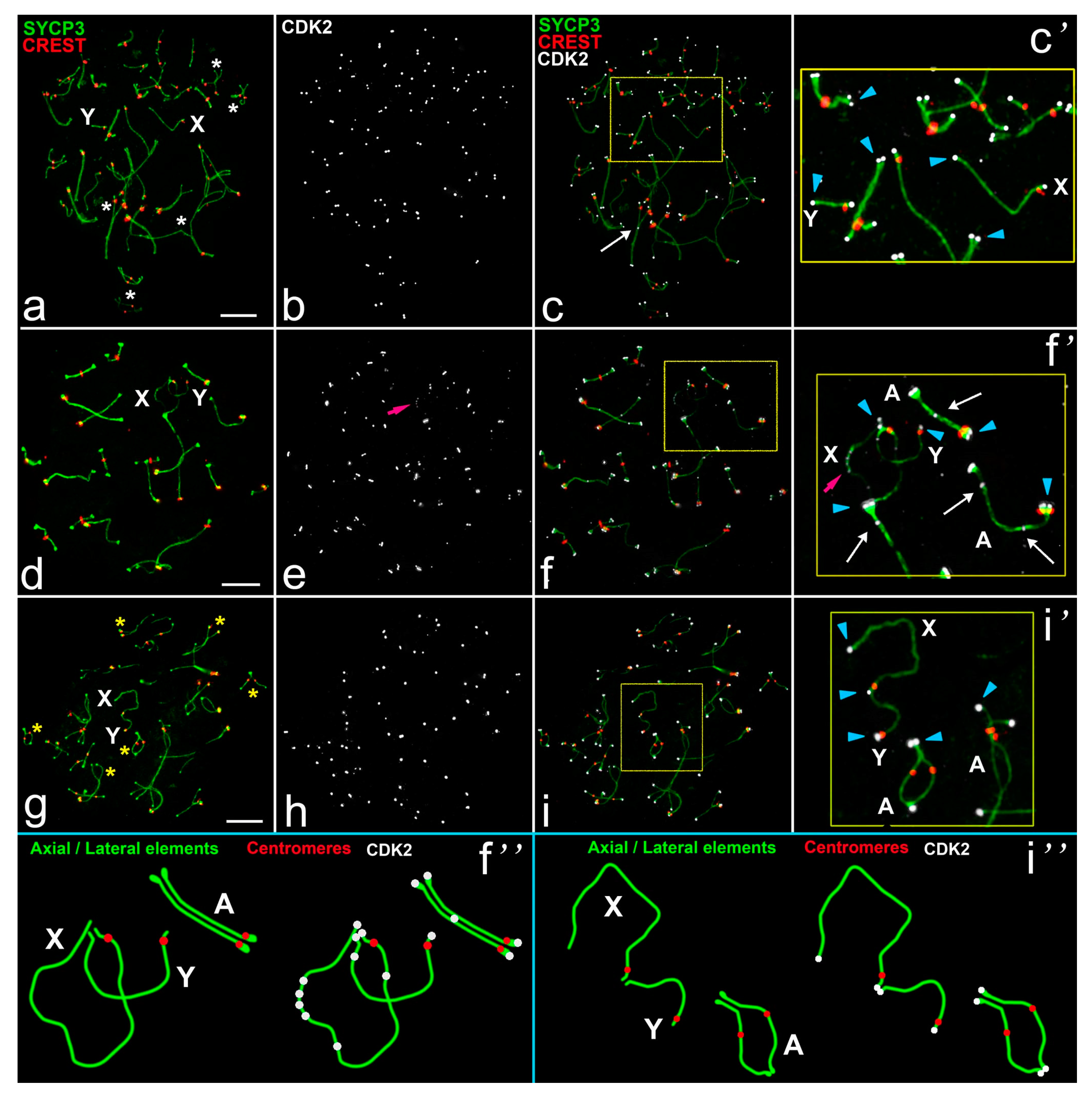
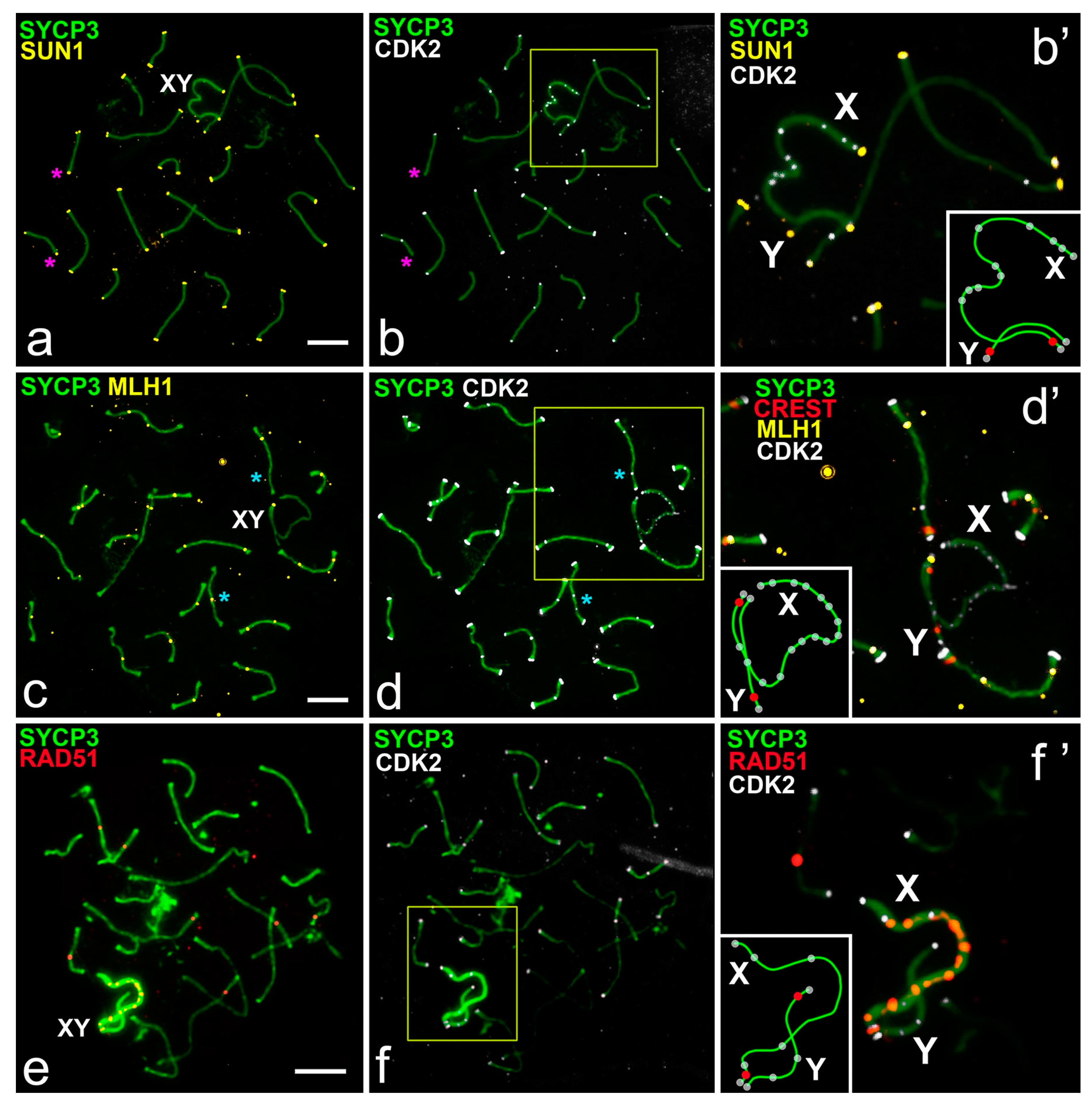
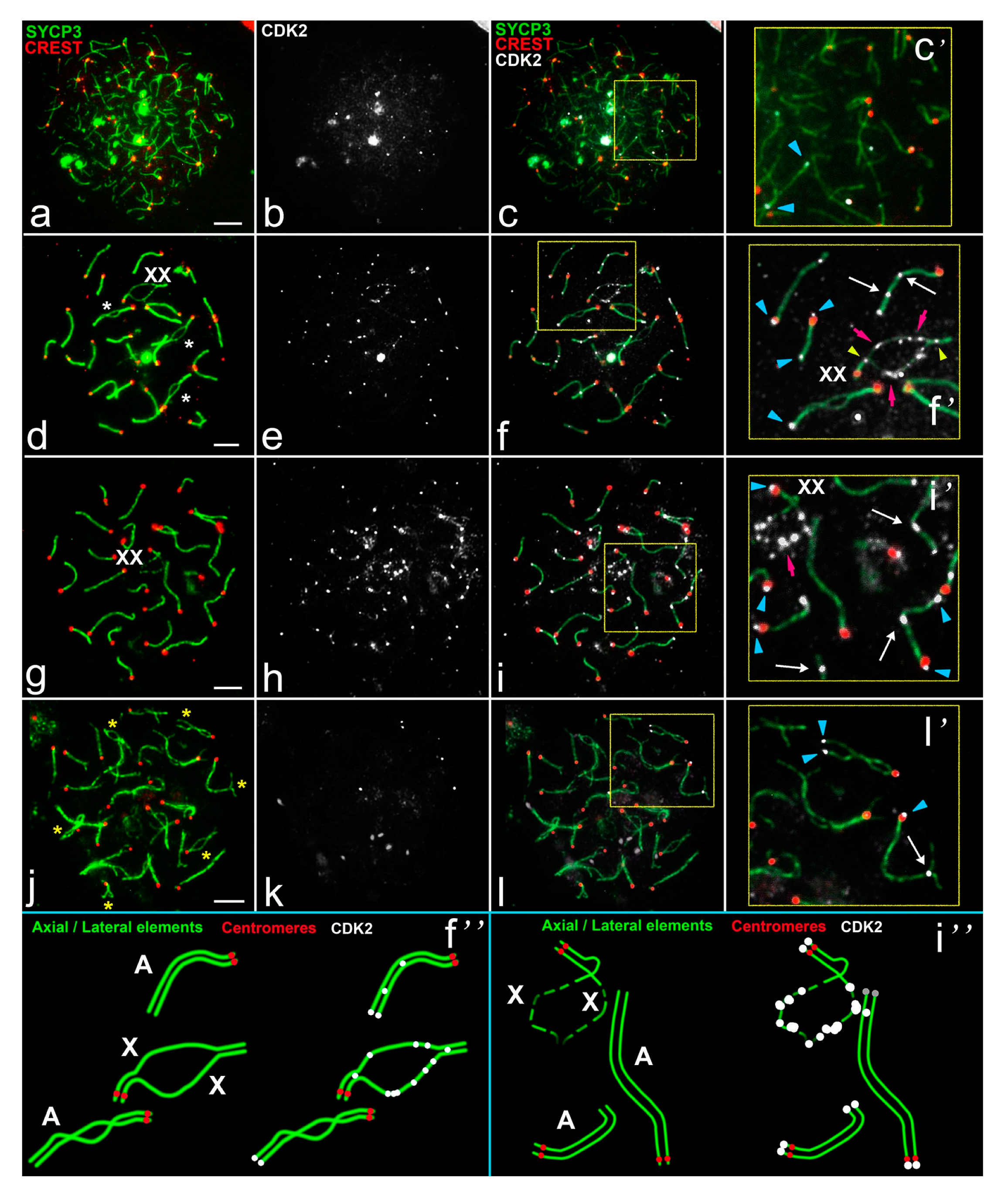
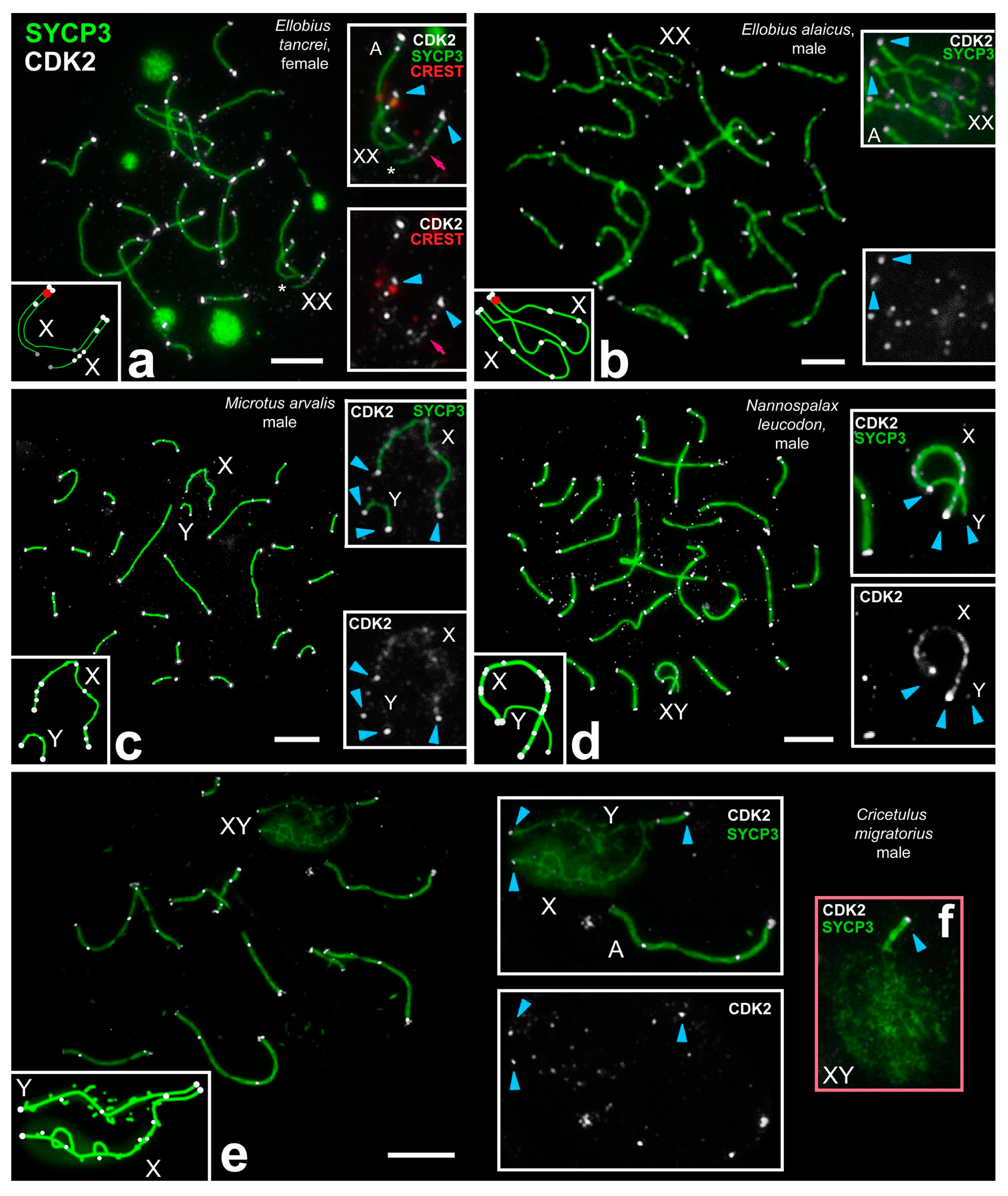
2. Results
3. Discussion
- At the unsynapsed regions, the X chromosome has a lot of CDK2 signals, Y chromosome has no or one weak CDK2 focus:
- Classical synaptic XY:
- PARs undergo synapsis, the centromeric region of the Y chromosomes is not involved in synapsis, as in R. norvegicus (compared with the mouse, in which the centromeric regions of both sex chromosomes are not involved in synapsis),
- Synapsis between sex chromosome starts in their telomeric regions and then Y is synapsed to its full length with co-orientated centromeres, as in N. leucodon,
- Fully asynaptic X and Y, as in M. arvalis.
- Exclusive male XX, as in E. talpinus and E. alaicus, partial two-site synapsis.
- At the unsynapsed regions, the X and Y chromosomes have an average number of CDK2 signals, including SYCP3 loops and fragments:
- 4.
- Synaptic XY: Sex chromosomes have the same or similar length with a long synaptic site and diffuse unsynapsed regions, as in C. migratorius.
- CDK2 signals are distributed along the sex bivalent as well as or close to autosomal bivalents:
- 5.
- Unusual female XX with a delayed synapsis, as in E. tancrei.

- -
- Telomeric ends are CDK2-positive;
- -
- If a recombination nodule is formed, then CDK2 is localized in it;
- -
- The X chromosome, as a rule, has a large or clearly visible CDK2 signals in the asynaptic region;
- -
- The short Y chromosome is deficient in CDK2 (single faint signals or none at all).
4. Material and Methods
4.1. Animals
4.2. Meiotic Samples Preparing and Its Analyses
4.3. Antibodies
4.4. Immunostaining Procedure
- The fluorescence of the dyes conjugated to the secondary antibody should be burned between rounds by keeping the slides under the halogen lamp and thorough washing in PBS. The duration of the washing between rounds is always longer than the washings within procedures of each round.
- It is necessary to consider the size of the intra-nuclear structure, the fluorescence intensity of each fluorochrome and the features of its burnout under a halogen lamp. In each case, the volume of primary and secondary antibodies, as well as the concentration of antibodies applied to the slides should be under controlled. For example, secondary antibodies Alexa Fluor 555 were more resistant to burnout, so we used them in the second or third rounds.
- In the first round, more miniature structures should be immuno-stained because such structures tend to have a less intense glow. The order of applying antibodies should be chosen due to the researcher’s experience of examining the staining slides. The illumination of the fluorochromes associated with small structures, as a rule, was eliminated faster and easier when burning under the halogen lamp. However, for example, CREST antibodies in many species demonstrated bright signals, the glow of which was able to obscure (overlay) the light of other signals in the next rounds, so we used these antibodies in the last steps of immunostaining.
- Multi-round immunostaining should be accompanied by a specific single-round one. The results of both approaches should be used to correctly assess distribution of immuno-signals in cells.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDK2 | Cyclin-dependent kinases 2 |
| PAR | Pseudoautosomal region |
| SC | Synaptonemal complex |
| SYCP3 | Synaptonemal complex protein 3 |
| DSB | Double-strand break |
| CREST | Calcinosis Raynaud’s phenomenon, Esophageal dysmotility, Sclerodactyly, and Telangiectasia |
| MLH1 | MutL homolog 1 |
| BRCA1 | Breast cancer 1 |
| MSCI | Meiotic sex chromosome inactivation |
| SUN1 | Sad1 and UNC84 domain-containing protein 1 |
| PBS | Phosphate buffered saline |
| RPA | Replication protein A |
References
- Morgan, D.O. The Cell Cycle: Principles of Control, New Science Press Ltd.: London, UK, 2007; 297p.
- Malumbres, M.; Harlow, E.; Hunt, T.; Hunter, T.; Lahti, J.M.; Manning, G.; Morgan, D.O.; Tsai, L.H.; Wolgemuth, D.J. Cyclin-dependent kinases: A family portrait. Nat. Cell Biol. 2009, 11, 1275. [Google Scholar] [CrossRef] [Green Version]
- Chotiner, J.Y.; Wolgemuth, D.J.; Wang, P.J. Functions of cyclins and CDKs in mammalian gametogenesis. Biol Reprod. 2019, ioz070. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [Green Version]
- Nigg, E.A. Cyclin-dependent protein kinases: Key regulators of the eukaryotic cell cycle. Bioessays 1995, 17, 471–480. [Google Scholar] [CrossRef]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nature Rev. Mol. Cell Biol. 2016, 17, 280. [Google Scholar] [CrossRef]
- Berthet, C.; Aleem, E.; Coppola, V.; Tessarollo, L.; Kaldis, P. Cdk2 knockout mice are viable. Curr. Biol. 2003, 13, 1775–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, T.; Bhattacharyya, T.; Handel, M.A. Genetics of meiotic Chromosome Dynamics and Fertility. In Human Reproductive and Prenatal Genetics; Leung, C.K., Qiao, J., Eds.; Academic Press: London, UK, 2019; pp. 51–84. [Google Scholar] [CrossRef]
- Ortega, S.; Prieto, I.; Odajima, J.; Martín, A.; Dubus, P.; Sotillo, R.; Barbero, J.L.; Malumbres, M.; Barbacid, M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 2003, 35, 25. [Google Scholar] [CrossRef]
- Viera, A.; Rufas, J.S.; Martínez, I.; Barbero, J.L.; Ortega, S.; Suja, J.A. CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J. Cell Sci. 2009, 122, 2149–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, S.; Diril, M.K.; Lee, J.H.; Bisteau, X.; Manoharan, V.; Adhikari, D.; Ratnacaram, C.K.; Janela, B.; Noffke, J.; Ginhoux, F.; et al. Cdk2 catalytic activity is essential for meiotic cell division in vivo. Biochem. J. 2016, 473, 2783–2798. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.; Talib, S.Z.; Singh, P.; Goh, C.M.; Liu, K.; Schimenti, J.C.; Kaldis, P. A novel function for CDK2 activity at meiotic crossover sites. PLoS Biol. 2020, 18, e3000903. [Google Scholar] [CrossRef]
- Morelli, M.A.; Cohen, P.E. Not all germ cells are created equal: Aspects of sexual dimorphism in mammalian meiosis. Reproduction 2005, 130, 761–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, P.E.; Pollack, S.E.; Pollard, J.W. Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocrine Rev. 2006, 27, 398–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppi, L.; Jasin, M.; Keeney, S. The tricky path to recombining X and Y chromosomes in meiosis. Ann. N. Y. Acad. Sci. 2012, 1267, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raudsepp, T.; Chowdhary, B.P. The eutherian pseudoautosomal region. Cytogenet. Genome Res. 2015, 147, 81–94. [Google Scholar]
- Turner, J.M.; Mahadevaiah, S.K.; Ellis, P.J.; Mitchell, M.J.; Burgoyne, P.S. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev. Cell. 2006, 10, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.Y.; Yu, X. Double-strand break repair on sex chromosomes: Challenges during male meiotic prophase. Cell Cycle. 2015, 14, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Ashley, T.; Walpita, D.; de Rooij, D.G. Localization of two mammalian cyclin dependent kinases during mammalian meiosis. J. Cell Sci. 2001, 114, 685–693. [Google Scholar]
- Fredga, K. Unusual sex chromosome inheritance in mammals. Philos. R. Soc. Lond. B 1970, 259, 15–36. [Google Scholar] [CrossRef]
- Graves, J.M. Sex chromosomes and sex determination in weird mammals. Cytogenet Genome Res. 2002, 96, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Dresser, M.; Pisetsky, D.; Warren, R.; McCarty, G.; Moses, M. A new method for the cytological analysis of autoantibody specificities using whole-mount, surface-spread meiotic nuclei. J. Immunol. Methods 1987, 104, 111–121. [Google Scholar] [CrossRef]
- Bonilla, B.; Hengel, S.R.; Grundy, M.K.; Bernstein, K.A. RAD51 gene family structure and function. Ann. Rev. Genetics 2020, 54, 25–46. [Google Scholar] [CrossRef]
- Hinch, A.G.; Becker, P.W.; Li, T.; Moralli, D.; Zhang, G.; Bycroft, C.; Green, C.; Keeney, S.; Shi, Q.; Davies, B.; et al. The configuration of RPA, RAD51, and DMC1 binding in meiosis reveals the nature of critical recombination intermediates. Mol. Cell. 2020, 79, 689–701. [Google Scholar] [CrossRef]
- Matveevsky, S.; Bakloushinskaya, I.; Kolomiets, O. Unique sex chromosome systems in Ellobius: How do male XX chromosomes recombine and undergo pachytene chromatin inactivation? Sci. Rep. 2016, 6, 29949. [Google Scholar] [CrossRef] [Green Version]
- Matveevsky, S.; Kolomiets, O.; Bogdanov, A.; Alpeeva, E.; Bakloushinskaya, I. Meiotic chromosome contacts as a plausible prelude for Robertsonian translocations. Genes 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Fernández, A.; Matveevsky, S.; Martín-Ruiz, M.; Ribagorda, M.; Teresa Parra, M.; Viera, A.; Rufas, J.S.; Kolomiets, O.; Bakloushinskaya, I.; Page, J. Sex differences in the meiotic behavior of an XX sex chromosome pair in males and females of the mole vole Ellobius tancrei: Turning an X into a Y chromosome? Chromosoma 2021. Submitted. [Google Scholar]
- Lammers, J.H.; Offenberg, H.H.; Van Aalderen, M.; Vink, A.C.; Dietrich, A.J.; Heyting, C. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol. Cell. Biol. 1994, 14, 1137–1146. [Google Scholar]
- Zinkowski, R.P.; Meyne, J.; Brinkley, B.R. The centromere-kinetochore complex: A repeat subunit model. J. Cell Biol. 1991, 113, 1091–1110. [Google Scholar] [CrossRef] [Green Version]
- Kolomiets, O.L.; Matveevsky, S.N.; Bakloushinskaya, I.Y. Sexual dimorphism in prophase I of meiosis in the Northern mole vole (Ellobius talpinus Pallas, 1770) with isomorphic (XX) chromosomes in males and females. Comp. Cytogenet. 2010, 4, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Xu, R.; Yu, J.; Xu, T.; Zhuang, Y.; Han, M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 2007, 12, 863–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viera, A.; Alsheimer, M.; Gómez, R.; Berenguer, I.; Ortega, S.; Symonds, C.E.; Santamaría, D.; Benavente, R.; Suja, J.A. CDK2 regulates nuclear envelope protein dynamics and telomere attachment in mouse meiotic prophase. J. Cell Sci. 2015, 128, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.K.; Reeves, A.; Webb, L.M.; Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 1999, 151, 1569–1579. [Google Scholar]
- Schwacha, A.; Kleckner, N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 1995, 83, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Matveevsky, S.N.; Chassovnikarova, T.G.; Zidarova, S.A.; Atanasov, N.I.; Kolomiets, O.L. New chromosomal form of mole-rat Nannospalax leucodon (Rodentia: Spalacidae) from Western Bulgaria. Synaptonemal complex karyotyping. Acta Zool Bulg. 2020, Suppl 15, 27–32. [Google Scholar]
- Palmer, N.; Talib, S.Z.A.; Kaldis, P. Diverse roles for CDK-associated activity during spermatogenesis. FEBS Lett. 2019, 593, 2925–2949. [Google Scholar]
- Page, J.; De La Fuente, R.; Manterola, M.; Parra, M.T.; Viera, A.; Berríos, S.; Fernández-Donoso, R.; Rufas, J.S. Inactivation or non-reactivation: What accounts better for the silence of sex chromosomes during mammalian male meiosis? Chromosoma 2012, 121, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Youds, J.L.; Boulton, S.J. The choice in meiosis–defining the factors that influence crossover or non-crossover formation. J. Cell Sci. 2011, 124, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.M. Meiotic Silencing in Mammals. Rev. Genet. 2015, 49, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Kolomiets, O.L.; Vorontsov, N.N.; Lyapunova, E.A.; Mazurova, T.F. Ultrastructure, meiotic behavior, and evolution of sex chromosomes of the genus Ellobius. Genetica 1991, 847, 179–189. [Google Scholar] [CrossRef]
- Morimoto, A.; Shibuya, H.; Zhu, X.; Kim, J.; Ishiguro, K.I.; Han, M.; Watanabe, Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J. Cell Biol. 2012, 198, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.G.; Worman, H.J. Nuclear positioning. Cell 2013, 152, 1376–1389. [Google Scholar] [CrossRef] [Green Version]
- Horn, H.F.; Kim, D.I.; Wright, G.D.; Wong, E.S.M.; Stewart, C.L.; Burke, B.; Roux, K.J. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J. Cell Biol. 2013, 202, 1023–1039. [Google Scholar] [CrossRef] [Green Version]
- Alsheimer, M. The dance floor of meiosis: Evolutionary conservation of nuclear envelope attachment and dynamics of meiotic telomeres. In Meiosis. Genome Dynamics; Benavente, R., Wolff, J.-N., Eds.; Karger Publishers: Basel, Switzerland, 2009; Volume 5, pp. 81–93. [Google Scholar] [CrossRef]
- Tu, Z.; Bayazit, M.B.; Liu, H.; Zhang, J.; Busayavalasa, K.; Risal, S.; Shao, J.; Satyanarayana, A.; Coppola, V.; Tessarollo, L.; et al. Speedy A–Cdk2 binding mediates initial telomere–nuclear envelope attachment during meiotic prophase I independent of Cdk2 activation. Proc. Natl. Acad. Sci. USA 2017, 114, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, L.; Zhao, W.; Song, G.; Xu, R.; Wang, G.; Sun, F. Phosphorylation of CDK2 at threonine 160 regulates meiotic pachytene and diplotene progression in mice. Dev. Biol. 2014, 392, 108–116. [Google Scholar]
- Link, J.; Jantsch, V. Meiotic chromosomes in motion: A perspective from Mus musculus and Caenorhabditis elegans. Chromosoma 2019, 128, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Matveevsky, S.; Ivanitskaya, E.; Spangenberg, V.; Bakloushinskaya, I.; Kolomiets, O. Reorganization of the Y chromosomes enhances divergence in Israeli mole rats Nannospalax ehrenbergi (Spalacidae, Rodentia): Comparative analysis of meiotic and mitotic Chromosomes. Genes 2018, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Borodin, P.M.; Basheva, E.A.; Torgasheva, A.A.; Dashkevich, O.A.; Golenishchev, F.N.; Kartavtseva, I.V.; Mekada, K.; Dumont, B.L. Multiple independent evolutionary losses of XY pairing at meiosis in the grey voles. Chromosome Res. 2012, 20, 259–268. [Google Scholar] [CrossRef]
- Martinerie, L.; Manterola, M.; Chung, S.S.; Panigrahi, S.K.; Weisbach, M.; Vasileva, A.; Geng, Y.; Sicinski, P.; Wolgemuth, D.J. Mammalian E-type cyclins control chromosome pairing, telomere stability and CDK2 localization in male meiosis. PLoS Genet. 2014, 10, e1004165. [Google Scholar] [CrossRef] [Green Version]
- Mikolcevic, P.; Isoda, M.; Shibuya, H.; del Barco Barrantes, I.; Igea, A.; Suja, J.A.; Shackleton, S.; Watanabe, Y.; Nebreda, A.R. Essential role of the Cdk2 activator RingoA in meiotic telomere tethering to the nuclear envelope. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.O.; Reinholdt, L.G.; Motley, W.W.; Niswander, L.M.; Deacon, D.C.; Griffin, L.B.; Langlais, K.K.; Backus, V.L.; Schimenti, K.J.; O’Brien, M.J.; et al. Mutation in mouse hei10, an e3 ubiquitin ligase, disrupts meiotic crossing over. PLoS Genet. 2007, 3, e139. [Google Scholar] [CrossRef] [Green Version]
- Stokes, W.S. Reducing unrelieved pain and distress in laboratory animals using humane endpoints. ILAR J. 2000, 41, 59–60. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.H.F.M.; Plug, A.W.; van Vugt, M.J.; de Boer, P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germ line. Chromosome Res. 1997, 5, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Page, J.; Berríos, S.; Rufas, J.S.; Parra, M.T.; Suja, J.Á.; Heyting, C.; Fernández-Donoso, R. The pairing of X and Y chromosomes during meiotic prophase in the marsupial species Thylamys elegans is maintained by a dense plate developed from their axial elements. J. Cell Sci. 2003, 116, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveevsky, S.; Chassovnikarova, T.; Grishaeva, T.; Atsaeva, M.; Malygin, V.; Bakloushinskaya, I.; Kolomiets, O. Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes. Int. J. Mol. Sci. 2021, 22, 1969. https://doi.org/10.3390/ijms22041969
Matveevsky S, Chassovnikarova T, Grishaeva T, Atsaeva M, Malygin V, Bakloushinskaya I, Kolomiets O. Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes. International Journal of Molecular Sciences. 2021; 22(4):1969. https://doi.org/10.3390/ijms22041969
Chicago/Turabian StyleMatveevsky, Sergey, Tsenka Chassovnikarova, Tatiana Grishaeva, Maret Atsaeva, Vasilii Malygin, Irina Bakloushinskaya, and Oxana Kolomiets. 2021. "Kinase CDK2 in Mammalian Meiotic Prophase I: Screening for Hetero- and Homomorphic Sex Chromosomes" International Journal of Molecular Sciences 22, no. 4: 1969. https://doi.org/10.3390/ijms22041969





