TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD
Abstract
:1. Introduction
2. Results
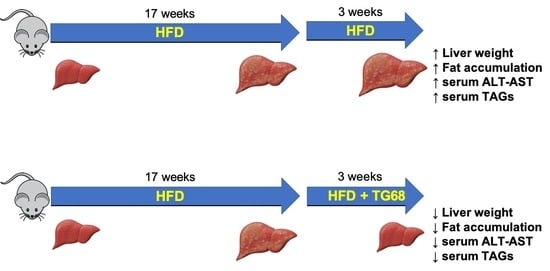
2.1. A High Dose of TG68 Caused a Reduction in Liver Volume and Hepatic Steatosis
2.2. The Strong Reduction in Intrahepatic Neutral Lipid Accumulation of HFD-Fed Mice Is Achieved Also by a Lower Dosage of TG68
2.3. TG68 Reduced Liver Damage and the Levels of Circulating Triacylglycerides
2.4. TG68 Specifically Induces Thyroid Hormone Receptor Target Genes
2.5. TG68 Showed Liver Specificity
3. Discussion
4. Materials and Methods
4.1. Mice and Drug Treatments
4.2. Analysis of Serum Triacylglycerides, Cholesterol, Aspartate Aminotransferase, and Alanine Aminotransferase
4.3. Histological Analyses
4.4. Determination of Hepatocyte Proliferation
4.5. Determination of Hepatic-Activated Fibrogenic Cells
4.6. RNA Extraction and qRT-PCR
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the Period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Trépo, E.; Valenti, L. Update on NAFLD Genetics: From New Variants to the Clinic. J. Hepatol. 2020, 72, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Viggiano, T.R.; Mcgill, D.B.; Oh, B.J. Nonalcoholic Steatohepatitis: Mayo Clinic Experiences with a Hitherto Unnamed Disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current Concepts and Future Challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; de Paolis, P.; Capussotti, L.; Salizzoni, M. Expanding the Natural History of Nonalcoholic Steatohepatitis: From Cryptogenic Cirrhosis to Hepatocellular Carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Day, C.P. From Fat to Inflammation. Gastroenterology 2006, 130, 207–210. [Google Scholar] [CrossRef]
- Gupta, A.; Das, A.; Majumder, K.; Arora, N.; Mayo, H.G.; Singh, P.P.; Beg, M.S.; Singh, S. Obesity Is Independently Associated with Increased Risk of Hepatocellular Cancer-Related Mortality: A Systematic Review and Meta-Analysis. Am. J. Clin. Oncol. 2018, 41, 874. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. The New Liver Epidemic. Nat. Biotechnol. 2019, 37, 209–214. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Am. Physiol. Soc. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.A. Thyroid Hormone Receptors: Multiple Forms, Multiple Possibilities. Endocr. Rev. 1993, 14, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, H.; Rudling, M.; Saltó, C.; Forrest, D.; Angelin, B.; Vennström, B. Requirement for Thyroid Hormone Receptor β in T3 Regulation of Cholesterol Metabolism in Mice. Mol. Endocrinol. 2002, 16, 1767–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brent, G.A. The Molecular Basis of Thyroid Hormone Action. N. Engl. J. Med. 2010, 331, 847–853. [Google Scholar] [CrossRef]
- Forrest, D.; Vennström, B. Functions of Thyroid Hormone Receptors in Mice. Thyroid 2009, 10, 41–52. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, C.-Y.; Tsai, M.-M.; Tsai, C.-Y.; Lin, K.-H. Molecular Functions of Thyroid Hormones and Their Clinical Significance in Liver-Related Diseases. BioMed Res. Int. 2013, 2013, 601361. [Google Scholar] [CrossRef] [Green Version]
- Krause, C.; Grohs, M.; el Gammal, A.T.; Wolter, S.; Lehnert, H.; Mann, O.; Mittag, J.; Kirchner, H. Reduced Expression of Thyroid Hormone Receptor β in Human Nonalcoholic Steatohepatitis. Endocr. Connect. 2018, 7, 1448. [Google Scholar] [CrossRef] [Green Version]
- Frau, C.; Loi, R.; Petrelli, A.; Perra, A.; Menegon, S.; Kowalik, M.A.; Pinna, S.; Leoni, V.P.; Fornari, F.; Gramantieri, L.; et al. Local Hypothyroidism Favors the Progression of Preneoplastic Lesions to Hepatocellular Carcinoma in Rats. Hepatology 2015, 61, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; de Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic Effects of Thyroid Hormone Derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Webb, P. Selective Activators of Thyroid Hormone Receptors. Thyroid 2005, 13, 489–500. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the Treatment of Non-Alcoholic Steatohepatitis: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; McCarty, K.; Frias, J.P.; Taub, R.; Alkhouri, N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH. Hepatol. Commun. 2021, 5, 573. [Google Scholar] [CrossRef]
- Runfola, M.; Sestito, S.; Bellusci, L.; la Pietra, V.; D’Amore, V.M.; Kowalik, M.A.; Chiellini, G.; Gul, S.; Perra, A.; Columbano, A.; et al. Design, Synthesis and Biological Evaluation of Novel TRβ Selective Agonists Sustained by ADME-Toxicity Analysis. Eur. J. Med. Chem. 2020, 188, 112006. [Google Scholar] [CrossRef]
- Perra, A.; Kowalik, M.A.; Cabras, L.; Runfola, M.; Sestito, S.; Migliore, C.; Giordano, S.; Chiellini, G.; Rapposelli, S.; Columbano, A. Potential Role of Two Novel Agonists of Thyroid Hormone Receptor-β on Liver Regeneration. Cell Prolif. 2020, 53, e12808. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.-E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.-S.; et al. Discovery of 2-[3,5-Dichloro-4-(5-Isopropyl-6-Oxo-1,6-Dihydropyridazin-3-Yloxy)Phenyl]-3,5-Dioxo-2,3,4,5-Tetrahydro [1, 2, 4]Triazine-6-Carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor β Agonist in Clinical Trials for the Treatment of Dyslipidemia. J. Med. Chem. 2014, 57, 3912–3923. [Google Scholar] [CrossRef]
- Kowalik, M.A.; Columbano, A.; Perra, A. Thyroid Hormones, Thyromimetics and Their Metabolites in the Treatment of Liver Disease. Front. Endocrinol. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaoka, K.; Nouchi, T.; Marumo, F.; Sato, C. α-Smooth-Muscle Actin Expression in Normal and Fibrotic Human Livers. Dig. Dis. Sci. 1993, 38, 1473–1479. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Ricci, C.; Fantauzzi, C.B.; Scipioni, A.; Salvi, L.; Cordone, S.; Delucchi, F.; Serino, M.; Federici, M.; et al. Galectin-3 Ablation Protects Mice from Diet-Induced NASH: A Major Scavenging Role for Galectin-3 in Liver. J. Hepatol. 2011, 54, 975–983. [Google Scholar] [CrossRef]
- Chalasani, N.; Abdelmalek, M.F.; Garcia-Tsao, G.; Vuppalanchi, R.; Alkhouri, N.; Rinella, M.; Noureddin, M.; Pyko, M.; Shiffman, M.; Sanyal, A.; et al. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients with Nonalcoholic Steatohepatitis with Cirrhosis and Portal Hypertension. Gastroenterology 2020, 158, 1334–1345.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Deng, S.; Wang, Y.; Li, P.; Tang, L.; Pang, Y. Specific Inhibition of Acyl-CoA Oxidase-1 by an Acetylenic Acid Improves Hepatic Lipid and Reactive Oxygen Species (ROS) Metabolism in Rats Fed a High Fat Diet. J. Biol. Chem. 2017, 292, 3800–3809. [Google Scholar] [CrossRef] [Green Version]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of Carnitine Palmitoyltransferase-1 in Skeletal Muscle Is Sufficient to Enhance Fatty Acid Oxidation and Improve High-Fat Diet–Induced Insulin Resistance. Diabetes 2009, 58, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, I.; Ojamaa, K. Thyroid Hormone and the Cardiovascular System. N. Engl. J. Med. 2009, 64, 2330–2338. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kütting, F.; Goeser, T.; Demir, M.; Steffen, H.-M. NAFLD and Cardiovascular Diseases: A Clinical Review. Clin. Res. Cardiol. 2021, 110, 921. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, F.; Ding, Y.; Hou, J.; Bi, J.; Zhang, Z. Association of Non-Alcoholic Fatty Liver Disease with Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 33386. [Google Scholar] [CrossRef] [Green Version]
- Weltman, N.Y.; Wang, D.; Redetzke, R.A.; Gerdes, A.M. Longstanding Hyperthyroidism Is Associated with Normal or Enhanced Intrinsic Cardiomyocyte Function despite Decline in Global Cardiac Function. PLoS ONE 2012, 7, 46655. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Bruinstroop, E.; Singh, B.K.; Yen, P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid 2019, 29, 1173. [Google Scholar] [CrossRef]
- Wafa, B.; Faten, H.; Mouna, E.; Fatma, M.; Mohamed, A. Hyperthyroidism and Hepatic Dysfunction: Report of 17 Cases. JGH Open 2020, 4, 876–879. [Google Scholar] [CrossRef] [Green Version]
- Scappaticcio, L.; Longo, M.; Maiorino, M.I.; Pernice, V.; Caruso, P.; Esposito, K.; Bellastella, G. Abnormal Liver Blood Tests in Patients with Hyperthyroidism: Systematic Review and Meta-Analysis. Thyroid 2021, 31, 922–932. [Google Scholar] [CrossRef]
- Kannt, A.; Wohlfart, P.; Madsen, A.N.; Veidal, S.S.; Feigh, M.; Schmoll, D. Activation of Thyroid Hormone Receptor-β Improved Disease Activity and Metabolism Independent of Body Weight in a Mouse Model of Non-Alcoholic Steatohepatitis and Fibrosis. Br. J. Pharmacol. 2021, 178, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ. 1997–2018; U.S. National Institutes of Health: Bethesda, MD, USA. Available online: https://imagej.nih.gov/ij/ (accessed on 30 November 2021).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021, 22, 13105. https://doi.org/10.3390/ijms222313105
Caddeo A, Kowalik MA, Serra M, Runfola M, Bacci A, Rapposelli S, Columbano A, Perra A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. International Journal of Molecular Sciences. 2021; 22(23):13105. https://doi.org/10.3390/ijms222313105
Chicago/Turabian StyleCaddeo, Andrea, Marta Anna Kowalik, Marina Serra, Massimiliano Runfola, Andrea Bacci, Simona Rapposelli, Amedeo Columbano, and Andrea Perra. 2021. "TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD" International Journal of Molecular Sciences 22, no. 23: 13105. https://doi.org/10.3390/ijms222313105