Semi-Continuous Desalination and Concentration of Small-Volume Samples
Abstract
:1. Introduction
2. Results
2.1. Determination of Outlet Concentrations
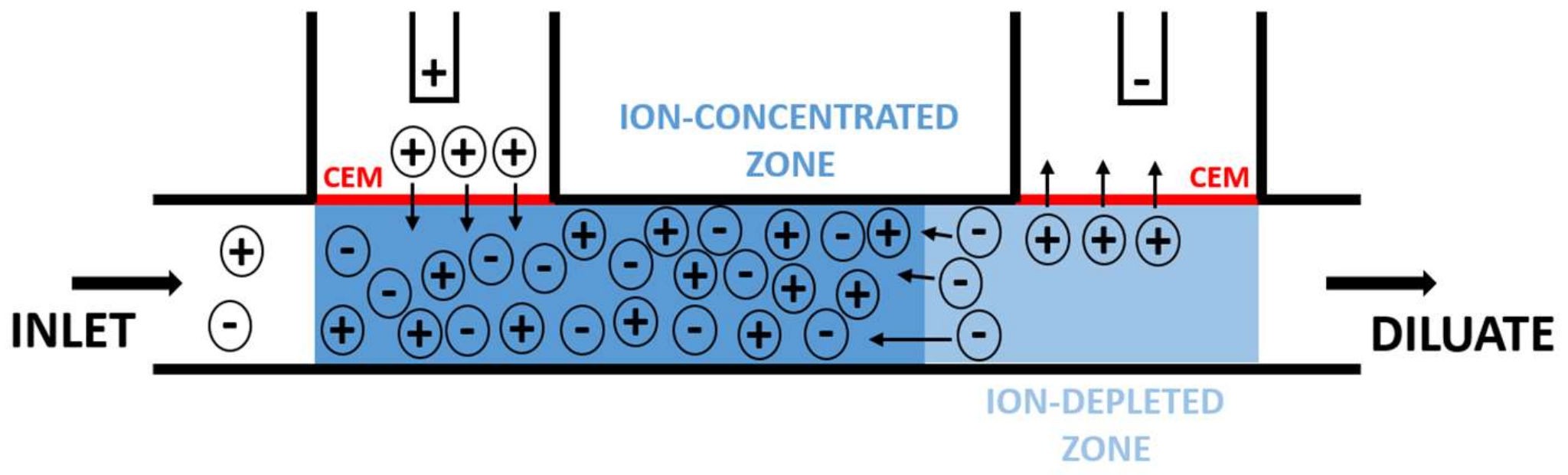
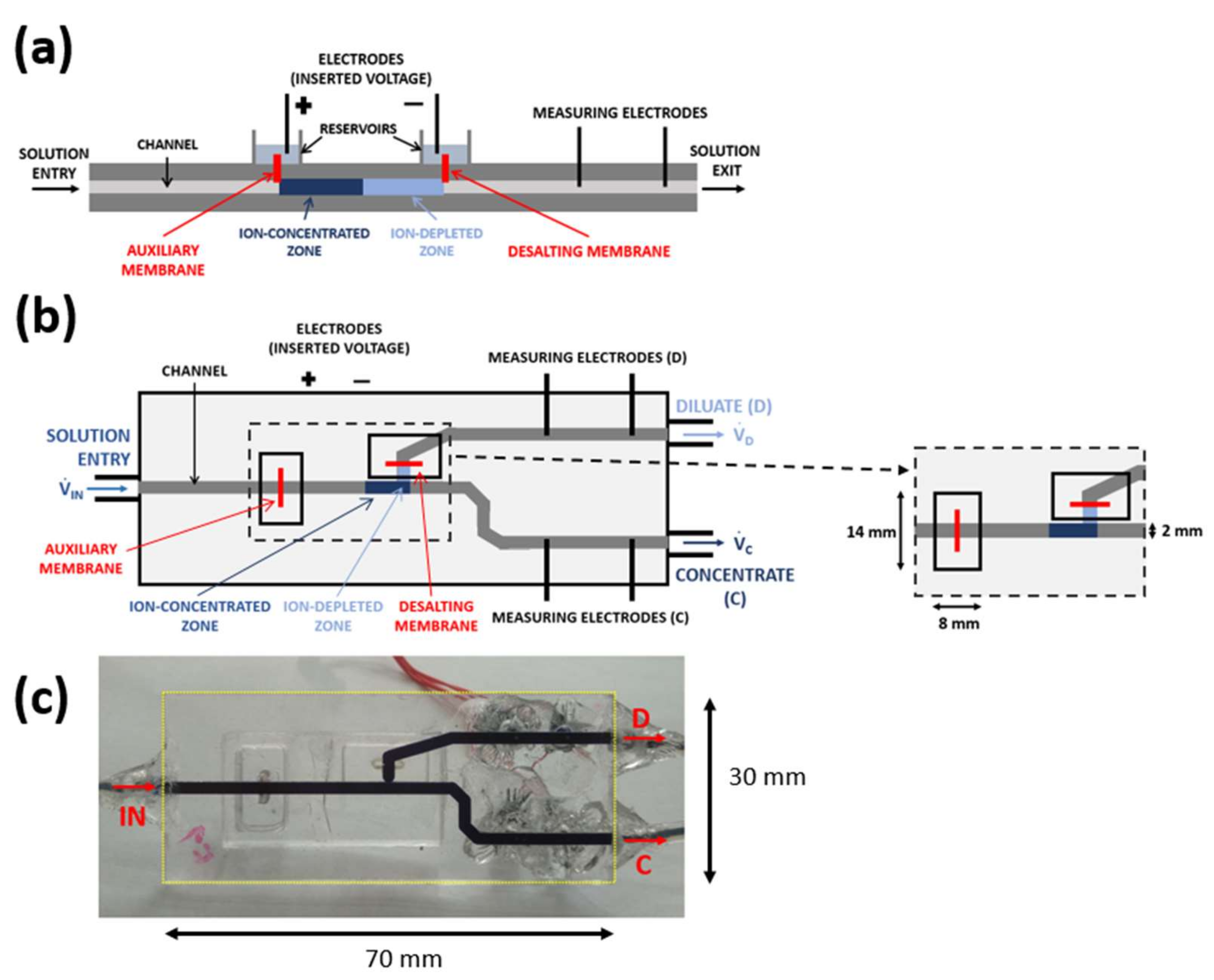
2.2. Principle of Operation
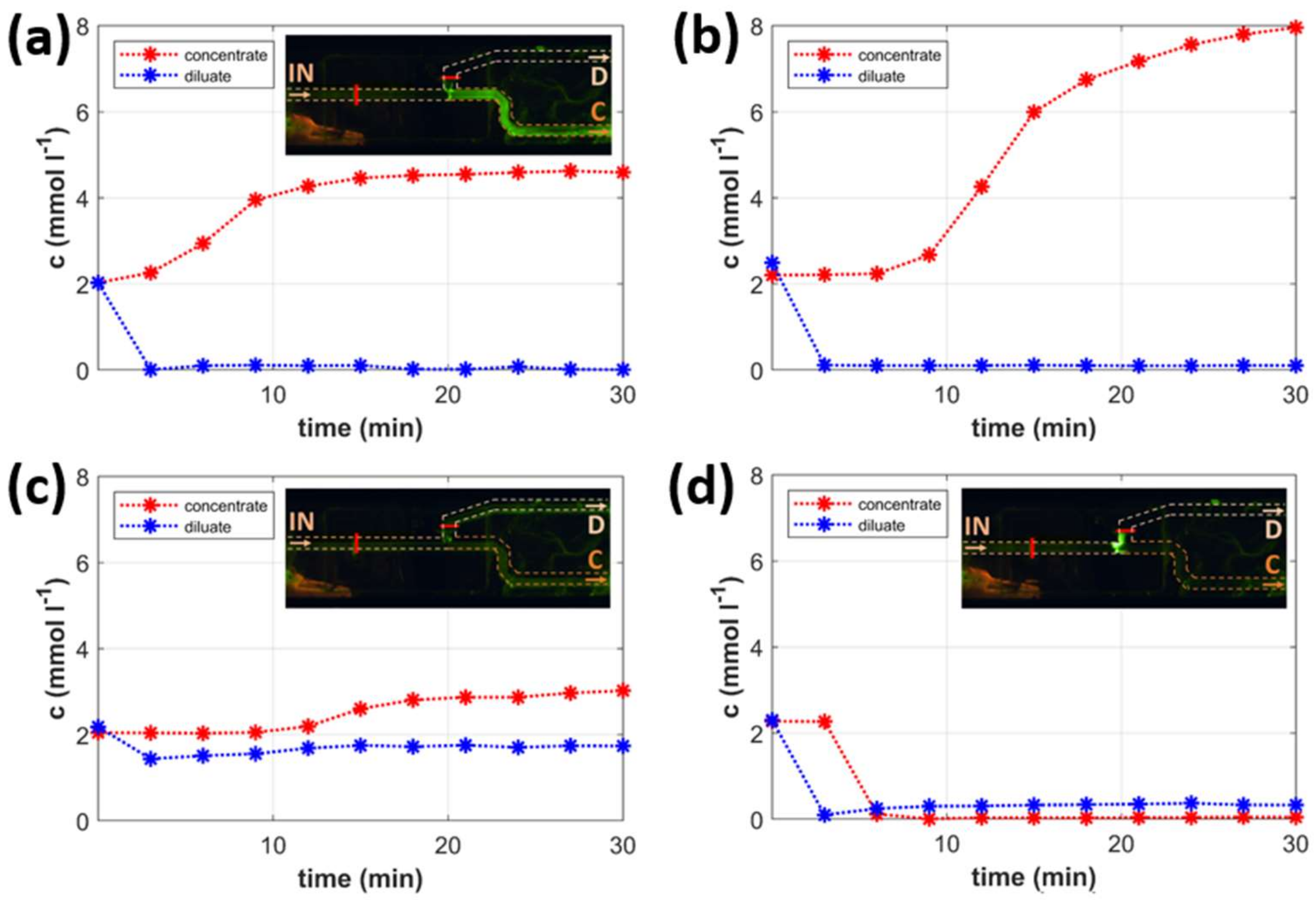
2.3. Types of Chip Operation
2.4. Mapping the Types of Operation
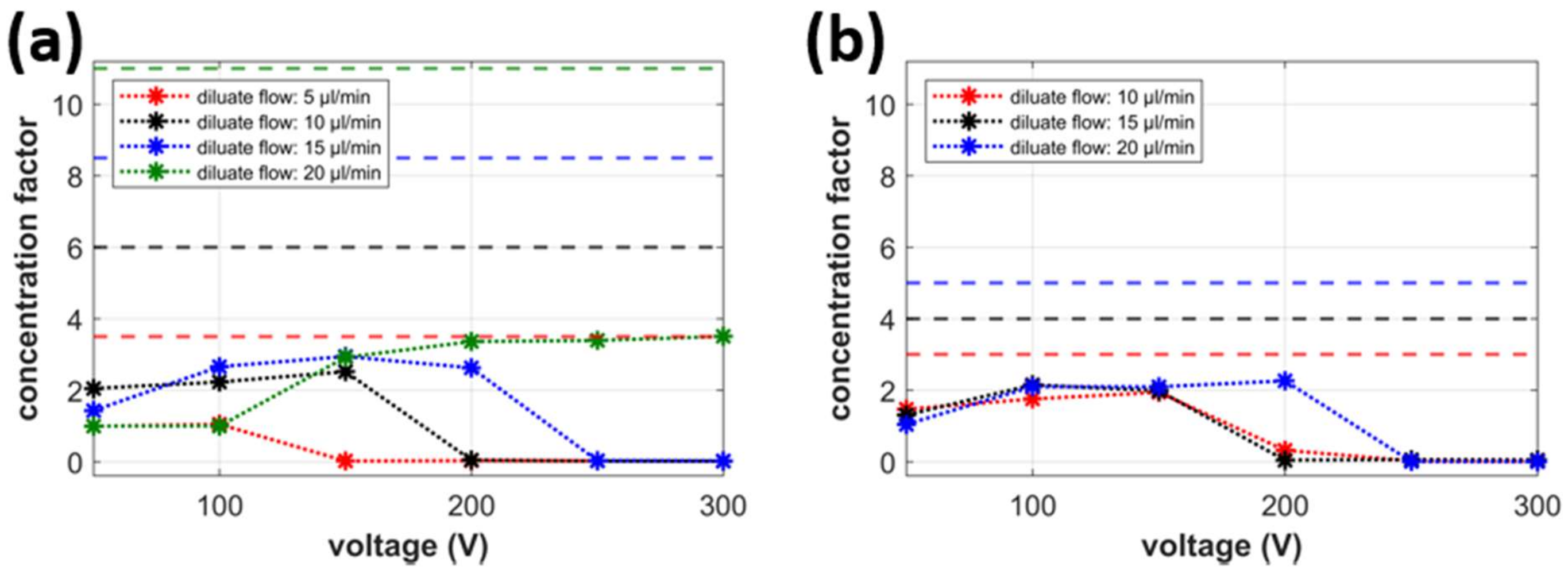
2.5. Desalination Factor
2.6. Concentration Factor
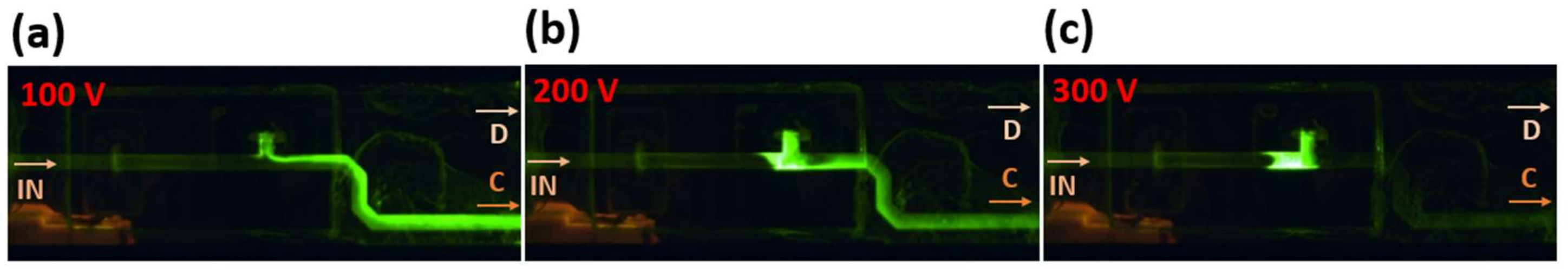
2.7. Fluorescein Experiments
3. Material and Methods
3.1. Material
3.2. Chip Design and Fabrication
3.3. Experimental Setup
3.4. Desalination Experiments
3.5. Results Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, T.W. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.B.; Yang, Z.J.; Wang, Y.M.; Jiang, C.X.; Ge, L.; Bakangura, E.; Xu, T.W. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Sea water desalination using electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- Arar, O.; Yuksel, U.; Kabay, N.; Yuksel, M. Various applications of electrodeionization (EDI) method for water treatment—A short review. Desalination 2014, 342, 16–22. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Huygebaert, M.; Van der Bruggen, B.; Ballet, R.; Meesschaert, B.; Pinoy, L. Desalination of an industrial saline water with conventional and bipolar membrane electrodialysis. Desalination 2013, 318, 9–18. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive delonization (CDI) for desalination and water treatment—Past, present and future (a review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- Luo, J.Y.; Wu, C.M.; Xu, T.W.; Wu, Y.H. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Yang, K.; Chu, X.M.; Zhang, X.J.; Li, X.F.; Zheng, J.F.; Li, S.H.; Li, N.W.; Sherazi, T.A.; Zhang, S.B. The effect of polymer backbones and cation functional groups on properties of anion exchange membranes for fuel cells. J. Membr. Sci. 2020, 603, 118025. [Google Scholar] [CrossRef]
- Wu, X.W.; Hu, J.P.; Liu, J.; Zhou, Q.M.; Zhou, W.X.; Li, H.Y.; Wu, Y.P. Ion exchange membranes for vanadium redox flow batteries. Pure Appl. Chem. 2014, 86, 633–649. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Li, M.; Anand, R.K. Recent advancements in ion concentration polarization. Analyst 2016, 141, 3496–3510. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Ihm, S.; Park, S.; Yu, Y.; Kwak, R. Concentric ion concentration polarization desalination for efficient En-bloc preconcentration and desalination. Desalination 2021, 499, 114810. [Google Scholar] [CrossRef]
- Bellon, T.; Polezhaev, P.; Vobecka, L.; Svoboda, M.; Slouka, Z. Experimental observation of phenomena developing on ion-exchange systems during current-voltage curve measurement. J. Membr. Sci. 2019, 572, 607–618. [Google Scholar] [CrossRef]
- Svoboda, M.; Slouka, Z.; Schrott, W.; Snita, D. Cation exchange membrane integrated into a microfluidic device. Microelectron. Eng. 2009, 86, 1371–1374. [Google Scholar] [CrossRef]
- Wang, Y.C.; Stevens, A.L.; Han, J.Y. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Slouka, Z.; Senapati, S.; Yan, Y.; Chang, H.C. Charge Inversion, Water Splitting, and Vortex Suppression Due to DNA Sorption on Ion-Selective Membranes and Their Ion-Current Signatures. Langmuir 2013, 29, 8275–8283. [Google Scholar] [CrossRef]
- Belova, E.; Lopatkova, G.; Pismenskaya, N.; Nikonenko, V.; Larchet, C. Role of water splitting in development in ion-exchange membrane of electroconvection systems. Desalination 2006, 199, 59–61. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Kwak, R. Controlling ion transport with pattern structures on ion exchange membranes in electrodialysis. Desalination 2021, 499, 114801. [Google Scholar] [CrossRef]
- Pundik, T.; Rubinstein, I.; Zaltzman, B. Bulk electroconvection in electrolyte. Phys. Rev. E 2005, 72, 061502. [Google Scholar] [CrossRef]
- Polezhaev, P.; Belloň, T.; Vobecká, L.; Slouka, Z. Molecular sieving of alkyl sulfate anions on strong basic gel-type anion-exchange resins. Sep. Purif. Technol. 2021, 276, 119382. [Google Scholar] [CrossRef]
- Simons, R. Origin and Elimination of Water Splitting in Ion-Exchange Membranes during Water Demineralization by Electrodialysis. Desalination 1979, 28, 41–42. [Google Scholar] [CrossRef]
- Sablani, S.S.; Goosen, M.F.A.; Al-Belushi, R.; Wilf, M. Concentration polarization in ultrafiltration and reverse osmosis: A critical review. Desalination 2001, 141, 269–289. [Google Scholar] [CrossRef]
- Slouka, Z.; Senapati, S.; Shah, S.; Lawler, R.; Shi, Z.G.; Stack, M.S.; Chang, H.C. Integrated, DC voltage-driven nucleic acid diagnostic platform for real sample analysis: Detection of oral cancer. Talanta 2015, 145, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.B.; Chen, X.Y. A review: Applications of ion transport in micro-nanofluidic systems based on ion concentration polarization. J. Chem. Technol. Biot. 2020, 95, 1622–1631. [Google Scholar] [CrossRef]
- Senapati, S.; Slouka, Z.; Shah, S.S.; Behura, S.K.; Shi, Z.G.; Stack, M.S.; Severson, D.W.; Chang, H.C. An ion-exchange nanomembrane sensor for detection of nucleic acids using a surface charge inversion Phenomenon. Biosens. Bioelectron. 2014, 60, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.C.; Slouka, Z.; Chang, H.C. Fluidic-based ion memristors and ionic latches. Small 2015, 11, 5206–5213. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef]
- Shen, M.; Yang, H.; Sivagnanam, V.; Gijs, M.A.M. Microfluidic protein preconcentrator using a microchannel-integrated nafion strip: Experiment and modeling. Anal. Chem. 2010, 82, 9989–9997. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Rhee, H.; Jeon, T.J.; Kim, D. Preconcentration of lipid vesicles using concentration polarization in a microfluidic chip. Sens. Actuators B Chem. 2016, 229, 276–280. [Google Scholar] [CrossRef]
- Han, S.I.; Yoo, Y.K.; Lee, J.; Kim, C.; Lee, K.; Lee, T.H.; Kim, H.; Yoon, D.S.; Hwang, K.S.; Kwak, R.; et al. High-ionic-strength pre-concentration via ion concentration polarization for blood-based biofluids. Sens. Actuators B Chem. 2018, 268, 485–493. [Google Scholar] [CrossRef]
- Kovar, P.; Tichy, D.; Slouka, Z. Effect of channel geometry on ion-concentration polarization-based preconcentration and desalination. Biomicrofluidics 2019, 13, 064102. [Google Scholar] [CrossRef]
- Abdulbari, H.A.; Basheer, E.A.M. Microfluidics chip for directional solvent extraction desalination of seawater. Sci. Rep. 2019, 9, 12576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ige, E.O.; Arun, R.K.; Singh, P.; Gope, M.; Saha, R.; Chanda, N.; Chakraborty, S. Water desalination using graphene oxide-embedded paper microfluidics. Microfluid. Nanofluidics 2019, 23, 80. [Google Scholar] [CrossRef]
- Li, H.B.; Zou, L. Ion-exchange membrane capacitive deionization: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- MacDonald, B.D.; Gong, M.M.; Zhang, P.; Sinton, D. Out-of-plane ion concentration polarization for scalable water desalination. Lab Chip 2014, 14, 681–685. [Google Scholar] [CrossRef]
- Schlumpberger, S.; Lu, N.B.; Suss, M.E.; Bazant, M.Z. Scalable and continuous water deionization by shock electrodialysis. Environ. Sci. Tech. Let. 2015, 2, 367–372. [Google Scholar] [CrossRef]
- Knust, K.N.; Hlushkou, D.; Anand, R.K.; Tallarek, U.; Crooks, R.M. Electrochemically mediated seawater desalination. Angew. Chem. Int. Edit 2013, 52, 8107–8110. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Li, L.D.; Han, J. Amplified electrokinetic response by concentration polarization near nanofluidic channel. Langmuir 2009, 25, 7759–7765. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Concentration Interval | (0; 0.2) mM | (0.2; 20) mM |
|---|---|---|
| Concentrate channel | cC = 4355∙(1/R2)2 − 8.593∙(1/R2) + 0.004 | cC = 1.0982∙(1/R2) − 0.0009 |
| Diluate channel | cD = 3533∙(1/R2)2 − 6.913∙(1/R2) + 0.0033 | cD = 1.0010∙(1/R2) − 0.0008 |
| VD/VC (μL/min) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5/2 | 10/2 | 10/5 | 15/2 | 15/5 | 20/2 | 20/5 | 20/10 | ||
| U (V) | 50 | C | X | C | X | X | X | X | X |
| 100 | C | C | C | C | C | X | C | C | |
| 150 | A | C | C | C | C | C | C | C | |
| 200 | A | A | A | C | A | C | C | C | |
| 250 | A | A | A | A | A | C | A | C | |
| 300 | A | A | A | A | A | A | A | A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichý, D.; Slouka, Z. Semi-Continuous Desalination and Concentration of Small-Volume Samples. Int. J. Mol. Sci. 2021, 22, 12904. https://doi.org/10.3390/ijms222312904
Tichý D, Slouka Z. Semi-Continuous Desalination and Concentration of Small-Volume Samples. International Journal of Molecular Sciences. 2021; 22(23):12904. https://doi.org/10.3390/ijms222312904
Chicago/Turabian StyleTichý, David, and Zdeněk Slouka. 2021. "Semi-Continuous Desalination and Concentration of Small-Volume Samples" International Journal of Molecular Sciences 22, no. 23: 12904. https://doi.org/10.3390/ijms222312904





