Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology
Abstract
:1. Introduction
2. The GABAergic System
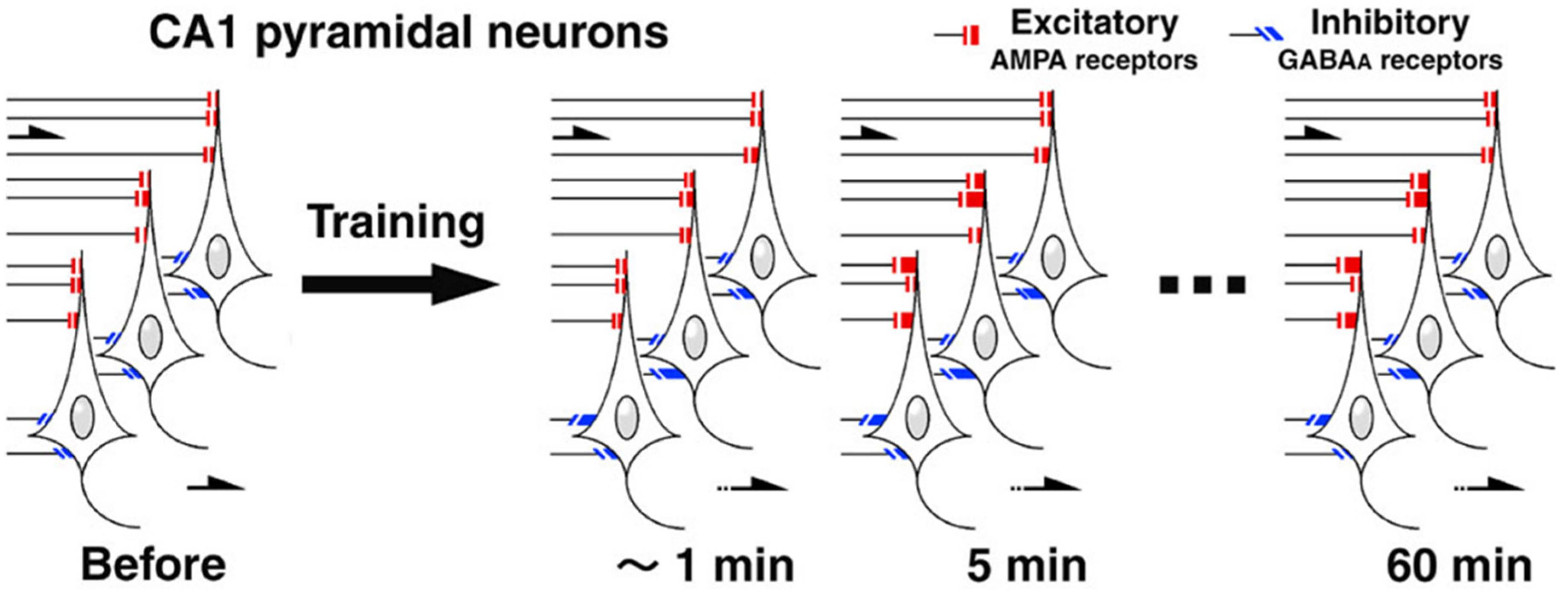
3. Contextual Fear Memory Triggers Rapid Synaptic Plasticity
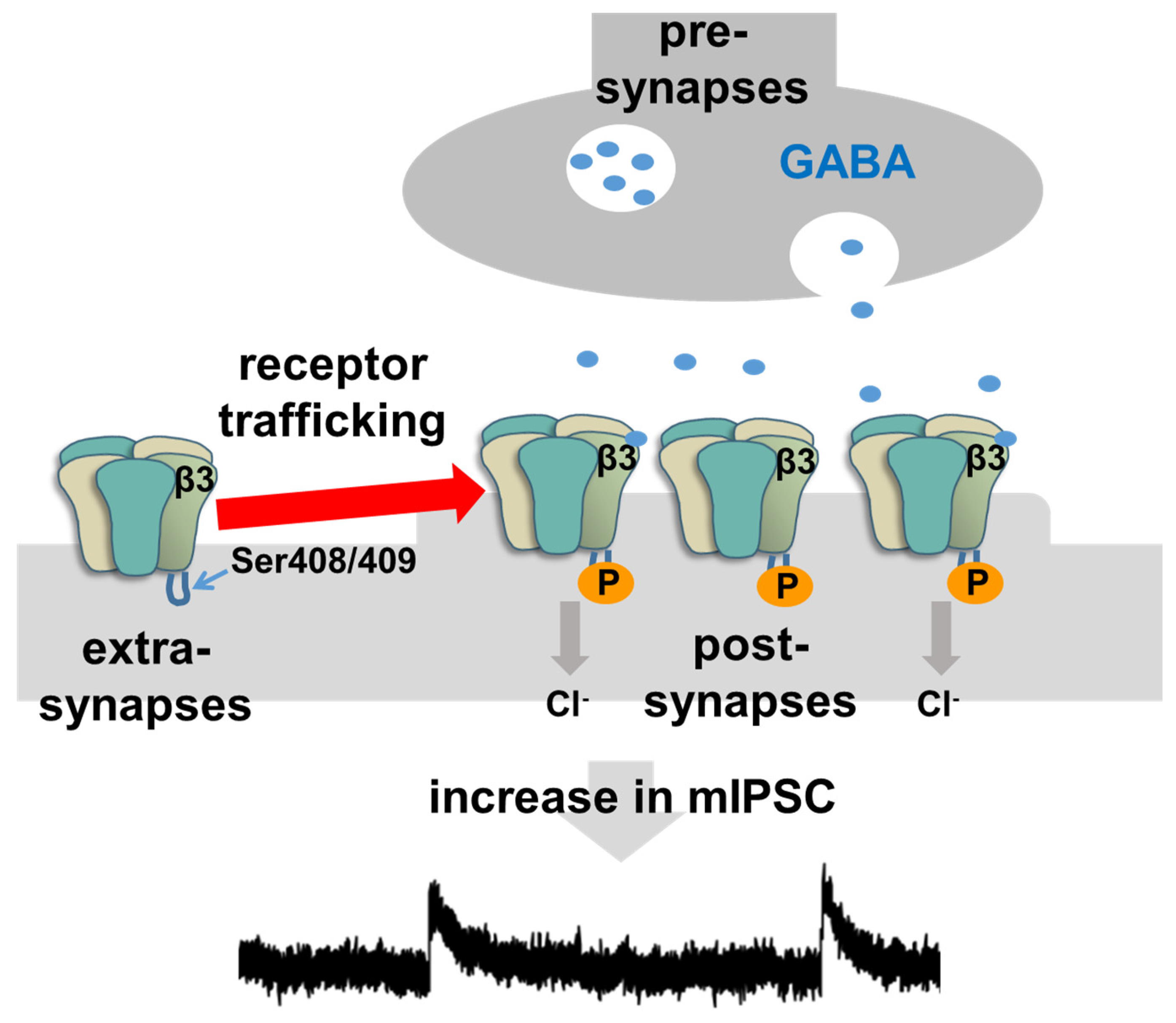
4. Intracellular Mechanism of Rapid Inhibitory Synaptic Plasticity
5. Alterations to GABAARβ3 in Cognitive Disease
5.1. AD
GABAA Receptor as Therapeutic Target in AD
5.2. ASD
GABAA Receptor as Therapeutical Target in ASD
5.3. SE
GABAA Receptor as Therapeutical Target in SE
5.4. PTSD
GABAA Receptor as Therapeutical Target in PTSD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- West, M.J.; Slomianka, L.; Gundersen, H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef] [PubMed]
- Bezaire, M.J.; Soltesz, I. Quantitative assessment of CA1 local circuits: Knowledge base for interneuron-pyramidal cell connectivity. Hippocampus 2013, 23, 751–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic inhibitory interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- DeFelipe, J.; López-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larranaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairen, A.; Feldmeyer, D.; et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, L.; Guo, F.; Han, X. The effects of GABAergic system under cerebral ischemia: Spotlight on cognitive function. Neural Plast. 2020, 2020, 8856722. [Google Scholar] [CrossRef]
- Megias, M.; Emri, Z.; Freund, T.F.; Gulyas, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 2001, 102, 527–540. [Google Scholar] [CrossRef]
- Bowery, N.G.; Hudson, A.L.; Price, G.W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience 1987, 20, 365–383. [Google Scholar] [CrossRef]
- Bormann, J.; Feigenspan, A. GABAC receptor. Trends Neurosci. 1995, 18, 515–519. [Google Scholar] [CrossRef]
- Cui, Y.; Costa, R.M.; Murphy, G.G.; Elgersma, Y.; Zhu, Y.; Gutmann, D.H.; Parada, L.F.; Mody, I.; Silva, A.J. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell 2008, 135, 549–560. [Google Scholar] [CrossRef] [Green Version]
- Mitsushima, D.; Sano, A.; Takahashi, T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat. Commun. 2013, 4, 2760. [Google Scholar] [CrossRef] [Green Version]
- Lovett-Barron, M.; Kaifosh, P.; Kheirbek, M.A.; Danielson, N.; Zaremba, J.D.; Reardon, T.R.; Turi, G.F.; Hen, R.; Zemelman, B.V.; Losonczy, A. Dendritic inhibition in the hippocampus supports fear learning. Science 2014, 343, 857–863. [Google Scholar] [CrossRef] [Green Version]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef] [PubMed]
- Telgkamp, P.; Padgett, D.E.; Ledoux, V.A.; Woolley, C.S.; Raman, I.M. Maintenance of high-frequency transmission at Purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron 2004, 41, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Pugh, J.R.; Raman, I.M. GABAA receptor kinetics in the cerebellar nuclei: Evidence for detection of transmitter from distant release sites. Biophys. J. 2005, 88, 1740–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, A.N.; Triller, A.; Sieghart, W. GABAA receptors: Post-synaptic co-localization and cross-talk with other receptors. Font. Cell Neurosci. 2011, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maingret, F.; Groc, L. Characterization of the functional cross-talk between surface GABAA and dopamine D5 receptors. Int. J. Mol. Sci. 2021, 22, 4867. [Google Scholar] [CrossRef] [PubMed]
- Castellano, C.; McGaugh, J.L. Effects of post-training bicuculline and muscimol on retention: Lack of state dependency. Behav. Neural Biol. 1990, 54, 156–164. [Google Scholar] [CrossRef]
- Jerusalinsky, D.; Ferreira, M.B.C.; Walz, R.; Da Silva, R.C.; Bianchin, M.; Ruschel, A.C.; Zanatta, M.S.; Medina, J.H.; Izquierdo, I. Amnesia by post-training infusion of glutamate receptor antagonists into the amygdala, hippocampus and entorhinal cortex. Behav. Neural Biol. 1992, 58, 76–80. [Google Scholar] [CrossRef]
- Bonini, J.S.; Rodrigues, L.; Kerr, D.S.; Bevilaqua, L.R.; Cammarota, M.; Izquierdo, I. AMPA/kainate and group-I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behav. Pharmacol. 2003, 14, 161–166. [Google Scholar] [CrossRef]
- Luft, T.; Pereira, G.S.; Cammarota, M.; Izquierdo, I. Different time course for the memory facilitating effect of bicuculline in hippocampus, entorhinal cortex, and posterior parietal cortex of rats. Neurobiol. Learn. Mem. 2004, 82, 52–56. [Google Scholar] [CrossRef]
- Izquierdo, I.; Bevilaqua, L.R.M.; Rossato, J.I.; Bonini, J.S.; Medina, J.H.; Cammarota, M. Different molecular cascades in different sites of the brain control consolidation. Trends Neurosci. 2006, 28, 496–505. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.M.; Park, S.J.; Cai, M.; Liu, X.; Lee, S.; Shin, C.Y.; Ryu, J.H. GABAA receptor blockade enhances memory consolidation by increasing hippocampal BDNF levels. Neuropsychopharmacology 2012, 37, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, Y.; Kida, H.; Mitsushima, D. Temporal dynamics of learning-promoted synaptic diversity in CA1 pyramidal neurons. FASEB J. 2019, 33, 14382–14393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyashita, T.; Kubik, S.; Haghighi, N.; Steward, O.; Guzowski, J.F. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J. Neurosci. 2009, 29, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, Y.; Mizuno, J.; Kida, H.; Kamiya, Y.; Ono, Y.; Mitsushima, D. Learning promotes subfield-specific synaptic diversity in hippocampal CA1 neurons. Cereb. Cortex 2019, 29, 2183–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsushima, D.; Ishihara, K.; Sano, A.; Kessels, H.W.; Takahashi, T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc. Natl. Acad. Sci. USA 2011, 108, 12503–12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, K.; Iwanari, H.; Tada, H.; Suyama, K.; Sano, A.; Nagai, T.; Hamakubo, T.; Takahashi, T. Optical inactivation of synaptic AMPA receptors erases fear memory. Nat. Biotechnol. 2017, 35, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kida, H.; Tsuda, Y.; Ito, N.; Yamamoto, Y.; Owada, Y.; Kamiya, Y.; Mitsushima, D. Motor training promotes both synaptic and intrinsic plasticity of layer II/III pyramidal neurons in the primary motor cortex. Cereb. Cortex 2016, 26, 3494–3507. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.T.; Chen, G.; Honing, S.; Bogdanov, Y.; McAinsh, K.; Arancibia-Carcamo, I.L.; Jovanovic, J.N.; Pangalos, M.N.; Hauche, V.; Yan, Z.; et al. Phospho-depenent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc. Natl. Acad. Sci. USA 2005, 102, 14871–14876. [Google Scholar] [CrossRef] [Green Version]
- DeLorey, T.M.; Handforth, A.; Anagnostaras, S.G.; Homanics, G.E.; Minassian, B.A.; Asatourian, A.; Ellison, G.D.; Olsen, R.W. Mice lacking the β3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J. Neurosci. 1998, 18, 8505–8514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luscher, B.; Fuchs, T.; Kilpatrick, C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 2011, 70, 385–409. [Google Scholar] [CrossRef] [Green Version]
- McDonald, B.J.; Moss, S.J. Differential phosphorylation of intracellular domains of gamma-aminobutyric acid type A receptor subunits by calcium/calmodulin type 2-dependent protein kinase and cGMP-dependent protein kinase. J. Biol. Chem. 1994, 269, 18111–18117. [Google Scholar] [CrossRef]
- McDonald, B.J.; Amato, A.; Connolly, C.N.; Benke, D.; Moss, S.J.; Smart, T.G. Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase. Nat. Neurosci. 1998, 1, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Brandon, N.J.; Delmas, P.; Kittler, J.T.; McDonald, B.J.; Sieghart, W.; Brown, D.A.; Smart, T.G.; Moss, S.J. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem. 2000, 275, 38856–38862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandon, N.J.; Jovanovic, J.N.; Smart, T.G.; Moss, S.J. Receptor for activated C kinase-1 facilitates protein kinase C-dependent phosphorylation and functional modulation of GABAA receptors with the activation of G-protein-coupled receptors. J. Neurosci. 2002, 22, 6353–6361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Mortensen, M.; Hosie, A.M.; Smart, T.G. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat. Neurosci. 2005, 8, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, Y.; Michels, G.; Armstrong-Gold, C.; Haydon, P.G.; Lindstrom, J.; Pangalos, M. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006, 25, 4381–4389. [Google Scholar] [CrossRef] [Green Version]
- Mele, M.; Leal, G.; Buarte, C.B. Role of GABAAR trafficking in the plasticity of inhibitory synapses. J. Neurochem. 2016, 139, 997–1018. [Google Scholar] [CrossRef] [PubMed]
- De Luca, E.; Ravasenga, T.; Petrini, E.M.; Polenghi, A.; Nieus, T.; Guazzi, S.; Barberis, A. Inter-synaptic lateral diffusion of GABAA receptors shapes inhibitory synaptic currents. Neuron 2017, 95, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tretter, V.; Mukherjee, J.; Maric, H.M.; Schindelin, H.; Sieghart, W.; Moss, S.J. Gephyrin, the enigmatic organizer at GABAergic synapses. Front. Cell Neurosci. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, S.; Winkelmann, A.; Smolinsky, B.; Förstera, B.; Neundorf, I.; Schwarz, G.; Meier, J.C. Direct binding of GABAA receptor β2 and β3 subunits to gephyrin. Eur. J. Neurosci. 2013, 37, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Kretschmannova, K.; Gouzer, G.; Maric, H.M.; Ramsden, S.; Tretter, V.; Harvey, K.; Davies, P.A.; Triller, A.; Schindelin, H.; et al. The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor α1 subunit to gephyrin. J. Neurosci. 2011, 31, 14677–14687. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, J.N.; Thomas, P.; Kittler, J.T.; Smart, T.G.; Moss, S.J. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABAA receptor phosphorylation, activity and cell-surface stability. J. Neurosci. 2004, 24, 522–530. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Cheng, P.L.; Lim, B.K.; Khoshnevisrad, N.; Poo, M.M. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 2010, 67, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrini, E.M.; Barberis, A. Diffusion dynamics of synaptic molecules during inhibitory postsynaptic plasticity. Front. Cell Neurosci. 2014, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson-Vest, N.M.; Waldvogel, H.J.; Rees, M.I.; Faull, R.L. GABAA receptor subunit and gephyrin protein changes differ in the globus pallidus in Huntington’s diseased brain. Brain Res. 2003, 994, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Möhler, H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 475–498. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Charry, L.; Nardi, L.; Methner, A.; Schmeisser, M.J. Abnormalities of synaptic mitochondria in autism spectrum disorder and related neurodevelopmental disorders. J. Mol. Med. 2020, 99, 161–178. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.O. Epileptic mechanisms shared by Alzheimer’s disease: Viewed via the unique lens of genetic epilepsy. Int. J. Mol Sci. 2021, 22, 7133. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Costa, R.O.; Duarte, C.B. Alterations in GABAA-receptor trafficking and synaptic dysfunction in brain disorders. Front. Cell Neurosci. 2019, 13, 77. [Google Scholar] [CrossRef]
- Lei, M.; Xu, H.; Li, Z.; Wang, Z.; O’Malley, T.T.; Zhang, D.; Walsh, D.M.; Xu, P.; Selkoe, D.J.; Li, S. Soluble Aβ oligomers impair hippocampal LTP by disrupting glutamatergic/GABAergic balance. Neurobiol. Dis. 2016, 85, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Vyas, Y.; Montgomery, J.M.; Cheyne, J.E. Hippocampal deficits in anyloidβ related rodent models of Alzheimer’s disease. Front. Neurosci. 2020, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; O’Gorman, J.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Counts, S.E.; Ikonomovic, M.D.; Mercado, N.; Vega, I.; Mufson, E.J. Biomarkers for the early detection and progression of Alzheimer’s disease. Neurotherapeutics 2017, 14, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Liu, W.; Yan, Z. β-amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase Ⅱ synaptic distribution. J. Biol. Chem. 2009, 284, 10639–10649. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.Q.; Santini, F.; Breese, R.; Ross, D.; Zhang, X.D.; Stone, D.J.; Ferrer, M.; Townsend, M.; Wolfe, A.L.; Seager, M.A.; et al. Inhibition of calcineurin-mediated endocytosis and AMPA receptor prevent amyloid β oligomer-induced synaptic disruption. J. Biol. Chem. 2010, 285, 7619–7632. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Lee, D.H.; D’Andrea, M.R.; Peterson, P.A.; Shank, R.P.; Reitz, A.B. β-Amyloid1-42 binds to α7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J. Biol. Chem. 2000, 275, 5626–5632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Townsend, M.; Whyment, A.; Walczak, J.S.; Jeggo, R.; van den Top, M.; Flood, D.G.; Leventhal, L.; Patzke, H.; Koening, G. α7-nAChR agonist enhances neural plasticity in the hippocampus via a GABAergic circuit. J. Neurophysiol. 2016, 116, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D. Amyloid-β impairs synaptic inhibition via GABAA receptor endocytosis. J. Neurosci. 2015, 35, 9205–9210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Kawai, H.; Berg, D.K. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 4734–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limon, A.; Reyes-Ruiz, J.M.; Miledi, R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. USA 2012, 109, 10071–10076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Calvo-Flores, G.B.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L. Gamma-aminobutyric acid A receptors in Alzheimer’s disease: Highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 2018, 13, 1362–1363. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Ban, J.Y.; Seong, Y.H. Chronic stimulation of GABAA receptor with muscimol reduces amyloid beta protein (23-35)-induced neurotoxicity in cultured rat cortical cells. Neurosci. Res. 2005, 52, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Obregon, D.; Ehrhart, J.; Deng, J.; Tian, J.; Hou, H.; Giunta, B.; Swamiller, D.; Tan, J.S.-Q.; Obregon, D.; et al. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer’s disease transgenic mouse model. J. Neurosci. Res. 2013, 91, 1239–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crocetti, L.; Guerrini, G. GABAA receptor subtype modulators in medicinal chemistry: An updated patent review (2014-present). Expert Opin. Ther. Pat. 2020, 30, 409–432. [Google Scholar] [CrossRef]
- Shimohama, T.; Taniguchi, T.; Fujiwara, M.; Kemeyama, M. Changes in benzodiazepine receptors in Alzheimer-type dementia. Ann. Neurol. 1998, 23, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Sakimoto, Y.; Tsukada, Y.; Kimura, R.; Kida, H.; Mitsushima, D. Adverse effects of Aβ1-42 oligomers: Contextual learning and GABAA synapses in CA1 pyramidal neurons. J. Physiol. Sci. 2021, 71 (Suppl. 1), 24. [Google Scholar]
- Alanis, B.A.V.; Iorio, M.T.; Silva, L.L.; Bampali, K.; Ernst, M.; Schnurch, M.; Mihovilovic, M.D. Allosteric GABAA receptor modulators—A review on the most recent heterocyclic chemotypes and their synthetic accessibility. Molecules 2020, 25, 999. [Google Scholar] [CrossRef] [Green Version]
- Pascual, B.; Prieto, E.; Arbizu, J.; Marti-Climent, J.M.; Penuelas, I.; Quincoces, G.; Zarauza, R.; Pappata, S.; Masdeu, J.C. Decreased carbon-11-flumazenil binding in early Alzheimer’s disease. Brain 2012, 135, 2817–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzikov, S.A.; Morozov, S.G.; Kubatiev, A.A. Intricacies of GABAA receptor function: The Critical Role of the β3 subunit in norm and pathology. Int. J. Mol. Sci. 2021, 22, 1457. [Google Scholar] [CrossRef]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [PubMed]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic causes and modifiers of autism spectrum disorder. Front. Cell Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Coghlan, S.; Horder, J.; Inkster, B.; Mendez, M.A.; Murphy, D.G.; Nutt, D. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci. Biobehav. Rev. 2012, 36, 2033–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Homanics, G.E.; DeLorey, T.M.; Firestone, L.L.; Quinlan, J.J.; Handforth, A.; Harrison, N.L.; Krasowski, M.D.; Rick, C.E.M.; Korpi, E.R.; Makela, R.; et al. Mice devoid of γ-aminobutyrate typeA receptor β3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl. Acad. Sci. USA 1997, 94, 4143–4148. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Lee, J.; Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 2017, 81, 838–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Thuras, P.D. GABAA receptor downregulation in brains of subjects with autism. J. Autism Dev. Disord. 2009, 39, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Barnea-Goraly, N.; Frazier, T.W.; Piacenza, L.; Minshew, N.J.; Keshavan, M.S.; Reiss, A.L.; Hardan, A.Y. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Bangerter, A.; Ness, S.; Aman, M.G.; Esbensen, A.J.; Goodwin, M.S.; Dawson, G.; Hendren, R.; Leventhal, B.; Khan, A.; Opler, M.; et al. Autism behavior inventory: A novel tool for assessing core and associated symptoms of autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2017, 27, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Culotta, L.; Penzes, P. Exploring the mechanisms underlying excitation/inhibition imbalance in human iPSC-derived models of ASD. Mol. Autism 2020, 11, 32. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Darnieder, L.M.; Deeb, T.Z.; Moss, S.J. Regulation of GABAARs by phosphorylation. Adv. Pharmacol. 2015, 72, 97–146. [Google Scholar] [PubMed] [Green Version]
- Han, S.; Tai, C.; Westenbroek, R.E.; Yu, F.H.; Cheah, C.S.; Potter, G.B.; Rubenstein, J.L.; Scheuer, T.; de la lglesia, H.O.; Catterall, W.A. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012, 489, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Tai, C.; Jones, C.J.; Scheuer, T.; Catterall, W.A. Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron 2014, 81, 1282–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherian, A.; Thomas, S.V. Status epilepticus. Ann. Indian Acad. Neurol. 2009, 12, 140–153. [Google Scholar]
- Asada, H.; Kawamura, Y.; Maruyama, K.; Kume, H.; Ding, R.; Ji, F.Y.; Kanbara, N.; Kuzume, H.; Sanbo, M.; Yagi, T.; et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 1996, 229, 891–895. [Google Scholar] [CrossRef]
- Janve, V.S.; Hernandez, C.C.; Verdier, K.M.; Hu, N.; Macdonald, R.L. Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function. Ann. Neurol. 2016, 79, 806–825. [Google Scholar] [CrossRef] [PubMed]
- Møller, R.S.; Wuttke, T.V.; Helbig, I.; Marini, C.; Johannesen, K.M.; Brilstra, E.H.; Vaher, U.; Borggraefe, I.; Talvik, I.; Talvik, T.; et al. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology 2017, 88, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Absalom, N.L.; Ahring, P.K.; Liao, V.W.; Balle, T.; Jiang, T.; Anderson, L.L.; Arnold, J.C.; McGregor, I.S.; Bowen, M.T.; Chebib, M. Functional genomics of epilepsy-associated mutations in the GABAA receptor subunits reveal that one mutation impairs function and two are catastrophic. J. Biol. Chem. 2019, 294, 6157–6171. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C. Alterations in synaptic function in epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.N., Avoli, M., Rogawski, M., Olsen, R., Delgado-Escueta, A., Eds.; Oxford University Press: New York, NJ, USA, 2012. [Google Scholar]
- Sayin, U.; Sutula, T.P.; Stafstrom, C.E. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia 2004, 45, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, M.; Xu, J.; Vithlani, M.; Sieghart, W.; Kittler, J.; Pangalos, M.; Haydon, P.G.; Coulter, D.A.; Moss, S.J. Deficits in phosphorylation of GABAA receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J. Neurosci. 2008, 28, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, F.I.; Tejos-Bravo, M.; Diaz-Veliz, G.; Pacheco, A.; Garcia-Rogo, G.; Corrales, W.; Olave, F.A.; Aliaga, E.; Ulloa, J.; Avalos, A.M.; et al. Hippocampal memory recovery after acute stress: Behavioral, morphological and molecular study. Front. Mol. Neurosci. 2018, 11, 283. [Google Scholar] [CrossRef]
- Uysal, N.; Sisman, A.R.; Dayi, A.; Ozbal, S.; Cetin, F.; Baykara, B.; Aksu, I.; Tas, A.; Cavus, S.A.; Gonenc-Arda, S.; et al. Acute foot-shock-stress increases spatial learning-memory and correlates to increased hippocampal BDNF and VEGF and cell numbers in adolescent male and female rats. Neurosci. Lett. 2012, 514, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Shizadian, A.; Ostadhadi, S.; Hassanipour, M.; Shafaroodi, H.; Khoshnoodi, M.; Haj-Mirzaian, A.; Sharifzadeh, M.; Amiri, S.; Ghasemi, M.; Dehpour, A.R. Acute foot-shock stress decreased seizure susceptibility against pentylenetetrazole-induced seizures in mice: Interaction between endogenous. Epilepsy Behav. 2018, 87, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.J., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptor. Seizure 2013, 22, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankovic, S.M.; Djesevic, M.; Jankovic, S.V. Experimental GABAA receptor agonists and allosteric modulators for the treatment of focal epilepsy. J. Exp. Pharmacol. 2021, 13, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Fritschy, J.M.; Kiener, T.; Bouilleret, V.; Loup, F. GABAergic neurons and GABAA-receptors in temporal lobe epilepsy. Neurochem. Int. 1999, 34, 435–445. [Google Scholar] [CrossRef]
- Stamboulian-Platel, S.; Legendre, A.; Chabrol, T.; Platel, J.C.; Pernot, F.; Duveau, V.; Roucard, C.; Baudry, M.; Depaulis, A. Activation of GABAA receptors controls mesiotemporal lobe epilepsy despite changes in chloride transporters expression: In vivo and in silico approach. Exp. Neurol. 2016, 284 (Pt A), 11–28. [Google Scholar] [CrossRef]
- Brophy, G.M.; Bell, R.; Claassen, J.; Alldredge, B.; Bleck, T.P.; Glauser, T.; Laroche, S.M.; Riviello, J., Jr.; Shutter, L.; Sperling, M.R.; et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012, 17, 3–23. [Google Scholar] [CrossRef]
- Wilkes, R.; Tasker, R.C. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit. Care Clin. 2013, 29, 239–257. [Google Scholar] [CrossRef]
- Olff, M. Sex and gender differences in post-traumatic stress disorder: An update. Eur. J. Psychotraumatol. 2017, 8, 1351204. [Google Scholar] [CrossRef]
- Teicher, M.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Lu, C.Y.; Liu, D.X.; Jiang, H.; Pan, F.; Ho, C.S.H.; Ho, R.C.M. Effects of traumatic stress induced in the juvenile period on the expression of GABAA receptor subunits in adult rat brain. Neural Plast. 2017, 2017, 5715816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girgenti, M.J.; Wang, J.; Ji, D.; Cruz, D.A.; Traumatic Stress Brain Research Group; Stein, M.B.; Gelernter, J.; Young, K.A.; Huber, B.R.; Williamnson, D.E.; et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 2021, 24, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kavushansky, A.; Vouimba, R.M.; Choen, H.; Richter-Levin, G. Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus 2006, 16, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sharvit, A.; Segal, M.; Kehat, O.; Stork, O.; Richter-Levin, G. Differential modulation of synaptic plasticity and local circuit activity in the dentate gyrus and CA1 regions of the rat hippocampus by corticosterone. Stress 2015, 18, 319–327. [Google Scholar] [CrossRef]
- Zhou, H.; Xiong, G.-J.; Jing, L.; Song, N.-N.; Pu, D.-L.; Tang, X.; He, X.-B.; Xu, F.-Q.; Huang, J.-F.; Li, L.-J.; et al. The interhemispheric CA1 circuit governs rapid generalization but not fear memory. Nat. Commun. 2017, 8, 2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vouimba, R.M.; Anunu, R.; Richter-Levin, G. GABAergic transmission in the basolateral amygdala differentially modulates plasticity in the dentate gyrus and the CA1 areas. Int. J. Mol. Sci. 2020, 21, 3786. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.; Demiray, Y.E.; Kliche, S.; Jing, L.; Hazra, S.; Hazra, J.D.; Richter-Levin, G.; Stork, O. Reducing glutamic acid decarboxylase in the dorsal dentate gyrus attenuates juvenile stress induced emotional and cognitive deficits. Neurobiol. Stress 2021, 15, 100350. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2020, 14, 100286. [Google Scholar] [CrossRef] [PubMed]
- Ardi, Z.; Richter-Levin, A.; Xu, L.; Cao, X.; Volkmer, H.; Stork, O.; Richter-Levin, G. The role of GABAA receptor α1 subunit in the ventral hippocampus in stress resilience. Sci. Rep. 2019, 9, 13513. [Google Scholar] [CrossRef] [PubMed]
- Barnard, E.A.; Skolnick, P.; Olsen, R.W.; Mohler, H.; Sieghart, W.; Biggio, G.; Braestrup, C.; Bateson, A.N.; Langer, S.A. International union of pharmacology. XV. Subtypes of GABAA receptor: Classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998, 50, 291–314. [Google Scholar]
- Geuze, E.; van Berchel, B.N.M.; Lammertsma, A.A.; Boellaard, R.; de Kloet, C.S.; Vermetten, E.; Westenberg, H.G.M. Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol. Psychiatry 2008, 13, 74–83. [Google Scholar] [CrossRef]
- Feusner, J.; Ritchie, T.; Lawford, B.; Young, R.; Kann, B.; Noble, E.P. GABAA receptorβ3 subunit gene and psychiatric morbidity in a post-traumatic stress disorder population. Psychiatry Res. 2001, 104, 109–117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakimoto, Y.; Oo, P.M.-T.; Goshima, M.; Kanehisa, I.; Tsukada, Y.; Mitsushima, D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. Int. J. Mol. Sci. 2021, 22, 12456. https://doi.org/10.3390/ijms222212456
Sakimoto Y, Oo PM-T, Goshima M, Kanehisa I, Tsukada Y, Mitsushima D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. International Journal of Molecular Sciences. 2021; 22(22):12456. https://doi.org/10.3390/ijms222212456
Chicago/Turabian StyleSakimoto, Yuya, Paw Min-Thein Oo, Makoto Goshima, Itsuki Kanehisa, Yutaro Tsukada, and Dai Mitsushima. 2021. "Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology" International Journal of Molecular Sciences 22, no. 22: 12456. https://doi.org/10.3390/ijms222212456




