In Vivo Renin Activity Imaging in the Kidney of Progeroid Ercc1 Mutant Mice
Abstract
:1. Introduction
2. Results
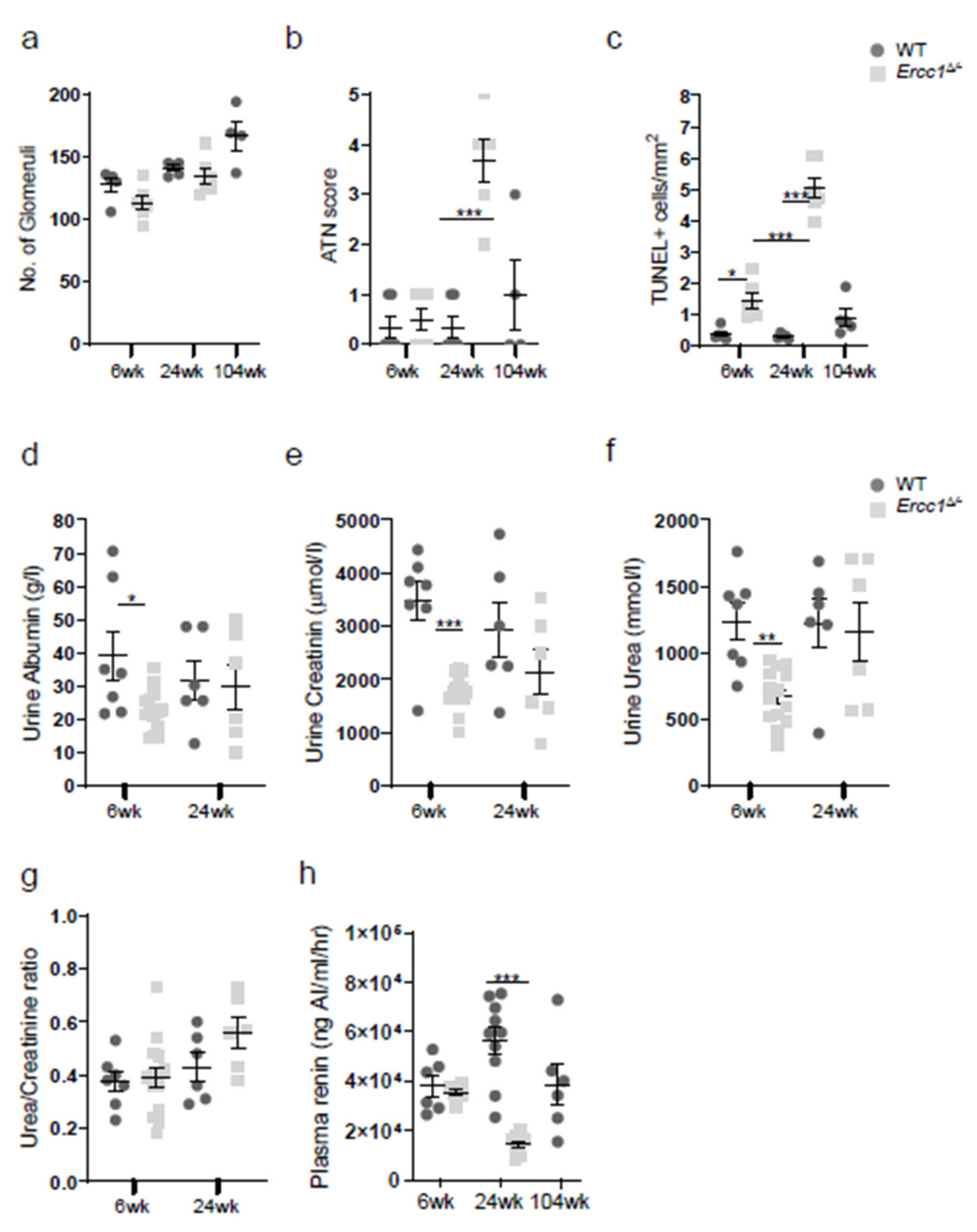
2.1. Progeroid Ercc1d/− Mice Display Age-Related Kidney Pathology
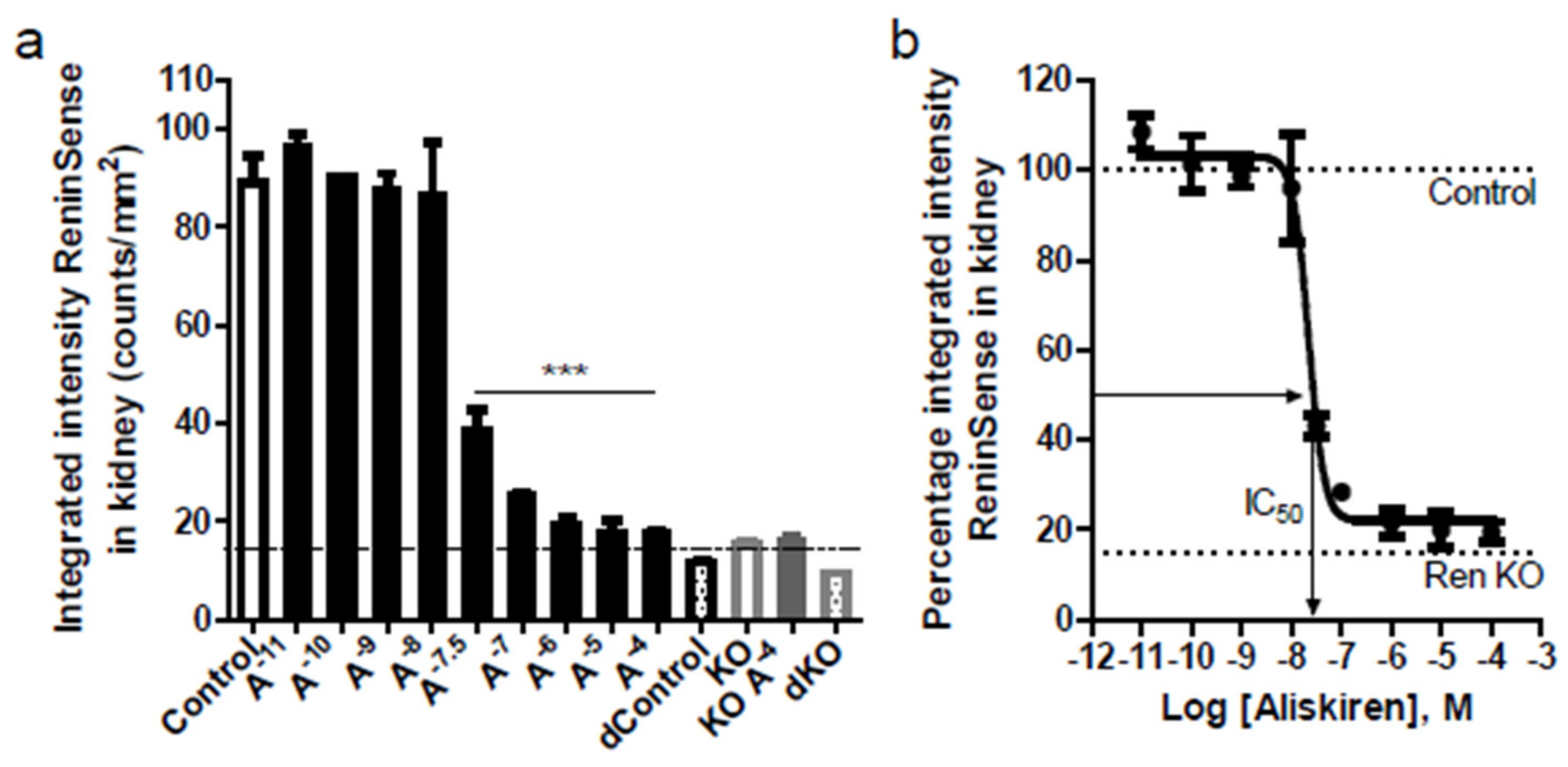
2.2. ReninSense Selectively Detects Renin Activity in the Kidney In Vitro
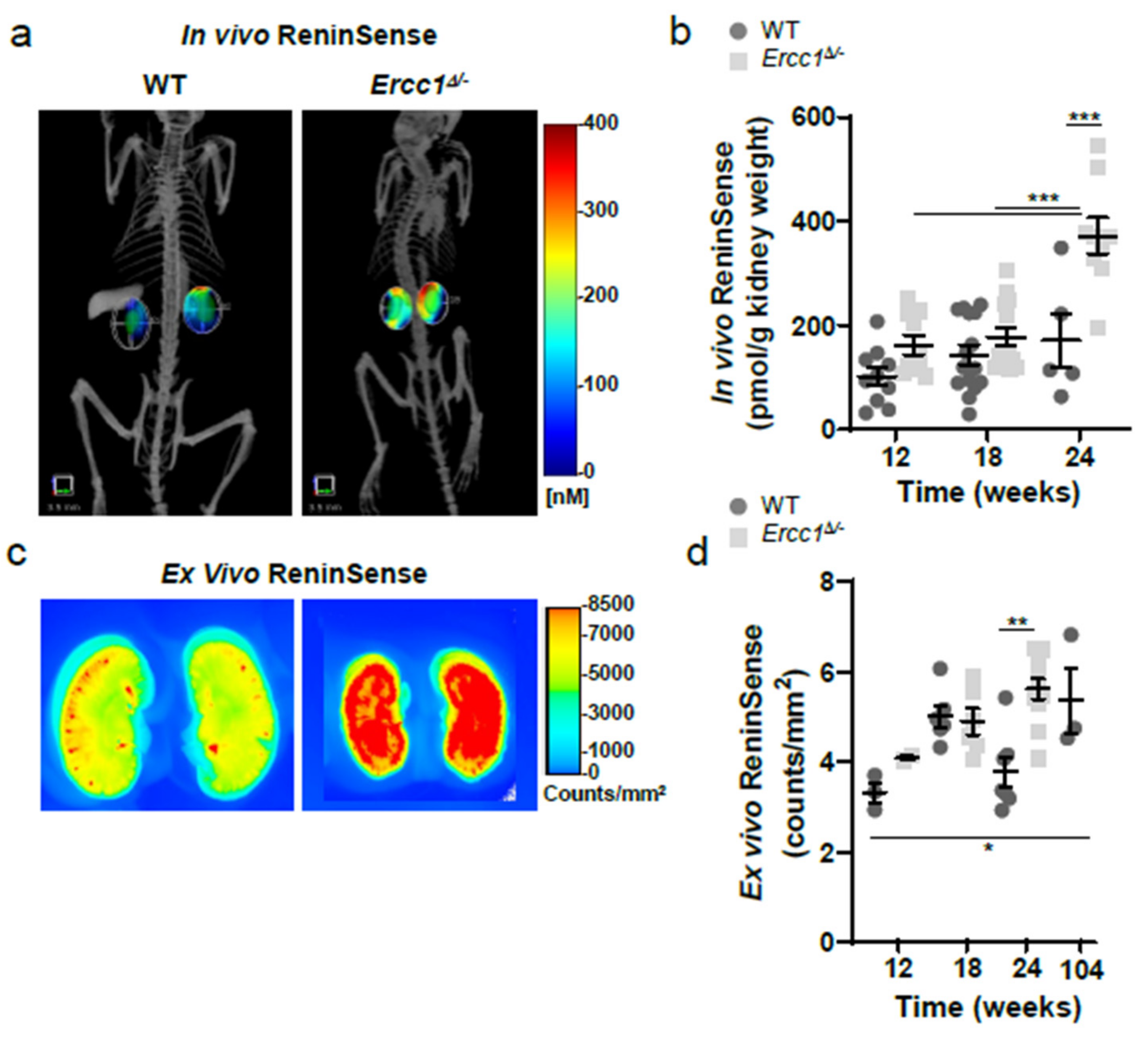
2.3. In Vivo Imaging of Renin Upregulation Shown by ReninSense
2.4. Increased Renin Activity in the Kidneys of Progeroid Ercc1d/− Mice In Vivo
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Histological Assessment
4.3. Plasma Renin Concentration Measured by Enzyme-Kinetic Assay
4.4. Urine Measurements Relevant to Renal Function
4.5. In Vivo MicroCT-FMT Imaging of Renin Activity
4.6. Tissue Collection and Ex Vivo Fluorescent Imaging of Excised Kidneys
4.7. In Vitro Fluorescent Imaging of Kidney and Plasma Renin Activity
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S. Ageing and the renin-angiotensin system. Nephrol. Dial. Transplant. 1997, 12, 1093–1094. [Google Scholar] [CrossRef]
- Conti, S.; Cassis, P.; Benigni, A. Aging and the renin-angiotensin system. Hypertension 2012, 60, 878–883. [Google Scholar] [CrossRef]
- Remuzzi, G.; Perico, N.; Macia, M.; Ruggenenti, P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. Suppl. 2005, 68, S57–S65. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Romo, R.; Benitez, K.; Barrera-Chimal, J.; Perez-Villalva, R.; Gomez, A.; Aguilar-Leon, D.; Rangel-Santiago, J.F.; Huerta, S.; Gamba, G.; Uribe, N.; et al. At1 receptor antagonism before ischemia prevents the transition of acute kidney injury to chronic kidney disease. Kidney Int. 2016, 89, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Weidmann, P.; De Myttenaere-Bursztein, S.; Maxwell, M.H.; de Lima, J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975, 8, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Dimmeler, S.; Rippmann, V.; Weiland, U.; Haendeler, J.; Zeiher, A.M. Angiotensin ii induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ. Res. 1997, 81, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Herbert, K.E.; Mistry, Y.; Hastings, R.; Poolman, T.; Niklason, L.; Williams, B. Angiotensin ii-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ. Res. 2008, 102, 201–208. [Google Scholar] [CrossRef] [PubMed]
- de Cavanagh, E.M.; Piotrkowski, B.; Basso, N.; Stella, I.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003, 17, 1096–1098. [Google Scholar] [CrossRef]
- Turgut, F.; Balogun, R.A.; Abdel-Rahman, E.M. Renin-angiotensin-aldosterone system blockade effects on the kidneys in the elderly: Benefits and limitations. Clin. J. Am. Soc. Nephrol. 2010, 5, 1330–1339. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.E.; Choi, B.S. The renin-angiotensin system and aging in the kidney. Korean J. Intern. Med. 2014, 29, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenberg, N.K.; Fisher, N.D.; Nussberger, J.; Moukarbel, G.V.; Barkoudah, E.; Danser, A.H. Renal responses to three types of renin-angiotensin system blockers in patients with diabetes mellitus on a high-salt diet: A need for higher doses in diabetic patients? J. Hypertens. 2011, 29, 2454–2461. [Google Scholar] [CrossRef] [Green Version]
- Messerli, F.H.; Sundgaard-Riise, K.; Ventura, H.O.; Dunn, F.G.; Glade, L.B.; Frohlich, E.D. Essential hypertension in the elderly: Haemodynamics, intravascular volume, plasma renin activity, and circulating catecholamine levels. Lancet 1983, 2, 983–986. [Google Scholar] [CrossRef]
- Gibbons, G.H. The pathophysiology of hypertension: The importance of angiotensin ii in cardiovascular remodeling. Am. J. Hypertens. 1998, 11, 177S–181S. [Google Scholar] [CrossRef] [Green Version]
- Navar, L.G.; Imig, J.D.; Zou, L.; Wang, C.T. Intrarenal production of angiotensin ii. Semin. Nephrol. 1997, 17, 412–422. [Google Scholar]
- Kobori, H.; Nangaku, M.; Navar, L.G.; Nishiyama, A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev. 2007, 59, 251–287. [Google Scholar] [CrossRef]
- Weeda, G.; Donker, I.; de Wit, J.; Morreau, H.; Janssens, R.; Vissers, C.J.; Nigg, A.; van Steeg, H.; Bootsma, D.; Hoeijmakers, J.H. Disruption of mouse ercc1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol. 1997, 7, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Niedernhofer, L.J.; Odijk, H.; Budzowska, M.; van Drunen, E.; Maas, A.; Theil, A.F.; de Wit, J.; Jaspers, N.G.; Beverloo, H.B.; Hoeijmakers, J.H.; et al. The structure-specific endonuclease ercc1-xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell Biol. 2004, 24, 5776–5787. [Google Scholar] [CrossRef] [Green Version]
- Dolle, M.E.; Kuiper, R.V.; Roodbergen, M.; Robinson, J.; de Vlugt, S.; Wijnhoven, S.W.; Beems, R.B.; de la Fonteyne, L.; de With, P.; van der Pluijm, I.; et al. Broad segmental progeroid changes in short-lived ercc1(-/delta7) mice. Pathobiol. Aging Age Relat. Dis. 2011, 1, 7219. [Google Scholar]
- Vermeij, W.P.; Hoeijmakers, J.H.; Pothof, J. Genome integrity in aging: Human syndromes, mouse models, and therapeutic options. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Schermer, B.; Bartels, V.; Frommolt, P.; Habermann, B.; Braun, F.; Schultze, J.L.; Roodbergen, M.; Hoeijmakers, J.H.; Schumacher, B.; Nurnberg, P.; et al. Transcriptional profiling reveals progeroid ercc1(-/delta) mice as a model system for glomerular aging. BMC Genom. 2013, 14, 559. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Preda, D.V.; Vasquez, K.O.; Morin, J.; Delaney, J.; Bao, B.; Percival, M.D.; Xu, D.; McKay, D.; Klimas, M.; et al. A fluorogenic near-infrared imaging agent for quantifying plasma and local tissue renin activity in vivo and ex vivo. Am. J. Physiol. Renal Physiol. 2012, 303, F593–F603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, D.L.; Jin, L.; Xuan, H.; Contrepas, A.; Zhou, Y.; Webb, R.L.; Mueller, D.N.; Feldt, S.; Cumin, F.; Maniara, W.; et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic tg(mren-2)27 rats. Hypertension 2008, 52, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, D.J.; Lawrence, A.C.; Towrie, A.; Kladis, A.; Valentijn, A.J. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension 1991, 18, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Gilliam-Davis, S.; Payne, V.S.; Kasper, S.O.; Tommasi, E.N.; Robbins, M.E.; Diz, D.I. Long-term at1 receptor blockade improves metabolic function and provides renoprotection in fischer-344 rats. Am. J. Physiol Heart Circ. Physiol. 2007, 293, H1327–H1333. [Google Scholar] [CrossRef]
- Thompson, M.M.; Oyama, T.T.; Kelly, F.J.; Kennefick, T.M.; Anderson, S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1787–R1794. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Does evidence support renin-angiotensin system blockade for slowing nephropathy progression in elderly persons? Ann. Intern. Med. 2009, 150, 731–733. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Kikuta, T.; Inoue, T.; Hamada, U. Time to re-evaluate effects of renin-angiotensin system inhibitors on renal and cardiovascular outcomes in diabetic nephropathy. World J. Nephrol. 2015, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.K.; Kamath, N.S.; El Kossi, M.; El Nahas, A.M. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 3977–3982. [Google Scholar] [CrossRef] [Green Version]
- Chaumont, M.; Pourcelet, A.; van Nuffelen, M.; Racape, J.; Leeman, M.; Hougardy, J.M. Acute kidney injury in elderly patients with chronic kidney disease: Do angiotensin-converting enzyme inhibitors carry a risk? J. Clin. Hypertens. 2016, 18, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Michael Dörks, S.H.-R.; Hoffmann, F.; Jobski, K. Combined use of drugs inhibiting the renin–angiotensin system: Prescribing patterns and risk of acute kidney injury in german nursing home residents. Clin. Interv. Aging 2018, 13, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuenzli, A.; Bucher, H.C.; Anand, I.; Arutiunov, G.; Kum, L.C.; McKelvie, R.; Afzal, R.; White, M.; Nordmann, A.J. Meta-analysis of combined therapy with angiotensin receptor antagonists versus ace inhibitors alone in patients with heart failure. PLoS ONE 2010, 5, e9946. [Google Scholar] [CrossRef] [PubMed]
- Mallat, S.G. Dual renin-angiotensin system inhibition for prevention of renal and cardiovascular events: Do the latest trials challenge existing evidence? Cardiovasc Diabetol. 2013, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- McAlister, F.A.; Zhang, J.; Tonelli, M.; Klarenbach, S.; Manns, B.J.; Hemmelgarn, B.R.; Alberta Kidney Disease, N. The safety of combining angiotensin-converting-enzyme inhibitors with angiotensin-receptor blockers in elderly patients: A population-based longitudinal analysis. CMAJ 2011, 183, 655–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.O.; Kashani, A.; Ko, D.K.; Francis, G.; Krumholz, H.M. Adverse effects of combination angiotensin ii receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: A quantitative review of data from randomized clinical trials. Arch. Intern. Med. 2007, 167, 1930–1936. [Google Scholar] [CrossRef] [Green Version]
- Aronow, W.S.; Fleg, J.L.; Pepine, C.J.; Artinian, N.T.; Bakris, G.; Brown, A.S.; Ferdinand, K.C.; Forciea, M.A.; Frishman, W.H.; Jaigobin, C.; et al. Accf/aha 2011 expert consensus document on hypertension in the elderly: A report of the american college of cardiology foundation task force on clinical expert consensus documents. Circulation 2011, 123, 2434–2506. [Google Scholar] [CrossRef]
- Bader, M.; Ganten, D. Update on tissue renin-angiotensin systems. J. Mol. Med. 2008, 86, 615–621. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Hollenberg, N.K.; Price, D.A.; Fisher, N.D.; Lansang, M.C.; Perkins, B.; Gordon, M.S.; Williams, G.H.; Laffel, L.M. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003, 63, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Carey, R.M.; Siragy, H.M. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol. Metab. 2003, 14, 274–281. [Google Scholar] [CrossRef]
- Correa-Rotter, R.; Hostetter, T.H.; Rosenberg, M.E. Renin and angiotensinogen gene expression in experimental diabetes mellitus. Kidney Int. 1992, 41, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffa, A.A.; Chai, K.X.; Chao, J.; Chao, L.; Mayfield, R.K. Effects of diabetes and insulin on expression of kallikrein and renin genes in the kidney. Kidney Int. 1992, 41, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimpelmann, J.; Kumar, D.; Levine, D.Z.; Wehbi, G.; Imig, J.D.; Navar, L.G.; Burns, K.D. Early diabetes mellitus stimulates proximal tubule renin mrna expression in the rat. Kidney Int. 2000, 58, 2320–2330. [Google Scholar] [CrossRef] [Green Version]
- Cowie, C.C.; Rust, K.F.; Ford, E.S.; Eberhardt, M.S.; Byrd-Holt, D.D.; Li, C.; Williams, D.E.; Gregg, E.W.; Bainbridge, K.E.; Saydah, S.H.; et al. Full accounting of diabetes and pre-diabetes in the U.S. Population in 1988–1994 and 2005–2006. Diabetes Care 2009, 32, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, R.R.; Egan, J.M. Diabetes and altered glucose metabolism with aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 333–347. [Google Scholar] [CrossRef] [Green Version]
- Roksnoer, L.C.; Heijnen, B.F.; Nakano, D.; Peti-Peterdi, J.; Walsh, S.B.; Garrelds, I.M.; van Gool, J.M.; Zietse, R.; Struijker-Boudier, H.A.; Hoorn, E.J.; et al. On the origin of urinary renin: A translational approach. Hypertension 2016, 67, 927–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roksnoer, L.C.; Verdonk, K.; van den Meiracker, A.H.; Hoorn, E.J.; Zietse, R.; Danser, A.H. Urinary markers of intrarenal renin-angiotensin system activity in vivo. Curr. Hypertens. Rep. 2013, 15, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Heuvel, M.; Batenburg, W.W.; Jainandunsing, S.; Garrelds, I.M.; van Gool, J.M.; Feelders, R.A.; van den Meiracker, A.H.; Danser, A.H. Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin-angiotensin-aldosterone system activity and the efficacy of renin-angiotensin-aldosterone system blockade in the kidney. J. Hypertens. 2011, 29, 2147–2155. [Google Scholar] [CrossRef]
- Sun, Y.; Goes Martini, A.; Janssen, M.J.; Garrelds, I.M.; Masereeuw, R.; Lu, X.; Danser, A.H.J. Megalin: A novel endocytic receptor for prorenin and renin. Hypertension 2020, 75, 1242–1250. [Google Scholar] [CrossRef]
- Tang, J.; Wysocki, J.; Ye, M.; Valles, P.G.; Rein, J.; Shirazi, M.; Bader, M.; Gomez, R.A.; Sequeira-Lopez, M.S.; Afkarian, M.; et al. Urinary renin in patients and mice with diabetic kidney disease. Hypertension 2019, 74, 83–94. [Google Scholar] [CrossRef]
- Fisher, N.D.; Price, D.A.; Litchfield, W.R.; Williams, G.H.; Hollenberg, N.K. Renal response to captopril reflects state of local renin system in healthy humans. Kidney Int. 1999, 56, 635–641. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, N.K.; Chenitz, W.R.; Adams, D.F.; Williams, G.H. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin ii in normal man. J. Clin. Investig. 1974, 54, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollenberg, N.K.; Williams, G.H.; Burger, B.; Chenitz, W.; Hoosmand, I.; Adams, D.F. Renal blood flow and its response to angiotensin ii. An interaction between oral contraceptive agents, sodium intake, and the renin-angiotensin system in healthy young women. Circ. Res. 1976, 38, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, N.K.; Williams, G.H.; Taub, K.J.; Ishikawa, I.; Brown, C.; Adams, D.F. Renal vascular response to interruption of the renin-angiotensin system in normal man. Kidney Int. 1977, 12, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Shoback, D.M.; Williams, G.H.; Moore, T.J.; Dluhy, R.G.; Podolsky, S.; Hollenberg, N.K. Defect in the sodium-modulated tissue responsiveness to angiotensin ii in essential hypertension. J. Clin. Investig. 1983, 72, 2115–2124. [Google Scholar] [CrossRef]
- van Kats, J.P.; Schalekamp, M.A.; Verdouw, P.D.; Duncker, D.J.; Danser, A.H. Intrarenal angiotensin II: Interstitial and cellular levels and site of production. Kidney Int. 2001, 60, 2311–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Esch, J.H.M.; Gembardt, F.; Sterner-Kock, A.; Heringer-Walther, S.; Le, T.H.; Lassner, D.; Stijnen, T.; Coffman, T.M.; Schultheiss, H.; jan Danser, A.H.; et al. Cardiac phenotype and angiotensin II Levels in AT1a, AT1b, and AT2 receptor single, double, and triple knockouts. Cardiovasc. Res. 2010, 86, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Lopez, M.L.; Cowhig, J.E.; Taylor, M.A., Jr.; Hatada, T.; Riggs, E.; Lee, G.; Gomez, R.A.; Kim, H.S.; Smithies, O. Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J. Am. Soc. Nephrol. 2005, 16, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The banff 2019 kidney meeting report (i): Updates on and clarification of criteria for t cell- and antibody-mediated rejection. Am. J. Trans. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Racusen, L.C.; Solez, K.; Colvin, R.B.; Bonsib, S.M.; Castro, M.C.; Cavallo, T.; Croker, B.P.; Demetris, A.J.; Drachenberg, C.B.; Fogo, A.B.; et al. The banff 97 working classification of renal allograft pathology. Kidney Int. 1999, 55, 713–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danser, A.H.; van Kesteren, C.A.; Bax, W.A.; Tavenier, M.; Derkx, F.H.; Saxena, P.R.; Schalekamp, M.A. Prorenin, renin, angiotensinogen, and angiotensin-converting enzyme in normal and failing human hearts. Evidence for renin binding. Circulation 1997, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Thiel, B.S.; van der Linden, J.; Ridwan, Y.; Garrelds, I.M.; Vermeij, M.; Clahsen-van Groningen, M.C.; Qadri, F.; Alenina, N.; Bader, M.; Roks, A.J.M.; et al. In Vivo Renin Activity Imaging in the Kidney of Progeroid Ercc1 Mutant Mice. Int. J. Mol. Sci. 2021, 22, 12433. https://doi.org/10.3390/ijms222212433
van Thiel BS, van der Linden J, Ridwan Y, Garrelds IM, Vermeij M, Clahsen-van Groningen MC, Qadri F, Alenina N, Bader M, Roks AJM, et al. In Vivo Renin Activity Imaging in the Kidney of Progeroid Ercc1 Mutant Mice. International Journal of Molecular Sciences. 2021; 22(22):12433. https://doi.org/10.3390/ijms222212433
Chicago/Turabian Stylevan Thiel, Bibi S., Janette van der Linden, Yanto Ridwan, Ingrid M. Garrelds, Marcel Vermeij, Marian C. Clahsen-van Groningen, Fatimunnisa Qadri, Natalia Alenina, Michael Bader, Anton J. M. Roks, and et al. 2021. "In Vivo Renin Activity Imaging in the Kidney of Progeroid Ercc1 Mutant Mice" International Journal of Molecular Sciences 22, no. 22: 12433. https://doi.org/10.3390/ijms222212433





