Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids
Abstract
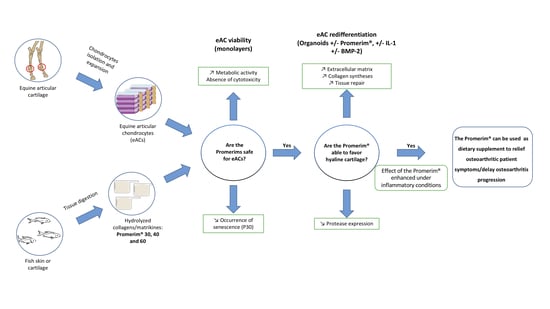
:1. Introduction
2. Results
2.1. Promerim®30, 40, and 60 Have no Cytotoxic Effects on Equine Articular Chondrocytes, They Promote Their Metabolic Activity and Promerim®30 Downregulates Cellular Senescence
2.2. Effect of Promerim® Hydrolysates on the Expression of mRNAs Encoding Characteristic Biomarkers of Chondrocytes
2.3. Effect of Promerim® Hydrolysates on the Protein Expression of Type II and I Collagens and Htra1
2.4. Promerim®30 and 40 Promote Proliferation, Whereas Promerim®60 Promotes Migration of eACs
3. Discussion
4. Materials and Methods
4.1. Collagen Hydrolysates
4.2. Isolation and Cell Culture
4.3. XTT Assay
4.4. Toxilight Assay
4.5. β-Galactosidase Activity
4.6. eAC Redifferentiation
4.7. RNA Isolation and RT-qPCR
4.8. Western Blots
4.9. Scratch Wound Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aigner, T.; Cook, N.J.L.; Gerwin, S.S.; Glasson, S.; Laverty, C.B.; Little, W.; McIlwraith, C.W.; Kraus, V.B. Histopathology Atlas of Animal Model Systems–Overview of Guiding Principles. Osteoarthr. Cartil. 2010, 18, 2–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aigner, T.; Stöve, J. Collagens major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug. Deliv. Rev. 2003, 55, 1569–1593. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Aubert-Foucher, E.; Demoor, M.; Camuzeaux, B.; Paumier, A.; Piperno, M.; Damour, O.; Duterque-Coquillaud, M.; Galéra, P.; Mallein-Gerin, F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. J. Cell. Biochem. 2010, 111, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jozkowicz, A.; Dulak, J. HIF-1 and HIF-2 transcription factors—Similar but not identical. Mol. Cells 2010, 29, 435–442. [Google Scholar] [CrossRef]
- Silver, I.A. Measurement of pH and ionic composition of pericellular sites. Philos. Trans. R Soc. Lond. B Biol. Sci. 1975, 271, 261–272. [Google Scholar]
- Pujol, J.P.; Galera, P.; Redini, F.; Mauviel, A.; Loyau, G. Role of cytokines in osteoarthritis: Comparative effects of interleukin 1 and transforming growth factor-beta on cultured rabbit articular chondrocytes. J. Rheumatol. Suppl. 1991, 27, 76–79. [Google Scholar]
- Pujol, J.P.; Chadjichristos, C.; Legendre, F.; Bauge, C.; Beauchef, G.; Andriamanalijaona, R.; Galera, P.; Boumediene, K. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect. Tissue Res. 2008, 49, 293–297. [Google Scholar] [CrossRef]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Demoor, M.; Ollitrault, D.; Gomez-Leduc, T.; Bouyoucef, M.; Hervieu, M.; Fabre, H.; Lafont, J.; Denoix, J.M.; Audigié, F.; Mallein-Gerin, F.; et al. Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim. Biophys. Acta 2014, 1840, 2414–2440. [Google Scholar] [CrossRef]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar]
- Malda, J.; Benders, K.E.M.; Klein, T.J.; de Grauw, J.C.; Kik, M.J.L.; Hutmacher, D.W.; Saris, D.B.F.; van Weeren, P.R.; Dhert, W.J.A. Comparative study of depth-dependent characteristics of equine and human osteochondraltissue from the medial and lateral femoral condyles. Osteoarthr. Cartil. 2012, 20, 1147–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, N.R.; Reid, S.W.; Morris, R.S. Effect of training location and time period on racehorse performance in New Zealand. 1. Descriptive analysis. N. Z. Vet. J. 2004, 52, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Dolvik, N.I.; Klemetsdal, G. The effect of arthritis in the carpal joint on performance in Norwegian cold-blooded trotters. Vet. Res. Commun. 1996, 20, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Allen, K.D.; Golightly, Y.M.; Goode, A.P.; Jordan, J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [Green Version]
- McIlwraith, C.W.; Lattermann, C. Intra-Articular Corticosteroids for Knee Pain—What Have We Learned from the Equine Athlete and Current Best Practice. J. Knee Surg. 2019, 32, 9–25. [Google Scholar]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Murray, J.M.D.; Hanna, E.; Hastie, P. Equine dietary supplements: An insight into their use and perceptions in the Irish equine industry. Ir. Vet. J. 2018, 71, 4. [Google Scholar] [CrossRef] [Green Version]
- Bassleer, C.; Rovati, L.; Franchimont, P. Stimulation of Proteoglycan Production by Glucosamine Sulfate in Chondrocytes Isolated from Human Osteoarthritic Articular Cartilage In Vitro. Osteoarthr. Cartil. 1998, 6, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, F.; Wang, C. Efficacy of Curcumin and Boswellia for Knee Osteoarthritis: Systematic Review and Meta-Analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract Synergically Inhibit Inflammatory and Catabolic Mediator’s Synthesis by Normal Bovine and Osteoarthritic Human Chondrocytes in Monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Noorbaloochi, S.; MacDonald, R.; Maxwell, L.J. Chondroitin for osteoarthritis. Cochrane Database Syst. Rev. 2015, 1, CD005614. [Google Scholar] [PubMed] [Green Version]
- Maquart, F.X.; Monboisse, J.C. Extracellular matrix and wound healing. Pathol. Biol. 2014, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef]
- Jiang, J.X.; Yu, S.; Huang, Q.R.; Zhang, X.L.; Zhang, C.Q.; Zhou, J.L.; Prawitt, J. Collagen peptides improve knee osteoarthritis in elderly women: A 6-month randomized, double blind, placebo-controlled study. Agro Food Ind. Hi Tech 2014, 25, 19–23. [Google Scholar]
- Lugo, J.P.; Saiyed, Z.M.; Lau, F.C.; Molina, J.P.; Pakdaman, M.N.; Shamie, A.N.; Udani, J.K. Undenatured type II collagen (UC-II®) for joint support: A randomized, double-blind, placebo-controlled study in healthy volunteers. J. Int. Soc. Sports Nutr. 2013, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Kilinc, B.E.; Oc, Y.; Alibakan, G.; Bilgin, E.; Kanar, M.; Eren, O.T. An Observational 1-Month Trial on the Efficacy and Safety of Promerim for Improving Knee Joint. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544118757496. [Google Scholar] [CrossRef]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Galéra, P.; Ollitrault, D.; Legendre, F.; Demoor, M.; Mallein-Gerin, F.; Boumediene, K.; Herbage, B.; Duterque-Coquillaud, M.; Damour, O. Brevet Francais FR 2965278 du 23/09/2010. Method for Obtaining Differentiated Articular Chondrocytes In Vitro or Ex Vivo and Uses of Same. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012038668 (accessed on 8 January 2021).
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Benateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Rakic, R.; Bourdon, B.; Hervieu, M.; Branly, T.; Legendre, F.; Saulnier, N.; Audigié, F.; Maddens, S.; Demoor, M.; Galera, P. RNA Interference and BMP-2 Stimulation Allows Equine Chondrocytes Redifferentiation in 3D-Hypoxia Cell Culture Model: Application for Matrix-Induced Autologous Chondrocyte Implantation. Int. J. Mol. Sci. 2017, 18, 1842. [Google Scholar] [CrossRef]
- Murray, C.; Marshall, M.; Rathod, T.; Bowen, C.J.; Menz, H.B.; Roddy, E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS ONE 2018, 13, e0193662. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Colombini, A.; Carimati, G.; Beggio, M.; De Girolamo, L.; Volpi, P. Intra-Articular Injection of Hydrolyzed Collagen to Treat Symptoms of Knee Osteoarthritis. A Functional In Vitro Investigation and a Pilot Retrospective Clinical Study. J. Clin. Med. 2019, 8, 975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, C.; Kaur, J.; Shishodia, S.; Aggarwal, B.B.; Ralhan, R. Curcumin down regulates smokeless tobacco-induced NF-kappaB activation and COX-2 expression in human oral premalignant and cancer cells. Toxicology 2006, 228, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kizhakkedath, R. Clinical evaluation of a formulation containing Curcuma longa and Boswellia serrata extracts in the management of knee osteoarthritis. Mol. Med. Rep. 2013, 8, 1542–1548. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, N.; Miller, M.J.; Haqqi, T.M. Effect of a Herbal-Leucine mix on the IL-1β-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complement. Altern. Med. 2011, 11, 66. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Machado, G.C.; Eyles, J.P.; Ravi, V.; Hunter, D.J. Dietary supplements for treating osteoarthritis: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef] [Green Version]
- DiNubile, N.A. A potential role for avocado- and soybean-based nutritional supplements in the management of osteoarthritis: A review. Phys. Sportsmed. 2010, 38, 71–81. [Google Scholar] [CrossRef]
- DiNubile, N.A. Glucosamine and Chondroitin Sulfate: What Has Been Learned Since the Glucosamine/chondroitin Arthritis Intervention Trial. Orthopedics 2018, 41, 200–207. [Google Scholar] [CrossRef]
- Dahmer, S.; Schiller, R.M. Glucosamine. Am. Fam. Physician 2008, 78, 471–476. [Google Scholar]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kashiuchi, S.; Miyazawa, R.; Nagata, H.; Shirai, M.; Shimizu, M.; Sone, H.; Kamiyama, S. Effects of administration of glucosamine and chicken cartilage hydrolysate on rheumatoid arthritis in SKG mice. Food Funct. 2019, 10, 5008–5017. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Stenehjem, J.; Park, J.; Endres, J.R.; Clewell, A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2012, 60, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Pivnenko, T.N.; Sukhoverkhova, G.; Epshteĭn, L.M.; Somova-Isachkova, L.M.; Timchenko, N.F.; Besednova, N.N. Experimental morphological study of the therapeutic effect of shark cartilage preparation in a model of infective allergic arthritis. Antibiot. Khimioter. 2005, 50, 20–23. [Google Scholar]
- Fan, L.; Cao, M.; Gao, S.; Wang, T.; Wu, H.; Peng, M.; Zhou, X.; Nie, M. Preparation and characterization of sodium alginate modified with collagen peptides. Carbohydr. Polym. 2013, 93, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ennaas, N.; Hammami, R.; Gomaa, A.; Bédard, F.; Biron, É.; Subirade, M.; Beaulieu, L.; Fliss, I. Collagencin, an antibacterial peptide from fish collagen: Activity, structure and interaction dynamics with membrane. Biochem. Biophys. Res. Commun. 2016, 473, 642–647. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Yang, F.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterisation of collagens from the skin, scale and bone of deep-sea redfish (Sebastes mentella). Food Chem. 2008, 108, 616–623. [Google Scholar] [CrossRef]
- Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of marine collagen peptides on physiological and neurobehavioral development of male rats with perinatal asphyxia. Mar. Drugs 2015, 13, 3653–3671. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Mizushige, T.; Jinno, S.; Sugihara, F.; Inoue, N.; Tanaka, H.; Kabuyama, Y. Absorption and metabolism of orally administered collagen hydrolysates evaluated by the vascularly perfused rat intestine and liver in situ. Biomed. Res. 2018, 39, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.I.; Carozza, M.; Klein, M.; Nantermet, P.; Luk, D.; Crowl, R.M. Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J. Biol. Chem. 1998, 273, 34406–34412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, C.; Tsujimoto, R.; Kajikawa, M.; Koshiba-Takeuchi, K.; Ina, J.; Yano, M.; Tsuchiya, A.; Ueta, Y.; Soma, A.; Kanda, H.; et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004, 131, 1041–1053. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Lin, H.; Zhu, L.; Liu, Z.; Hu, F.; Shi, J.; Yang, T.; Shi, X.; Guo, H.; Tan, X.; et al. The Inhibitory Effect of IFN-γ on Protease HTRA1 Expression in Rheumatoid Arthritis. J. Immunol. 2014, 193, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Comblain, F.; Dubuc, J.E.; Lambert, C.; Sanchez, C.; Lesponne, I.; Serisier, S.; Henrotin, Y. Identification of Targets of a New Nutritional Mixture for Osteoarthritis Management Composed by Curcuminoids Extract, Hydrolyzed Collagen and Green Tea Extract. PLoS ONE 2016, 11, e0156902. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Drobnic, M.; Madry, H.; Jelic, M.; Van Dijk, N.; Della Villa, S. Non-surgical management of early knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 436–449. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Murakami, A.; Song, M.; Katsumata, S.; Uehara, M.; Suzuki, K.; Ohigashi, H. Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: Possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 2007, 30, 179–192. [Google Scholar] [CrossRef]
- Ahmed, S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: Progress and promise. Arthritis Res. Ther. 2010, 12, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boe, C.; Vangsness, C.T. Fish Oil and Osteoarthritis: Current Evidence. Am. J. Orthop. 2015, 44, 302–305. [Google Scholar] [PubMed]
- Frondoza, C.G.; Sohrabi, A.; Polotsky, A.; Phan, P.V.; Hungerford, D.S.; Lindmark, L. An in vitro screening assay for inhibitors of proinflammatory mediators in herbal extracts using human synoviocyte cultures. Vitr. Cell. Dev. Biol. Anim. 2004, 40, 95–101. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Muñoz, E.; Rose, T.; Weiss, G.; McGregor, G.P. Molecular targets of the antiinflammatory Harpagophytum procumbens (devil’s claw): Inhibition of TNFα and COX-2 gene expression by preventing activation of AP-1. Phytother. Res. 2012, 26, 806–811. [Google Scholar] [CrossRef]
- Kahan, A.; Uebelhart, D.; De Vathaire, F.; Delmas, P.D.; Reginster, J.Y. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: The study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009, 60, 524–533. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential anti-inflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Latire, T.; Legendre, F.; Bouyoucef, M.; Marin, F.; Carreiras, F.; Rigot-Jolivet, M.; Lebel, J.M.; Galéra, P.; Serpentini, A. Shell extracts of the edible mussel and oyster induce an enhancement of the catabolic pathway of human skin fibroblasts, in vitro. Cytotechnology 2017, 69, 815–829. [Google Scholar] [CrossRef]
- Bouyoucef, M.; Rakic, R.; Gómez-Leduc, T.; Latire, T.; Marin, F.; Leclercq, S.; Carreiras, F.; Serpentini, A.; Lebel, J.M.; Galéra, P.; et al. Regulation of Extracellular Matrix Synthesis by Shell Extracts from the Marine Bivalve Pecten maximus in Human Articular Chondrocytes—Application for Cartilage Engineering. Mar. Biotechnol. 2018, 20, 436–450. [Google Scholar] [CrossRef]
- Sivaraman, K.; Shanthi, C. Matrikines for therapeutic and biomedical applications. Life Sci. 2018, 214, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003, 311, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.G.; Samarasinghe, R.K. Review article: Glucosamine. J. Orthop. Surg. 2009, 17, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Sanchez, C.; Deberg, M.A.; Piccardi, N.; Guillou, G.B.; Msika, P.; Reginster, J.Y. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J. Rheumatol. 2003, 30, 1825–1834. [Google Scholar]
- Latire, T.; Legendre, F.; Bigot, N.; Carduner, L.; Kellouche, S.; Bouyoucef, M.; Carreiras, F.; Marin, F.; Lebel, J.M.; Galéra, P.; et al. Shell extracts from the marine bivalve Pecten maximus regulate the synthesis of extracellular matrix in primary cultured human skin fibroblasts. PLoS ONE 2014, 9, e99931. [Google Scholar] [CrossRef] [Green Version]
- Grimaud, E.; Heymann, D.; Rédini, F. Recent advances in TGF-beta effects on chondrocyte metabolism. Potential therapeutic roles of TGF-beta in cartilage disorders. Cytokine Growth Factor Rev. 2002, 13, 241–257. [Google Scholar] [CrossRef]
- Joyce, M.E.; Roberts, A.B.; Sporn, M.B.; Bolander, M.E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J. Cell Biol. 1990, 110, 2195–2207. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, Y. A Molecular Cascade Underlying Articular Cartilage Degeneration. Curr. Drug Targets 2020, 21, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Manning, L.B.; Li, Y.; Chickmagalur, N.S.; Li, X.; Xu, L. Discoidin Domain Receptor 2 as a Potential Therapeutic Target for Development of Disease-Modifying Osteoarthritis Drugs. Am. J. Pathol. 2016, 186, 3000–3010. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, T.; Fukuda, K.; Yamazaki, K.; Hashimoto, K.; Ueda, H.; Mori, S.; Hamanishi, C. Cyclic compression loaded on cartilage explants enhances the production of reactive oxygen species. J. Rheumatol. 2007, 34, 556–562. [Google Scholar]
- Cha, B.H.; Lee, J.S.; Kim, S.W.; Cha, H.J.; Lee, S.H. The modulation of the oxidative stress response in chondrocytes by Wip1 and its effect on senescence and dedifferentiation during in vitro expansion. Biomaterials 2013, 34, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P.; Pujol, J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.K.; Gibson, M.A.; Elisseeff, J.H.; Trice, M.E. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed. Res. Int. 2014, 2014, 272481. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Contentin, R.; Demoor, M.; Concari, M.; Desancé, M.; Audigié, F.; Branly, T.; Galéra, P. Comparison of the Chondrogenic Potential of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord Blood Intended for Cartilage Tissue Engineering. Stem Cell Rev. Rep. 2020, 16, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem. Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Branly, T.; Bertoni, L.; Contentin, R.; Rakic, R.; Gomez-Leduc, T.; Desancé, M.; Hervieu, M.; Legendre, F.; Jacquet, S.; Audigié, F.; et al. Characterization and use of equine bone marrow mesenchymal stem cells in equine cartilage engineering. Study of their hyaline cartilage forming potential when cultured under hypoxia within a biomaterial in the presence of BMP-2 and TGF-ß1. Stem Cell Rev. 2017, 5, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Branly, T.; Contentin, R.; Desancé, M.; Jacquel, T.; Bertoni, L.; Jacquet, S.; Mallein-Gerin, F.; Denoix, J.M.; Audigié, F.; Demoor, M.; et al. Improvement of the Chondrocyte-Specific Phenotype upon Equine Bone Marrow Mesenchymal Stem Cell Differentiation: Influence of Culture Time, Transforming Growth Factors and Type I Collagen siRNAs on the Differentiation Index. Int. J. Mol. Sci. 2018, 19, 435. [Google Scholar] [CrossRef] [Green Version]
- Desancé, M.; Contentin, R.; Bertoni, L.; Gomez-Leduc, T.; Branly, T.; Jacquet, S.; Betsch, J.M.; Batho, A.; Legendre, F.; Audigié, F.; et al. Chondrogenic Differentiation of Defined Equine Mesenchymal Stem Cells Derived from Umbilical Cord Blood for Use in Cartilage Repair Therapy. Int. J. Mol. Sci. 2018, 19, 537. [Google Scholar] [CrossRef] [Green Version]
- Rakic, R.; Bourdon, B.; Demoor, M.; Maddens, S.; Saulnier, N.; Galéra, P. Differences in the intrinsic chondrogenic potential of equine umbilical cord matrix and cord blood mesenchymal stromal/stem cells for cartilage regeneration. Sci. Rep. 2018, 8, 13799. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galéra, P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. Int. J. Mol. Sci. 2021, 22, 580. https://doi.org/10.3390/ijms22020580
Bourdon B, Contentin R, Cassé F, Maspimby C, Oddoux S, Noël A, Legendre F, Gruchy N, Galéra P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. International Journal of Molecular Sciences. 2021; 22(2):580. https://doi.org/10.3390/ijms22020580
Chicago/Turabian StyleBourdon, Bastien, Romain Contentin, Frédéric Cassé, Chloé Maspimby, Sarah Oddoux, Antoine Noël, Florence Legendre, Nicolas Gruchy, and Philippe Galéra. 2021. "Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids" International Journal of Molecular Sciences 22, no. 2: 580. https://doi.org/10.3390/ijms22020580