Performance of Polydopamine Complex and Mechanisms in Wound Healing
Abstract
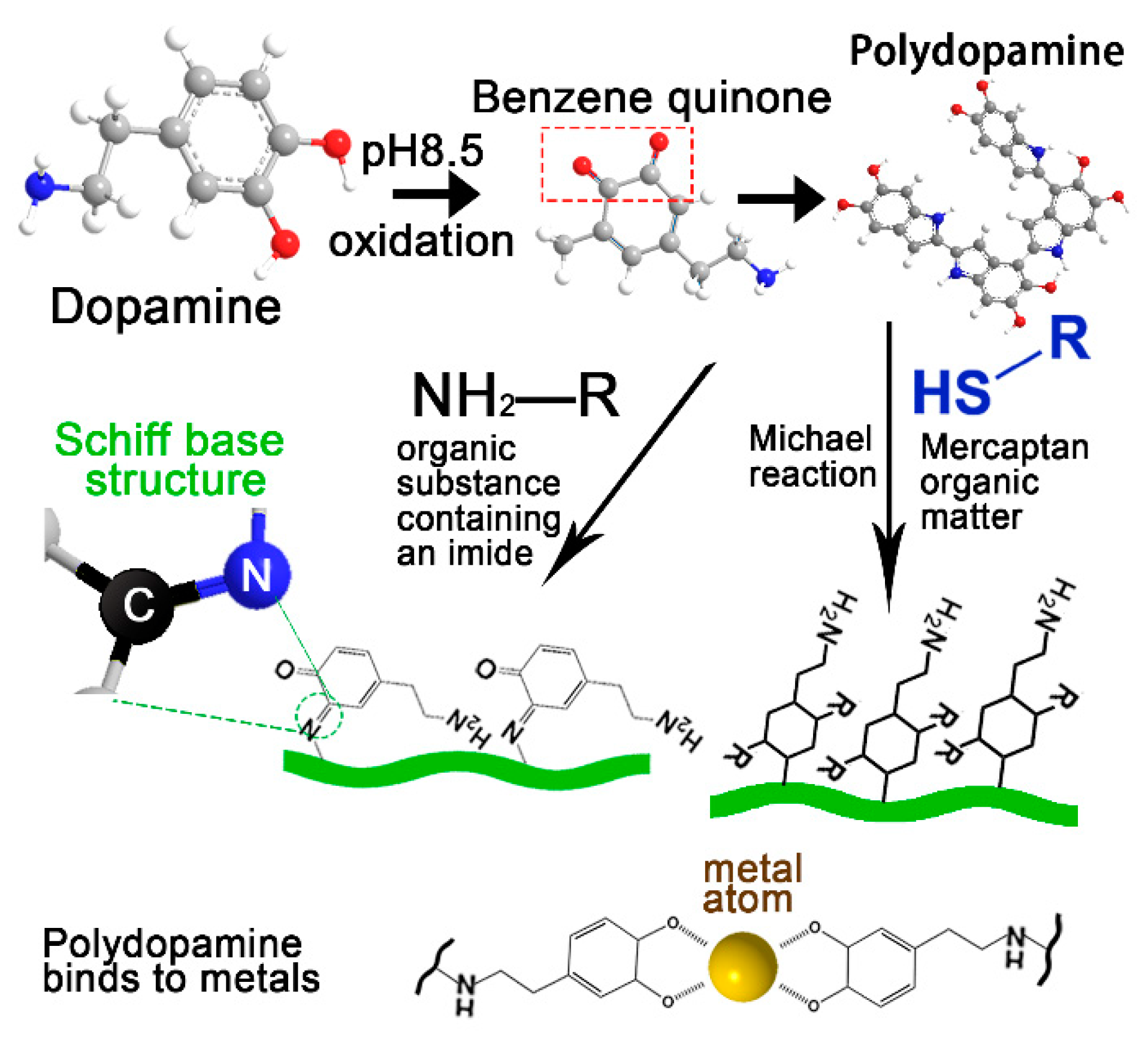
:1. Introduction
2. Construction Methods and Physicochemical Properties of PDA Complex
2.1. Mechanical Properties of PDA Complex
2.2. Healing Properties of PDA Complex
2.3. Electrical Conductivity of PDA Complex
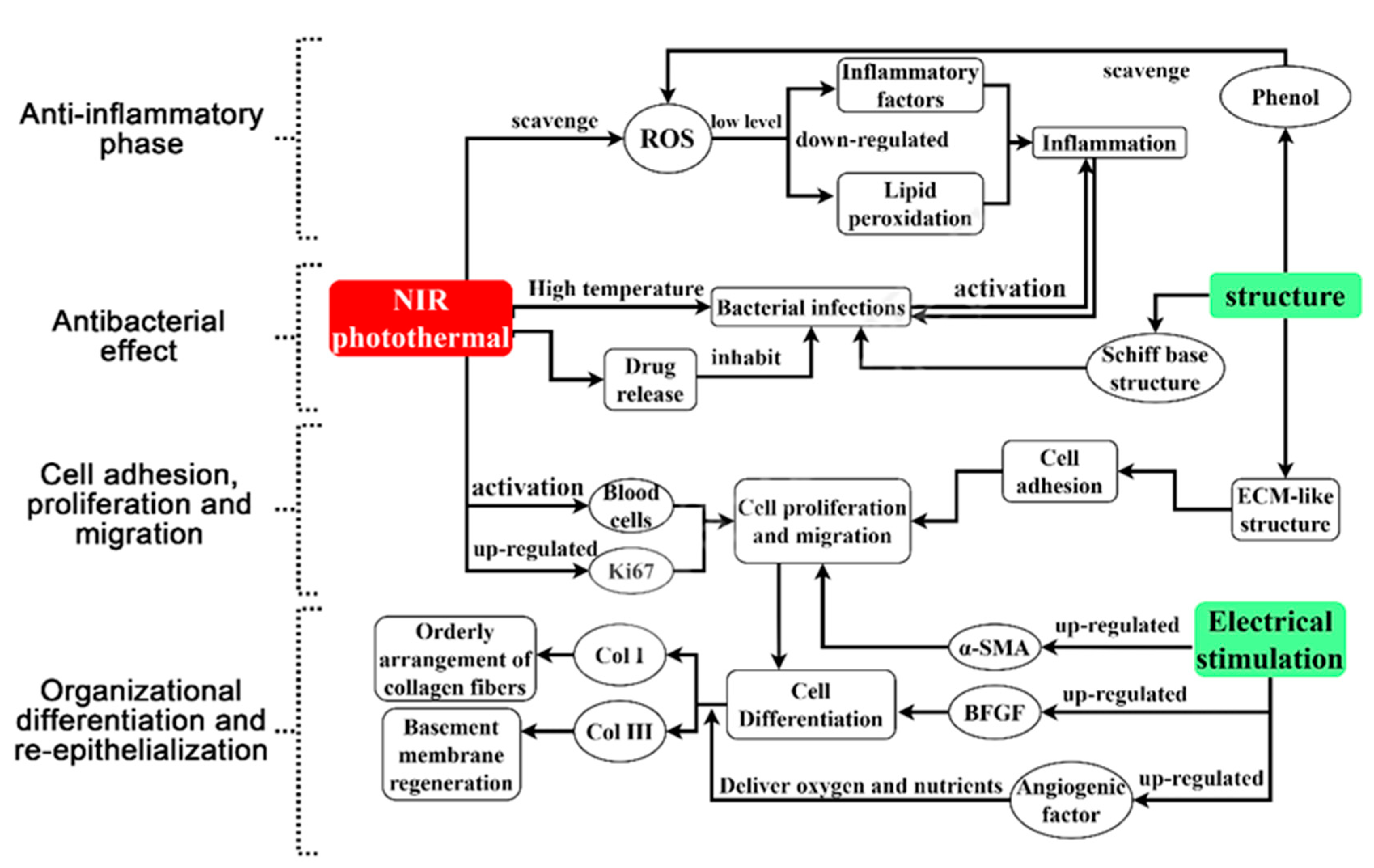
3. The Mechanism of PDA Complex for Promoting Wound Healing
3.1. Antibacterial Effect
3.2. Anti-Inflammatory Effect
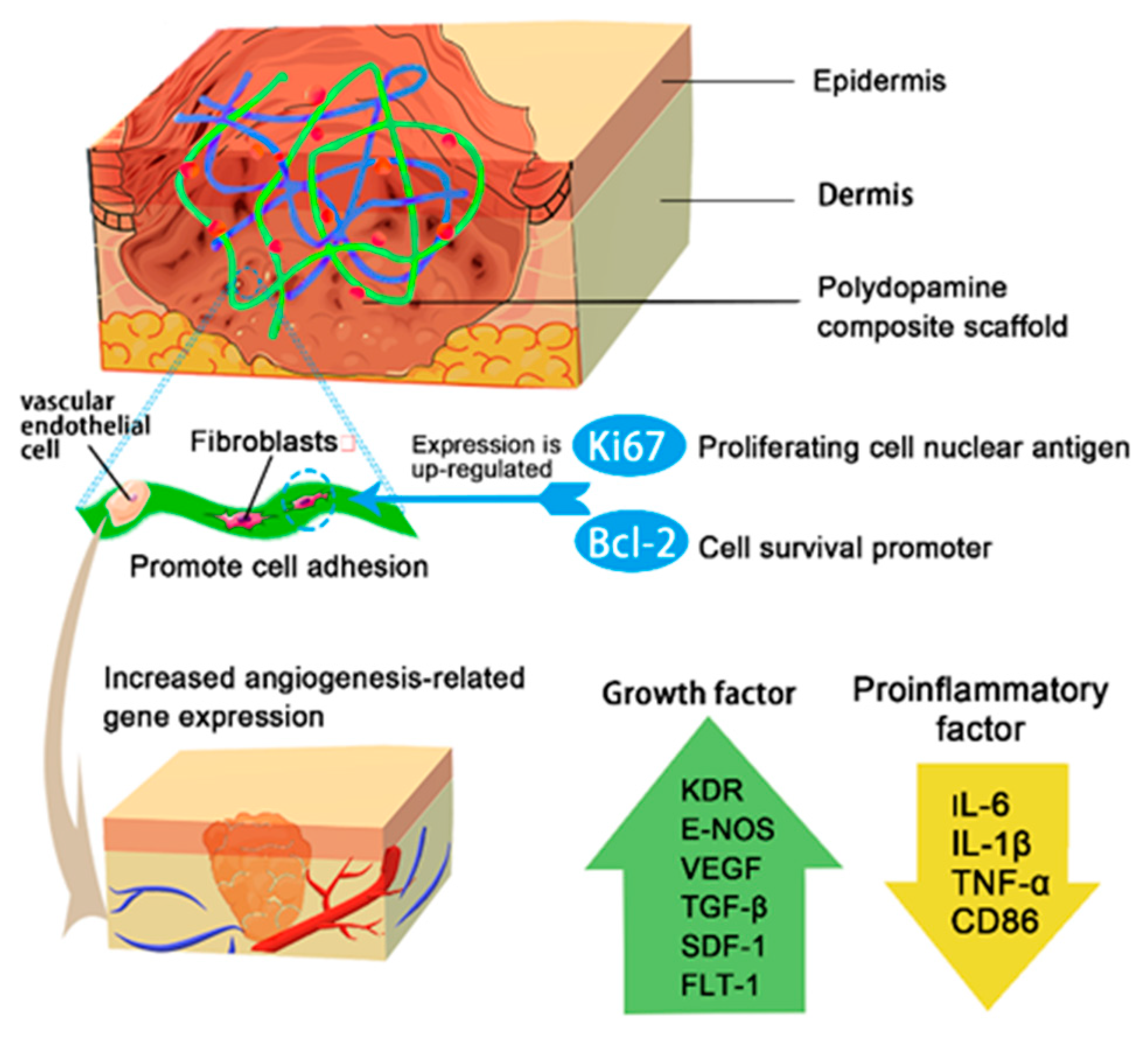
3.3. Cell Adhesion, Proliferation and Migration
3.3.1. Simulation of the Extracellular Matrix (ECM)
3.3.2. Activated Blood Cells
3.3.3. NIR Irradiation Promotes Cell Proliferation
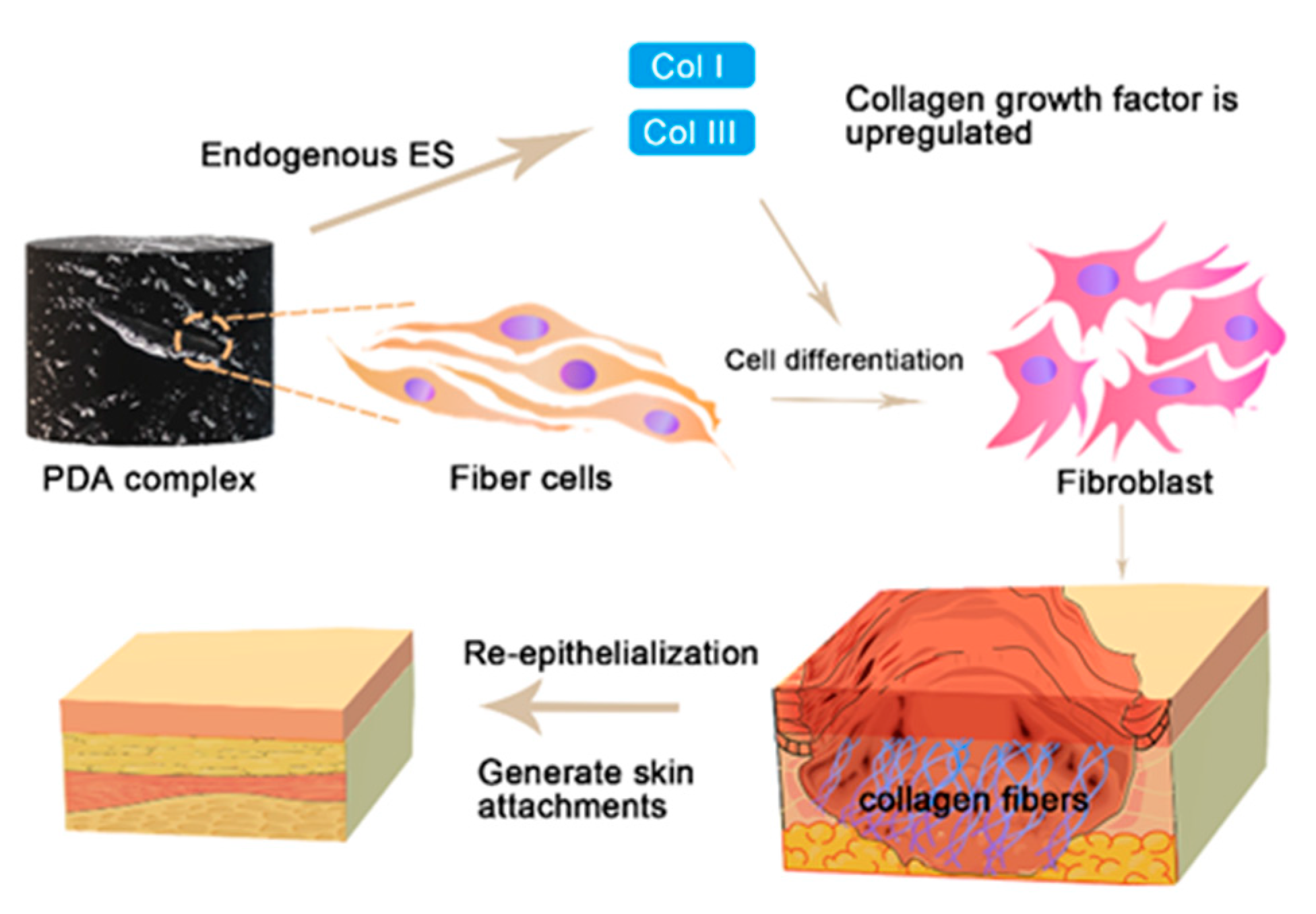
3.4. Organizational Differentiation and Re-Epithelialization
4. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Holcomb, J.B. Methods for improved hemorrhage control. Crit. Care 2004, 8, S57. [Google Scholar] [CrossRef] [Green Version]
- Gegel, B.; Burgert, J.; Gasko, J.; Campbell, C.; Martens, M.; Keck, J.; Reynolds, H.; Loughren, M.; Johnson, D. The Effects of QuikClot Combat Gauze and Movement on Hemorrhage Control in a Porcine Model. Mil. Med. 2012, 177, 1543–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilardo, N.; Feinberg, J.; Black, J.; Ratner, E. The use of QuikClot combat gauze in cervical and vaginal hemorrhage. Gynecol. Oncol. Rep. 2017, 21, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Lu, B.; Wang, T.; Wang, L.; Chen, J.; Yu, K.; Liu, J.; Dai, F.; Wu, D. Chitosan/gelatin composite sponge is an absorbable surgical hemostatic agent. Colloids Surf. B Biointerfaces 2015, 136, 1026–1034. [Google Scholar] [CrossRef]
- Hsu, B.B.; Conway, W.; Tschabrunn, C.M.; Mehta, M.; Perez-Cuevas, M.B.; Zhang, S.; Hammond, P.T. Clotting Mimicry from Robust Hemostatic Bandages Based on Self-Assembling Peptides. ACS Nano 2015, 9, 9394–9406. [Google Scholar] [CrossRef]
- Meron, G.; Kurkciyan, I.; Sterz, F.; Susani, M.; Domanovits, H.; Tobler, K.; Bohdjalian, A.; Laggner, A.N. Cardiopulmonary resuscitation-associated major liver injury. Resuscitation 2007, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Mclnnis, W.D.; Richardson, J.D.; Aust, J.B. Hepatic Trauma: Pitfalls in Management. Arch. Surg. 1977, 112, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, V.S.; German, A.I.; Moghul, T.H.; Scarano, D. Liver, Spleen, and Kidney Wounds: Experimental Repair With Topical Adhesive. Arch. Surg. 1964, 89, 237–243. [Google Scholar] [CrossRef]
- Lamb, C.A. Rupture of the Liver. N. Engl. J. Med. 1939, 221, 855–859. [Google Scholar] [CrossRef]
- Mlekusch, W.; Dick, P.; Haumer, M.; Sabeti, S.; Minar, E.; Schillinger, M. Arterial Puncture Site Management after Percutaneous Transluminal Procedures Using a Hemostatic Wound Dressing (Clo-Sur P.A.D.) versus Conventional Manual Compression: A Randomized Controlled Trial. J. Endovasc. Ther. 2006, 13, 23–31. [Google Scholar] [CrossRef]
- Deuling, J.H.H.; Vermeulen, R.P.; Anthonio, R.A.; van den Heuvel, A.F.M.; Jaarsma, T.; Jessurun, G.; de Smet, B.J.G.L.; Tan, E.S.; Zijlstra, F. Closure of the femoral artery after cardiac catheterization: A comparison of Angio-Seal, StarClose, and manual compression. Catheter. Cardiovasc. Interv. 2008, 71, 518–523. [Google Scholar] [CrossRef]
- Wagner, S.C.; Gonsalves, C.F.; Eschelman, D.J.; Sullivan, K.L.; Bonn, J. Complications of a Percutaneous Suture-mediated Closure Device versus Manual Compression for Arteriotomy Closure: A Case-controlled Study. J. Vasc. Interv. Radiol. 2003, 14, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Tron, C.; Koning, R.; Eltchaninoff, H.; Douillet, R.; Chassaing, S.; Sanchez-Giron, C.; Cribier, A. A Randomized Comparison of a Percutaneous Suture Device versus Manual Compression for Femoral Artery Hemostasis after PTCA. J. Interv. Cardiol. 2003, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Tarnuzzer, R.W.; Schultz, G.S. Biochemical analysis of acute and chronic wound environments. Wound Repair Regen. 1996, 4, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Do, D.C.; Garcia, M.; Kim, J.; Mirebrahim, S.H.; Lyubovitsky, J.; Lonardi, S.; Nothnagel, E.A.; Schiller, N.; Martins-Green, M. Generating and Reversing Chronic Wounds in Diabetic Mice by Manipulating Wound Redox Parameters. J. Diabetes Res. 2014, 2014, 562625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Li, J.; Xie, W.; Mo, Y.; Ouyang, L.; Zhao, F.; Fu, X.; Chen, X. Bioactive glass promotes the barrier functional behaviors of keratinocytes and improves the Re-epithelialization in wound healing in diabetic rats. Bioact. Mater. 2021, 6, 3496–3506. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, J.; Chen, X.; Wang, R.; Zhang, M.; Zhang, Q.; Zhang, Y.; Wang, S.; Cheng, Y. Bioactive skin-mimicking hydrogel band-aids for diabetic wound healing and infectious skin incision treatment. Bioact. Mater. 2021, 6, 3962–3975. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ersen, O.; Voegel , J.-C.; Jan, E.; Kotov, N.A.; Ball, V. Melanin-Containing Films: Growth from Dopamine Solutions versus Layer-by-Layer Deposition. Chem. Phys. Chem. 2010, 11, 3299–3305. [Google Scholar] [CrossRef] [Green Version]
- Mayandi, V.; Choong, A.C.W.; Dhand, C.; Lim, F.P.; Aung, T.T.; Sriram, H.; Dwivedi, N.; Periayah, M.H.; Sridhar, S.; Fazil, M.H.U.T.; et al. Multifunctional Antimicrobial Nanofiber Dressings Containing epsilon-Polylysine for the Eradication of Bacterial Bioburden and Promotion of Wound Healing in Critically Colonized Wounds. ACS Appl. Mater. Interfaces 2020, 12, 15989–16005. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Hong, W.; Sun, W.; Liu, X.; Tong, Z. Infrared-driving actuation based on bilayer graphene oxide-poly(N-isopropylacrylamide) nanocomposite hydrogels. J. Mater. Chem. A 2014, 2, 15633–15639. [Google Scholar] [CrossRef]
- He, X.; Liu, L.; Han, H.; Shi, W.; Yang, W.; Lu, X. Bioinspired and Microgel-Tackified Adhesive Hydrogel with Rapid Self-Healing and High Stretchability. Macromolecules 2019, 52, 72–80. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Pardeshi, S.; Sharma, J.G.; Lee, S.H.; Choi, E.H. Biomedical and Clinical Importance of Mussel-Inspired Polymers and Materials. Mar. Drugs 2015, 13, 6792–6817. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Chen, L.; Li, T.; Xu, Z.-R. A polydopamine-coated mesoporous nanocomposite with robust affinity to horseradish peroxidase based on catecholic adhesion. Colloid Interface Sci. Commun. 2021, 40, 100340. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional Tissue-Adhesive Cryogel Wound Dressing for Rapid Nonpressing Surface Hemorrhage and Wound Repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Guo, B.; Ma, P.X. Mussel-inspired injectable supramolecular and covalent bond crosslinked hydrogels with rapid self-healing and recovery properties via a facile approach under metal-free conditions. J. Mater. Chem B 2016, 4, 6644–6651. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.N.; Hou, S.; Park, S.; Shalek, B.A.; Jeong, K.J. Gelatin Hydrogel Combined with Polydopamine Coating To Enhance Tissue Integration of Medical Implants. ACS Biomater. Sci. Eng. 2018, 4, 3471–3477. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shi, D.; Dong, L.; Zhang, Z.; Li, X.; Chen, M. Fabrication of polydopamine nanoparticles knotted alginate scaffolds and their properties. J. Biomed. Mater. Res. A 2018, 106, 3255–3266. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Swartjes, J.J.T.M.; Das, T.; Sharifi, S.; Subbiahdoss, G.; Sharma, P.K.; Krom, B.P.; Busscher, H.J.; van der Mei, H.C. A Functional DNase I Coating to Prevent Adhesion of Bacteria and the Formation of Biofilm. Adv. Funct. Mater. 2013, 23, 2843–2849. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Yao, Y.; Liu, Z.; Gong, F.; Yan, F. High-Performance Dopamine Sensors Based on Whole-Graphene Solution-Gated Transistors. Adv. Funct. Mater. 2014, 24, 1036. [Google Scholar] [CrossRef]
- Yu, X.; He, D.; Zhang, X.; Zhang, H.; Song, J.; Shi, D.; Fan, Y.; Luo, G.; Deng, J. Surface-Adaptive and Initiator-Loaded Graphene as a Light-Induced Generator with Free Radicals for Drug-Resistant Bacteria Eradication. ACS Appl. Mater. Interfaces 2019, 11, 1766–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive Antibacterial Hemostatic Multifunctional Scaffolds Based on Ti3C2Tx MXene Nanosheets for Promoting Multidrug-Resistant Bacteria-Infected Wound Healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef]
- Habault, D.; Zhang, H.; Zhao, Y. Light-triggered self-healing and shape-memory polymers. Chem. Soc. Rev. 2013, 42, 7244–7256. [Google Scholar] [CrossRef]
- Studer, K.; Decker, C.; Beck, E.; Schwalm, R. Overcoming oxygen inhibition in UV-curing of acrylate coatings by carbon dioxide inerting. Part I Prog. Org. Coat. 2003, 48, 92–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, W.; Yang, K.; Shi, H.; Li, D.; Liu, J.; Ji, J.; Chu, P.K. Antibacterial and osteoinductive capability of orthopedic materials via cation-pi interaction mediated positive charge. J. Mater. Chem. B 2015, 3, 733–737. [Google Scholar] [CrossRef]
- Gan, D.; Xu, T.; Xing, W.; Ge, X.; Fang, L.; Wang, K.; Ren, F.; Lu, X. Mussel-Inspired Contact-Active Antibacterial Hydrogel with High Cell Affinity, Toughness, and Recoverability. Adv. Funct. Mater. 2019, 29, 1805964. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.; Cui, H. Crafting Polymeric and Peptidic Hydrogels for Improved Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900104. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Djamgoz, M.B. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J. Cell Sci. 2004, 117 Pt 9, 1631–1639. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Wei, C.; Chow, J.K.; Nguy, L.; Nguyen, H.K.; Schmidt, C.E. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J. Neural Eng. 2013, 10, 046011. [Google Scholar] [CrossRef]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Tai, G.; Tai, M.; Zhao, M. Electrically stimulated cell migration and its contribution to wound healing. Burn. Trauma 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, Z. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar]
- Mahoney, M.; Sadowski, S.; Brennan, D.; Pikander, P.; Saukko, P.; Wahl, J.; Aho, H.; Heikinheimo, K.; Bruckner-Tuderman, L.; Fertala, A. Compound Heterozygous Desmoplakin Mutations Result in a Phenotype with a Combination of Myocardial, Skin, Hair, and Enamel Abnormalities. J. Investig. Dermatol. 2010, 130, 968–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Gong, J.; Zeng, Y.; Ran, J.; Liu, J.; Wang, K.; Xie, C.; Lu, X.; Wang, J. Bioinspired Conductive Silk Microfiber Integrated Bioelectronic for Diagnosis and Wound Healing in Diabetes. Adv. Funct. Mater. 2021, 31, 2010461. [Google Scholar] [CrossRef]
- Nuccitelli, R. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem. Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar]
- Korupalli, C.; Li, H.; Nguyen, N.; Mi, F.L.; Chang, Y.; Lin, Y.J.; Sung, H.W. Conductive Materials for Healing Wounds: Their Incorporation in Electroactive Wound Dressings, Characterization, and Perspectives. Adv. Healthc. Mater. 2021, 10, e2001384. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Yuwen, L.; Sun, Y.; Tan, G.; Xiu, W.; Zhang, Y.; Weng, L.; Teng, Z.; Wang, L. MoS2@polydopamine-Ag nanosheets with enhanced antibacterial activity for effective treatment of Staphylococcus aureus biofilms and wound infection. Nanoscale 2018, 10, 16711–16720. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Lei, M.; Tan, H.; Chen, Z.; Antoshin, A.; Payne, G.F.; Qu, X.; Liu, C. Redox-Channeling Polydopamine-Ferrocene (PDA-Fc) Coating To Confer Context-Dependent and Photothermal Antimicrobial Activities. ACS Appl. Mater. Interfaces 2020, 12, 8915–8928. [Google Scholar] [CrossRef]
- Long, Y.; Wei, H.; Li, J.; Yao, G.; Yu, B.; Ni, D.; Gibson, A.L.; Lan, X.; Jiang, Y.; Cai, W.; et al. Effective Wound Healing Enabled by Discrete Alternative Electric Fields from Wearable Nanogenerators. ACS Nano 2018, 12, 12533–12540. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zeng, Z.; Liu, Y.; Wang, J.; Maitz, M.F.; Wang, Y.; Liu, S.; Chen, J.; Huang, N. Surface Modification with Dopamine and Heparin/Poly-L-Lysine Nanoparticles Provides a Favorable Release Behavior for the Healing of Vascular Stent Lesions. ACS Appl. Mater. Interfaces 2014, 6, 8729–8743. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, C.; Zhang, X.; Lin, K.; Wang, X.; Shen, S.G. Mussel-Inspired Polydopamine Coating: A General Strategy To Enhance Osteogenic Differentiation and Osseointegration for Diverse Implants. ACS Appl. Mater. Interfaces 2019, 11, 7615–7625. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhou, G.; Chen, Y.; Mao, C.; Yang, M. Polydopamine-Coated Antheraea pernyi (A. pernyi) Silk Fibroin Films Promote Cell Adhesion and Wound Healing in Skin Tissue Repair. ACS Appl. Mater. Interfaces 2019, 11, 34736–34743. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lu, X.; Liu, K.; Wang, K.; Fang, L.; Weng, L.-T.; Zhang, H.; Tang, Y.; Ren, F.; Zhao, C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, A.; Chen, Y.; Yang, Y.; Zhang, Q.; Luo, S.; Ye, M.; Zhou, Y.; An, Y.; Huang, W.; et al. Lotus leaf inspired antiadhesive and antibacterial gauze for enhanced infected dermal wound regeneration. Chem. Eng. J. 2020, 402, 126202. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.; Dai, L.; He, Y.; Hu, J.; Xu, K.; Tao, B.; Liu, P.; Cai, K. Near-Infrared Light-Activatable Dual-Action Nanoparticle Combats the Established Biofilms of Methicillin-Resistant Staphylococcus aureus and Its Accompanying Inflammation. Small 2021, 17, e2007522. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Deokar, A.R.; Lin, L.Y.; Chang, C.C.; Ling, Y.C. Single-walled carbon nanotube coated antibacterial paper: Preparation and mechanistic study. J. Mater. Chem B 2013, 1, 2639–2646. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Yuan, Z.; He, Y.; Chen, M.; Li, K.; Hu, J.; Yang, Y.; Xia, Z.; Cai, K. Near infrared light-triggered on-demand Cur release from Gel-PDA@Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Controlled-temperature photothermal and oxidative bacteria killing and acceleration of wound healing by polydopamine-assisted Au-hydroxyapatite nanorods. Acta Biomater. 2018, 77, 352–364. [Google Scholar] [CrossRef]
- Xu, Q.; Chang, M.; Zhang, Y.; Wang, E.; Xing, M.; Gao, L.; Huan, Z.; Guo, F.; Chang, J. PDA/Cu Bioactive Hydrogel with “Hot Ions Effect” for Inhibition of Drug-Resistant Bacteria and Enhancement of Infectious Skin Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 31255–31269. [Google Scholar] [CrossRef]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Y.; Zheng, S.; Zhong, L.; Xue, J. Preparation and properties of conductive bacterial cellulose-based graphene oxide-silver nanoparticles antibacterial dressing. Carbohydr. Polym. 2021, 257, 117671. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Zheng, B.; Guo, W.; Li, C.; He, W.; Wei, Y.; Du, Y.; Wang, H.; Wu, D.; et al. Highly Stretchable, Adhesive, Biocompatible, and Antibacterial Hydrogel Dressings for Wound Healing. Adv. Sci. 2021, 8, 2003627. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Liu, X.; Yeung, K.W.K.; Zheng, Y.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; et al. Photothermy-strengthened photocatalytic activity of polydopamine-modified metal-organic frameworks for rapid therapy of bacteria-infected wounds. J. Mater. Sci. Technol. 2021, 62, 83–95. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable Gelatin-Based IPN Cryogel Hemostat for Rapidly Stopping Deep Noncompressible Hemorrhage and Simultaneously Improving Wound Healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- He, D.; Yang, T.; Qian, W.; Qi, C.; Mao, L.; Yu, X.; Zhu, H.; Luo, G.; Deng, J. Combined photothermal and antibiotic therapy for bacterial infection via acidity-sensitive nanocarriers with enhanced antimicrobial performance. Appl. Mater. Today 2018, 12, 415–429. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, 1900322. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Tiwari, A.P. Mussel-inspired polydopamine-enabled in situ-synthesized silver nanoparticle-anchored porous polyacrylonitrile nanofibers for wound-healing applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 1857281. [Google Scholar] [CrossRef]
- Ma, K.; Dong, P.; Liang, M.; Yu, S.; Chen, Y.; Wang, F. Facile Assembly of Multifunctional Antibacterial Nanoplatform Leveraging Synergistic Sensitization between Silver Nanostructure and Vancomycin. ACS Appl. Mater. Interfaces 2020, 12, 6955–6965. [Google Scholar] [CrossRef]
- Tavakoli, S.; Mokhtari, H.; Kharaziha, M.; Kermanpur, A.; Talebi, A.; Moshtaghian, J. A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110837. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Qian, Y.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. A surface-engineered polyetheretherketone biomaterial implant with direct and immunoregulatory antibacterial activity against methicillin-resistant Staphylococcus aureus. Biomaterials 2019, 208, 8–20. [Google Scholar] [CrossRef]
- Wang, B.L.; Ren, K.F.; Chang, H.; Wang, J.L.; Ji, J. Construction of degradable multilayer films for enhanced antibacterial properties. ACS Appl Mater. Interfaces 2013, 5, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, Y.; Guo, B.; Yin, Z.; Zhu, D.; Han, Y. Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration. Chem. Eng. J. 2021, 403, 126329. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar] [CrossRef]
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef]
- Li, M.; Liang, Y.; He, J.; Zhang, H.; Guo, B. Two-Pronged Strategy of Biomechanically Active and Biochemically Multifunctional Hydrogel Wound Dressing To Accelerate Wound Closure and Wound Healing. Chem. Mater. 2020, 32, 9937–9953. [Google Scholar] [CrossRef]
- Guc, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-Inspired Electroactive and Antioxidative Scaffolds with Incorporation of Polydopamine-Reduced Graphene Oxide for Enhancing Skin Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714. [Google Scholar] [CrossRef]
- Moseley, R.; Hilton, J.R.; Waddington, R.J.; Harding, K.G.; Stephens, P.; Thomas, D.W. Comparison of oxidative stress biomarker profiles between acute and chronic wound environments. Wound Repair Regen. 2004, 12, 419–429. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef]
- Kopf, M.; Baumann, H.; Freer, G.; Freudenberg, M.; Lamers, M.; Kishimoto, T.; Zinkernagel, R.; Bluethmann, H.; Kohler, G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 1994, 368, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Omar, J.; Shafii, N.; Zainan, A.E.; Sirajudeen, K.N.S.; Abdullah, M.R. Evaluation of wound healing biomarkers of Interleukin 6 (IL-6), Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinases 9 (MMP-9) in post Lower Segment Caesarean Section (LSCS) patients consuming Channa Striatus extract. Bangladesh J. Med. Sci. 2020, 19, 520–526. [Google Scholar] [CrossRef]
- Salek-Maghsoudi, A.; Vakhshiteh, F.; Torabi, R.; Hassani, S.; Ganjali, M.R.; Norouzi, P.; Hosseini, M.; Abdollahi, M. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens. Bioelectron. 2018, 99, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Gagger, A.; Blalock, J.E. MMP generated matrikines. Matrix Biol. 2015, 44–46, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.; Wang, X.Y.; Zhou, L.Y.; Lao, G.J.; Liu, D.; Wang, C.; Hu, M.D.; Zeng, T.T.; Yan, L.; et al. MicroRNA-129 and-335 Promote Diabetic Wound Healing by Inhibiting Sp1-Mediated MMP-9 Expression. Diabetes 2018, 67, 1627–1638. [Google Scholar] [CrossRef] [Green Version]
- Tardaguila-Garcia, A.; Garcia-Morales, E.; Garcia-Alamino, J.M.; Javier Alvaro-Afonso, F.; Juan Molines-Barroso, R.; Luis Lazaro-Martinez, J. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated levels of matrix metalloproteinases and chronic wound healing: An updated review of clinical evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Et Biophys. Acta-Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef]
- Chen, L.; Xing, Q.; Zhai, Q.; Tahtinen, M.; Zhou, F.; Chen, L.; Xu, Y.; Qi, S.; Zhao, F. Pre-vascularization Enhances Therapeutic Effects of Human Mesenchymal Stem Cell Sheets in Full Thickness Skin Wound Repair. Theranostics 2017, 7, 117–131. [Google Scholar] [CrossRef]
- Hemmati, S.; Zangeneh, M.M.; Zangeneh, A. CuCl2 anchored on polydopamine coated-magnetic nanoparticles (Fe3O4@PDA/Cu(II)): Preparation, characterization and evaluation of its cytotoxicity, antioxidant, antibacterial, and antifungal properties. Polyhedron 2020, 177, 114327. [Google Scholar] [CrossRef]
- Bock, N.; Truc Le-Buu, P.; Tran Bao, N.; Trong Binh, N.; Tran, H.A.; Tran, P.A. Polydopamine coating of uncrosslinked chitosan as an acellular scaffold for full thickness skin grafts. Carbohydr. Polym. 2020, 245, 116524. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Extracell. Matrix 2019, 63, 349–364. [Google Scholar]
- Kim, H.; Nakamura, F.; Lee, W.; Shifrin, Y.; Arora, P.; McCulloch, C.A. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 2010, 298, C221–C236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Xi, Y.; Xue, Y.; Wang, M.; Liu, Y.; Guo, Y.; Lei, B. Injectable Self-Healing Antibacterial Bioactive Polypeptide-Based Hybrid Nanosystems for Efficiently Treating Multidrug Resistant Infection, Skin-Tumor Therapy, and Enhancing Wound Healing. Adv. Funct. Mater. 2019, 29, 1806883. [Google Scholar] [CrossRef]
- Liang, L.; Hou, T.; Ouyang, Q.; Xie, L.; Zhong, S.; Li, P.; Li, S.; Li, C. Antimicrobial sodium alginate dressing immobilized with polydopamine-silver composite nanospheres. Compos. Part B Eng. 2020, 188, 107877. [Google Scholar] [CrossRef]
- De Angelis, B.; D’Autilio, M.; Orlandi, F.; Pepe, G.; Garcovich, S.; Scioli, M.G.; Orlandi, A.; Cervelli, V.; Gentile, P. Wound Healing: In Vitro and In Vivo Evaluation of a Bio-Functionalized Scaffold Based on Hyaluronic Acid and Platelet-Rich Plasma in Chronic Ulcers. J. Clin. Med. 2019, 8, 1486. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Yang, P.; Jin, Z.; Jiang, Q.; Guo, R.; Xie, R.; He, Q.; Yang, W. Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials 2019, 197, 268–283. [Google Scholar] [CrossRef]
- Babitha, S.; Korrapati, P.S. Biodegradable zein-polydopamine polymeric scaffold impregnated with TiO2 nanoparticles for skin tissue engineering. Biomed. Mater. 2017, 12, 055008. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Li, J.; Han, Y.; Zhang, P.; Yi, Z.; Ke, Q.; Xu, H. A Mussel-Inspired Extracellular Matrix-Mimicking Composite Scaffold for Diabetic Wound Healing. ACS Appl. Bio Mater. 2020, 3, 4052–4061. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, M.; Shi, P.; Gao, Y.; Ma, J.; Li, Y.; Huang, L.; Yang, Z.; Yang, L. Polydopamine-modified collagen sponge scaffold as a novel dermal regeneration template with sustained release of platelet-rich plasma to accelerate skin repair: A one-step strategy. Bioact. Mater. 2021, 6, 2613–2628. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr. Polym. 2021, 257, 117598. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, P.; Wang, X.; Zhang, Q.; Zhang, Y.; Fan, H.; Li, R.; Zhang, M. Polydopamine-assisted PDGF-BB immobilization on PLGA fibrous substrate enhances wound healing via regulating anti-inflammatory and cytokine secretion. PLoS ONE 2020, 15, e0239366. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Deng, Z.; Yang, X.; Li, L.; Gao, Y.; Li, W. Ultrathin two-dimensional polydopamine nanosheets for multiple free radical scavenging and wound healing. Chem. Commun. 2020, 56, 10875–10878. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Maharajan, K.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Bacterial cellulose matrix with in situ impregnation of silver nanoparticles via catecholic redox chemistry for third degree burn wound healing. Carbohydr. Polym. 2020, 245, 116573. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, S.; Zhang, X.; Yu, J.; Wang, C. Mussel-inspired polydopamine-assisted bromelain immobilization onto electrospun fibrous membrane for potential application as wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110624. [Google Scholar] [CrossRef]
- Chai, C.; Guo, Y.; Huang, Z.; Zhang, Z.; Yang, S.; Li, W.; Zhao, Y.; Hao, J. Antiswelling and Durable Adhesion Biodegradable Hydrogels for Tissue Repairs and Strain Sensors. Langmuir 2020, 36, 10448–10459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sun, X.; Zhao, X.; Wang, L.; Yu, J.; Pan, G.; Li, B.; Yang, H.; Zhang, Y.; Cui, W. Surface biofunctional drug-loaded electrospun fibrous scaffolds for comprehensive repairing hypertrophic scars. Biomaterials 2016, 83, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cheng, L.; Zhao, J.; Jin, R.; Sun, B.; Shi, Y.; Zhang, L.; Zhang, Y.; Cui, W. bFGF-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J. Mater. Chem. B 2014, 2, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Leng, E.G.T.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.H.U.T.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153–168. [Google Scholar] [CrossRef]
- Liu, M.; Luo, G.; Wang, Y.; Xu, R.; Wang, Y.; He, W.; Tan, J.; Xing, M.; Wu, J. Nano-silver-decorated microfibrous eggshell membrane: Processing, cytotoxicity assessment and optimization, antibacterial activity and wound healing. Sci. Rep. 2017, 7, 436. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-inspired cryogels for promoting wound regeneration through photobiostimulation, modulating inflammatory responses and suppressing bacterial invasion. Nanoscale 2019, 11, 15846–15861. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.A.; Ly, K.L.; Fox, K.E.; Tran, P.A.; Thi-Hiep, N. Immobilization of Antimicrobial Silver and Antioxidant Flavonoid as a Coating for Wound Dressing Materials. Int. J. Nanomed. 2019, 14, 9929–9939. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Cui, Z.; Song, R.; Lv, B.; Tang, Z.; Meng, X.; Chen, X.; Zheng, X.; Zhang, J.; Yao, Z.; et al. SnWO4-based nanohybrids with full energy transfer for largely enhanced photodynamic therapy and radiotherapy. Biomaterials 2018, 155, 135–144. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Yao, C.; Wang, W.; Zhao, M.; El-Toni, A.M.; Zhang, F. Orthogonal near-infrared upconversion co-regulated site-specific O2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers. Biomaterials 2017, 125, 90–100. [Google Scholar] [CrossRef]
- Tang, J.B.; Cao, Y.; Xie, R.G.; Zhu, B.; Ke-Qin, X.; Tian, W.X.; Paul, L. BFGF gene transfer through AAV vectors to digital flexor tendons significantly increases healing strength: An in vivo study. J. Am. Coll. Surg. 2006, 203, S57–S58. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, H.Q.; Sun, X.P. Tests on wave-induced dynamic response and instability of silty clay seabeds around a semi-circular breakwater. Appl. Ocean Res. 2018, 78, 1–13. [Google Scholar] [CrossRef]
- Hamada, Y.; Katoh, S.; Hibino, N.; Kosaka, H.; Hamada, D.; Yasui, N. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J. Hand Surg. Am. 2006, 31, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Rickert, M.; Jung, M.; Adiyaman, M.; Richter, W.; Simank, H.G. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors 2001, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, F.; Liu, J.; Smatt, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, N.; Zhang, T.; Yin, X.; Shen, J. Fabrication of doxorubicin-gated mesoporous polydopamine nanoplatforms for multimode imaging-guided synergistic chemophotothermal therapy of tumors. Drug Deliv. 2020, 27, 367–377. [Google Scholar] [CrossRef]
- Xia, G.; Zhai, D.; Sun, Y.; Hou, L.; Guo, X.; Wang, L.; Li, Z.; Wang, F. Preparation of a novel asymmetric wettable chitosan-based sponge and its role in promoting chronic wound healing. Carbohydr. Polym. 2020, 227, 115296. [Google Scholar] [CrossRef]
- Bf, B.; Rb, B.; Nd, B.; Jm, B.; Mra, B.; Srb, C.; Spna, B.; Atv, D.; Sgka, B.J.B.M. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.J.N.C. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6, 6575. [Google Scholar] [CrossRef]
- Pei, X.; Jin, H.; Dong, S.; Lou, D.; Ma, L.; Wang, X.; Cheng, W.; Wong, H. Flexible wireless skin impedance sensing system for wound healing assessment. Vacuum 2019, 168, 108808. [Google Scholar] [CrossRef]
- Leung, C.M.; Dhand, C.; Mayandi, V.; Ramalingam, R.; Lim, F.P.; Barathi, V.A.; Dwivedi, N.; Orive, G.; Beuerman, R.W.; Ramakrishna, S.; et al. Wound healing properties of magnesium mineralized antimicrobial nanofibre dressings containing chondroitin sulphate-a comparison between blend and core-shell nanofibres. Biomater. Sci. 2020, 8, 3454–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hussain, M.; Wang, M.; Li, Z.; He, N. Embedded 3D printing of multi-internal surfaces of hydrogels. Addit. Manuf. 2020, 32, 101097. [Google Scholar] [CrossRef]


| Material | ΔTs/°C | NIR Antibacterial Rate | Anti-Bacterial Rate | Reference |
|---|---|---|---|---|
| Dibenzaldehyde-grafted poly (ethylene glycol) (PEGDA), lauric acid-terminated chitosan (Chi-LA), and Cur-loaded mesoporous PDA nanoparticles (PDA@Cur) | 24.8 | E. coli, 97.8%; S. aureus, 94.2% | E. coli, 37.1%; S. aureus, 20.4% | [68] |
| PDA@gold nanoparticles-hydroxyapatite PBS liquid | 18 | E. coli, 96.8; S. aureus, 95.2% | E. coli, 34.6%; S. aureus, 13.7% | [69] |
| Quaternized chitosan/PDA | 27.2 | S. aureus, 100%; E. coli, 100% | S. aureus, 100%; E. coli, 70% | [25] |
| TiO2 nanorods-PDA-Ferrocene | 38 | MRSA 1, ≥99%; E. coli, >99% | - | [56] |
| PDA/Cu-CS | 43 | MRSA, 97.64%; E. coli, 96.27% | MRSA, 13.89%, E. coli, 48.82% | [70] |
| Poly(L-lactic acid)-poly(citrate siloxane)-curcumin@PDA hybrid nanofibrous scaffold (denoted as PPCP matrix) | 21 | E. coli, 93.3 ± 1.2%; S. aureus, 97.7 ± 0.7% | - | [71] |
| Ag-pDA/BC (rGO) | - | E. coli, >84% | - | [72] |
| Deoxyribonuclease (DNase)-carbon monoxide (CO)@mesoporous PDA nanoparticles (MPDA NPs) | 23 | MRSA, 92% | - | [65] |
| bacterial cellulose/PDA/polyacrylamide hydrogels | - | - | S. aureus, 100% | [73] |
| MOF-PDA | 31 | S. aureus, 99.62%; E. coli, 99.97% | 0 | [74] |
| Gelatin/dopamine cryogels | 24.2 | S. aureus, 100%; E. coli, 100% | S. aureus, 70%; E. coli, 68% | [75] |
| DAP-GCS-PDA@GNRs | 35 | MRSA, 100% | - | [76] |
| MoS2@PDA-Ag | 43 | S. aureus, 99.99% | - | [55] |
| Carbon quantum dot (CQD)-decorated ZnO (C/ZnO) composites were chosen as the functional NPs | 30 | S. aureus, 99.9996%%; E. coli, 99.9998% | S. aureus, 70.8%; E. coli, 60.2% | [77] |
| GT-DA/CS/CNT gelatin-grafted-dopamine (GT-DA) and PDA-coated carbon nanotubes (CNT-PDA) | 26.7 | S. aureus, 100%; E. coli, 100% | S. aureus, 5.9%; E. coli, 2.1% | [78] |
| Materials | Healing Time/d | Healing Condition | Reference |
|---|---|---|---|
| Bioactive glass/PDA-modified electrospun scaffolds | 15 | The wound was almost completely healed and the remaining wound area was 0.98%. There were more dermal papillae and hair follicles on the wound surface and the epithelial structure was mature. | [115] |
| PDA-NPs/PNIPAM gel | 15 | The wound was closed, mature skin tissue was regenerated, and collagen fibers and hair follicles were arranged. | [35] |
| QCS/PDA | 10 | The wound healed completely. | [25] |
| PDA-reduced graphene oxide (pGO)-incorporated chitosan (CS) and silk fibroin | 21 | Blood stagnation and wound closure were observed. | [94] |
| PDA-coated Antheraea pernyi | 14 | The wound was completely healed and new skin and hair formed. | [60] |
| Poly(glycerol-ethylenimine),Ti3C2TxMXene@PDA (MXene@PDA) nanosheets and oxidized hyaluronic acid (HCHO) | 14 | The wound healing rate was 96.31%. | [34] |
| PDA (PDA) coating on hydroxyapatite (HAp) incorporated with gold nanoparticles (Au-HAp) | 10 | The wound healed. | [69] |
| Poly(3,4-ethylenedioxythiophene)-PDA-silk microfibers | 15 | The wound healed. | [50] |
| PDA functionalized bioactive glass nanoparticles (BGN@PDA)-F127-ε-Poly-l-lysine hydrogel | 14 | The wound was largely healed and abundant granulation tissue was visible. | [110] |
| PDA@Ag NPs), polyaniline-polyvinyl alcohol | 20 | The wound completely healed. | [40] |
| PDA/collagen sponge scaffolds | 21 | There was a full-thickness skin defect on the wound surface. After 21 d, the full thickness skin of each group survived, the wound was closed, and there was no obvious gap between the skin and regenerated tissue. | [116] |
| pDA-epsilon PL/NFDS | 32 | The average burn wound healing rate was 88.3 ± 16.0%. Angiogenesis and granulation tissue regeneration were increased. | [25] |
| Chitosan 20 mg/PDA 4.5 mg | 15 | The wound closure rate was 100%. | [85] |
| Bacterial cellulose/PDA/polyacrylamide hydrogels | 15 | Granulation tissue deposition was dense and collagen bundles were regular. | [73] |
| Dibenzaldehyde-grafted poly(ethylene glycol) (PEGDA), lauric acid-terminated chitosan (Chi-LA), and curcumin (Cur)-loaded mesoporous PDA nanoparticles (PDA@Cur) | 14 | Most of the wound healed. | [68] |
| Agarose-PDA hydrogel (APG) | 14 | The collagen density increased and most of the wound healed. | [117] |
| MOF-PDA | 12 | The wound healed. | [74] |
| Educed PDA nanoparticles (rPDA NPs) incorporated oxidized dextran/chitosan hybrid hydrogels | 15 | The wound healed with scar tissue. | [118] |
| pDA/PLGA nanofibrous/platelet-derived growth factor-bb | 7 | The wound was reduced by >80%. | [119] |
| Carpacara methacrylate-ZnO/PDA | 14 | There was complete epithelialization. | [81] |
| Ordinary medical gauze sequentially with PDA, perfluorocarbon, and silver nanoparticle | 14 | The wound area was reduced to 8.1 ± 5.7%. | [64] |
| QCS/reduction graphene oxide-PDA/poly(N-isopropylacrylamide) | 14 | The wound healed completely with re-epithelialization and no scar tissue was visible. | [90] |
| 2D PDA nanosheets | 14 | The wound of the high-dose group was healed without obvious scarring. | [120] |
| PDA coated BC with in situ silver nanoparticle reduction | 25 | The third degree burn wound healed completely without scarring. | [121] |
| Gelatin/dopamine cryogels | 14 | The wound was completely healed and re-epithelialized without scarring. | [75] |
| Bromelain immobilized electrospun poly(ε-caprolactone) (PCL) fibers (BrPDA-PCL fibers) | 11 | The wound was completely closed with scarring. | [122] |
| PTA/PDA | 18 | The wound was completely closed in the full-thickness skin defect model. | [123] |
| PDA-RGD peptide-bFGF | 60 | The rabbit ear wound was completely healed and epithelialized without scarring. | [124] |
| Basic fibroblast growth factor (bFGF)/PDA/poly(lactide-co-glycolide) (PLGA) fibers | 14 | The wound healing rate was 92% and scar tissue was obvious. | [125] |
| Zein/PDA/TiO2 | 15 | There was complete re-epithelialization with partial scar tissue. | [114] |
| Van-gel-PDA | 46 | The burn wound was closed without re-epithelialization. | [126] |
| Eggshell membrane/PDA | 7 | The wound healing rate was 81.9%. | [127] |
| MoS2@PDA-Ag | 8 | Most of the wound healed. | [55] |
| EGF-loaded PDA-NP-CS/SF cryogel | 21 | The wound healed completely and was re-epithelialized. | [128] |
| H2O2/HPR (horseradish peroxidase)-PDA-rGo | 14 | The hydrogel group had relatively more skin appendages and blood vessels such as hair follicles. | [129] |
| Carbon quantum dot (CQD)-decorated ZnO (C/ZnO) composites were chosen as the functional NPs | 10 | The skin was intact and the subcutaneous tissue structure was normal. | [77] |
| Cotton gauge (CG)-coated with quercetin and silver | 12 | On day 12, a thin layer of dermis was observed complete with glands and hair roots forming as per normal tissue. Connective tissue deposition and adipose tissue formation were enhanced. | [130] |
| GT-DA/CS/CNT gelatin-grafted-dopamine (GT-DA) and PDA-coated carbon nanotubes (CNT-PDA) | 14 | The wound surface was almost completely closed and smooth new epidermal tissue appeared. | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Huang, C.; Zhu, X.; Huang, H.; Xu, C. Performance of Polydopamine Complex and Mechanisms in Wound Healing. Int. J. Mol. Sci. 2021, 22, 10563. https://doi.org/10.3390/ijms221910563
Zheng D, Huang C, Zhu X, Huang H, Xu C. Performance of Polydopamine Complex and Mechanisms in Wound Healing. International Journal of Molecular Sciences. 2021; 22(19):10563. https://doi.org/10.3390/ijms221910563
Chicago/Turabian StyleZheng, Dantong, Chongxing Huang, Xuhao Zhu, Haohe Huang, and Chenglong Xu. 2021. "Performance of Polydopamine Complex and Mechanisms in Wound Healing" International Journal of Molecular Sciences 22, no. 19: 10563. https://doi.org/10.3390/ijms221910563







