Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
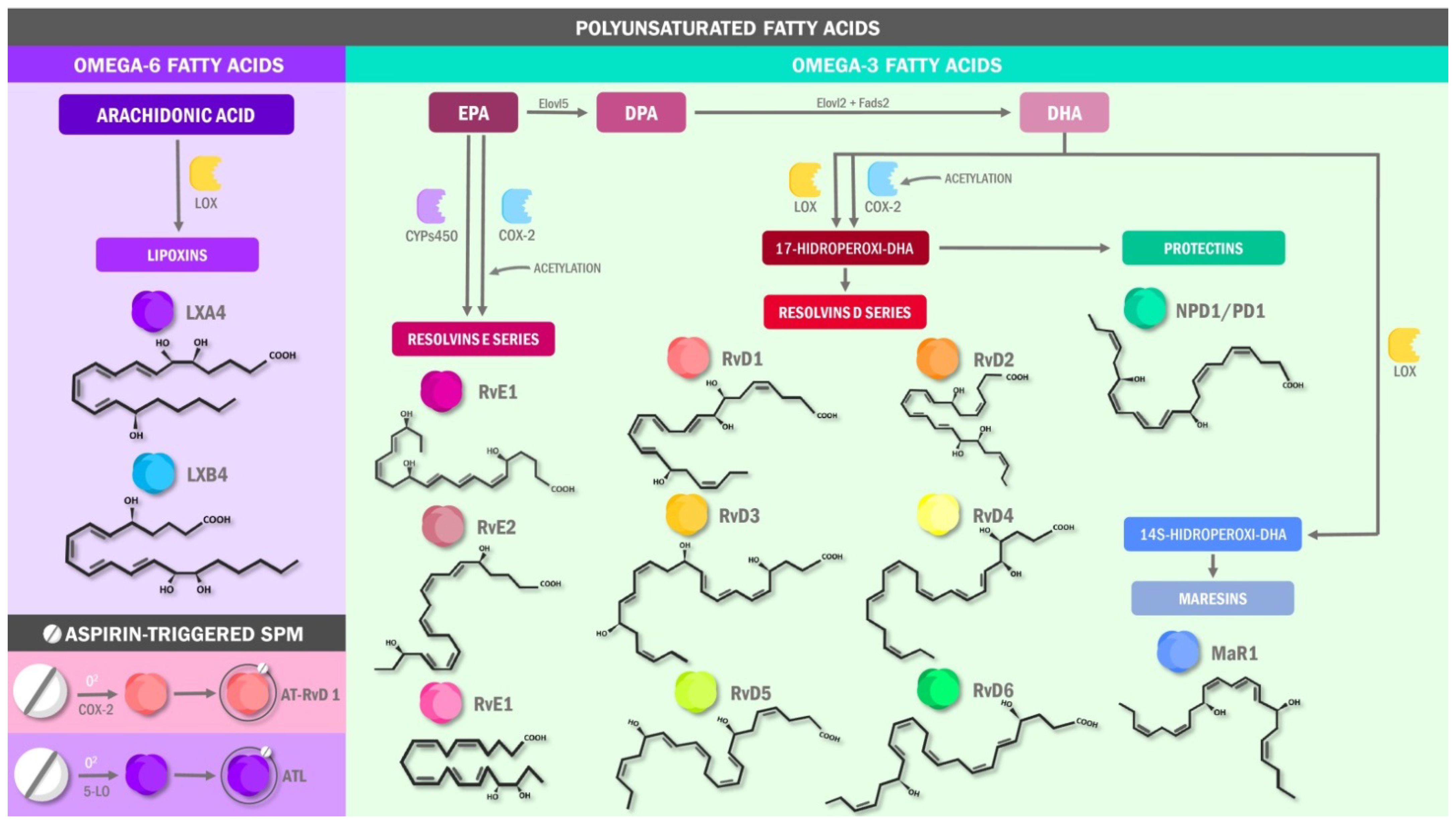
3.1. Specialized Pro-Resolving Lipids Mediators in Pain: The Molecular Basis
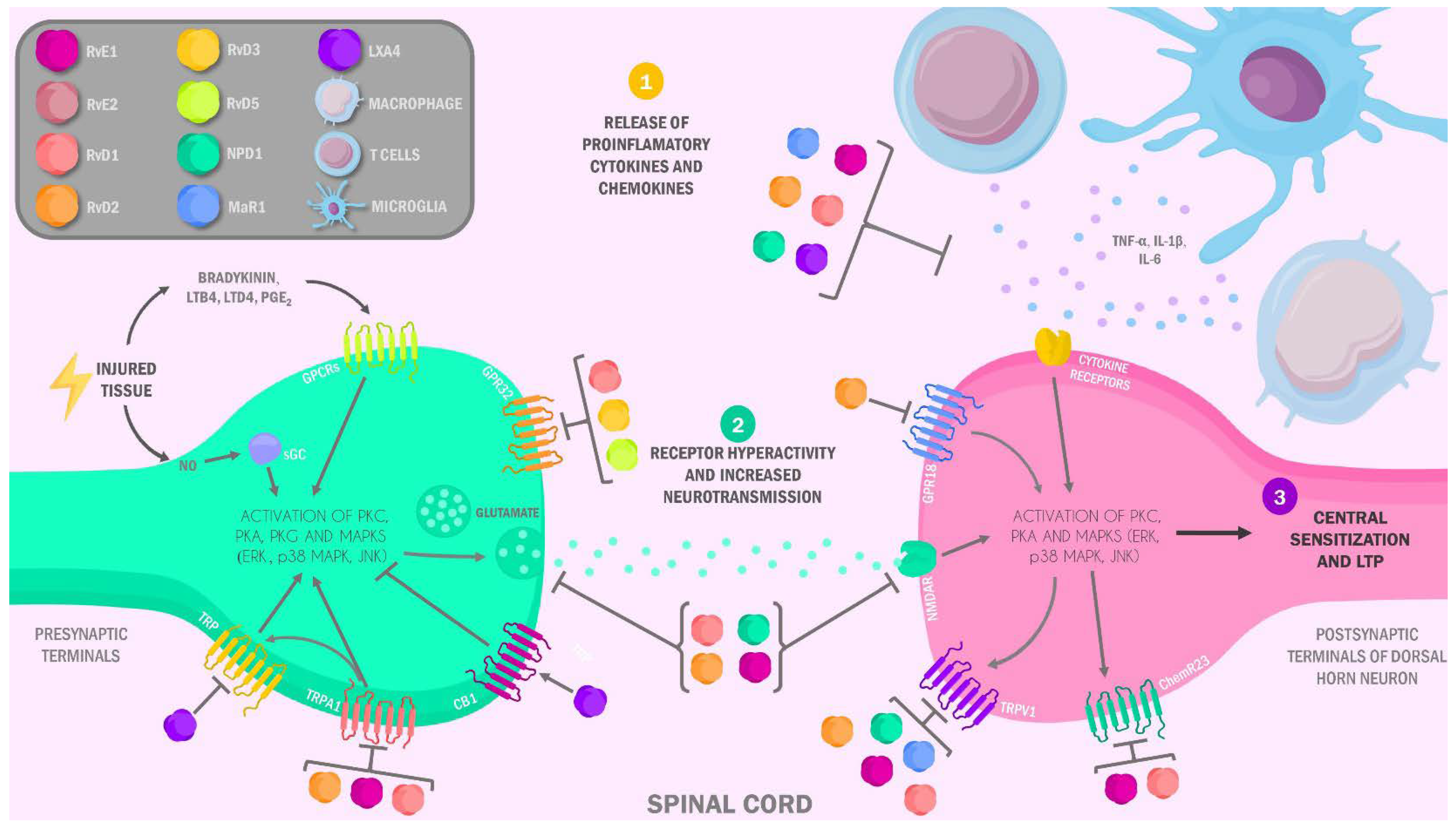
3.2. Specialized Pro-Resolving Lipids Mediators in the Neurobiology of Pain: Anti-Inflammatory and Analgesic Mechanisms
3.3. Preclinical and Clinical Evidence on Specialized Pro-Resolving Lipids Mediators in the Management of Pain
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geurts, J.W.; Willems, P.C.; Lockwood, C.; van Kleef, M.; Kleijnen, J.; Dirksen, C. Patient Expectations for Management of Chronic Non-Cancer Pain: A Systematic Review. Health Expect. 2017, 20, 1201–1217. [Google Scholar] [CrossRef] [Green Version]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of Chronic Pain in the UK: A Systematic Review and Meta-Analysis of Population Studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef] [PubMed]
- Kheiry, F.; Rakhshan, M.; Shaygan, M. The prevalence and associated factors of chronic pain in nurses Iran. Latinoam. Hipertens. 2019, 14, 20–25. [Google Scholar]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, V.J.A.; Dos Santos, F.; Dyzinger, W.; Herzog, S. Medicina Del Estilo de Vida: Trabajando Juntos Para Revertir La Epidemia de Las Enfermedades Crónicas En Latinoamérica. Cienc. Innovación Salud 2017, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Opioid Overdose. Available online: https://www.who.int/news-room/fact-sheets/detail/opioid-overdose (accessed on 21 June 2021).
- Hern, O.; Saumeth, K.T.; Cabrera, J.L.; Pinz, M. Consumos y Costos de Medicamentos: Herramienta para la Gestión de Suministro del Servicio Farmacéutico. Cienc. Innovación Salud 2015, 3, 45–52. [Google Scholar] [CrossRef]
- Freire, L.F.L.; Chingo, D.J.A.; Saldarriaga, L.C.Z.; Mera, L.M.I.; Escalante, V.C.G.; Villacres, A.X.Z.; Sanguil, A.T.A.; Bucheli, F.J.J.; Velasco, S.J.S. Alternativas emergentes en la farmacoterapia de la neuralgia del trigémino. AVFT—Arch. Venez. Farmacol. Ter. 2019, 38, 34–39. [Google Scholar]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging Targets in Neuroinflammation-Driven Chronic Pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Ravipati, S.; Menni, C.; Abhishek, A.; Metrustry, S.; Harris, J.; Nessa, A.; Williams, F.M.K.; Spector, T.D.; Doherty, M.; et al. Association of the Resolvin Precursor 17-HDHA, but Not D- or E- Series Resolvins, with Heat Pain Sensitivity and Osteoarthritis Pain in Humans. Sci. Rep. 2017, 7, 10748. [Google Scholar] [CrossRef]
- Chiang, N.; Serhan, C.N. Structural Elucidation and Physiologic Functions of Specialized Pro-Resolving Mediators and Their Receptors. Mol. Asp. Med. 2017, 58, 114–129. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: A New Class of Non-Immunosuppressive and Non-Opioid Analgesic Drugs. Pharmacol. Res. 2020, 151, 104549. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.C.; Berta, T.; Forner, S.; Chen, G.; Bento, A.F.; Ji, R.-R.; Rae, G.A. Lipoxin A4 Inhibits Microglial Activation and Reduces Neuroinflammation and Neuropathic Pain after Spinal Cord Hemisection. J. Neuroinflamm. 2016, 13, 75. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, R.J.; Katz, J. A Meta-Analysis of the Analgesic Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation for Inflammatory Joint Pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Polyunsaturated Fatty Acids and Inflammatory Processes: New Twists in an Old Tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Jia, M.-R.; Sun, T. The Roles of Special Proresolving Mediators in Pain Relief. Rev. Neurosci. 2018, 29, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.S.; Zahner, G.J.; Gasper, W.J.; Harris, W.S.; Conte, M.S.; Hills, N.K.; Grenon, S.M. Relationship between the Omega-3 Index and Specialized pro-Resolving Lipid Mediators in Patients with Peripheral Arterial Disease Taking Fish Oil Supplements. J. Clin. Lipidol. 2017, 11, 1289–1295. [Google Scholar] [CrossRef]
- Levy, B.D. Resolvins and Protectins: Natural Pharmacophores for Resolution Biology. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 327–332. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, P.; Patrono, C. Cyclooxygenase Inhibitors: From Pharmacology to Clinical Read-Outs. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 422–432. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Recchiuti, A.; Serhan, C.N. Pro-Resolving Lipid Mediators (SPMs) and Their Actions in Regulating MiRNA in Novel Resolution Circuits in Inflammation. Front. Immunol. 2012, 3, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalli, J.; Serhan, C.N. Pro-Resolving Mediators in Regulating and Conferring Macrophage Function. Front. Immunol. 2017, 8, 1400. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Resolution Phase of Inflammation: Novel Endogenous Anti-Inflammatory and Proresolving Lipid Mediators and Pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid Mediator Class Switching during Acute Inflammation: Signals in Resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [Green Version]
- Fiore, S.; Ryeom, S.W.; Weller, P.F.; Serhan, C.N. Lipoxin Recognition Sites. Specific Binding of Labeled Lipoxin A4 with Human Neutrophils. J. Biol. Chem. 1992, 267, 16168–16176. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel Anti-Inflammatory--pro-Resolving Mediators and Their Receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- McMahon, B.; Godson, C. Lipoxins: Endogenous Regulators of Inflammation. Am. J. Physiol. Renal Physiol. 2004, 286, F189–F201. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Taddeo, B.; Varai, G.; Varga, J.; Fiore, S. Mutations of Serine 236–237 and Tyrosine 302 Residues in the Human Lipoxin A4 Receptor Intracellular Domains Result in Sustained Signaling. Biochemistry 2000, 39, 13551–13557. [Google Scholar] [CrossRef]
- Bonnekoh, H.; Scheffel, J.; Wu, J.; Hoffmann, S.; Maurer, M.; Krause, K. Skin and Systemic Inflammation in Schnitzler’s Syndrome Are Associated With Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Barbu, E.A.; Mendelsohn, L.; Samsel, L.; Thein, S.L. Pro-Inflammatory Cytokines Associate with NETosis during Sickle Cell Vaso-Occlusive Crises. Cytokine 2020, 127, 154933. [Google Scholar] [CrossRef]
- Li, X.; Yuan, K.; Zhu, Q.; Lu, Q.; Jiang, H.; Zhu, M.; Huang, G.; Xu, A. Andrographolide Ameliorates Rheumatoid Arthritis by Regulating the Apoptosis-NETosis Balance of Neutrophils. Int. J. Mol. Sci. 2019, 20, 5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headland, S.E.; Norling, L.V. The Resolution of Inflammation: Principles and Challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and Aspirin-Triggered Lipoxins in Resolution of Inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
- Chiang, N.; Bermudez, E.A.; Ridker, P.M.; Hurwitz, S.; Serhan, C.N. Aspirin Triggers Antiinflammatory 15-Epi-Lipoxin A4 and Inhibits Thromboxane in a Randomized Human Trial. Proc. Natl. Acad. Sci. USA 2004, 101, 15178–15183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Meara, S.J.; Rodgers, K.; Godson, C. Lipoxins: Update and Impact of Endogenous pro-Resolution Lipid Mediators. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 47–70. [Google Scholar] [CrossRef]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, Actions, and Therapeutic Potential of Specialized Pro-Resolving Lipid Mediators for the Treatment of Inflammation in Cystic Fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Ryan, A.; Godson, C. Lipoxins: Regulators of Resolution. Curr. Opin. Pharmacol. 2010, 10, 166–172. [Google Scholar] [CrossRef]
- Maderna, P.; Cottell, D.C.; Berlasconi, G.; Petasis, N.A.; Brady, H.R.; Godson, C. Lipoxins Induce Actin Reorganization in Monocytes and Macrophages but Not in Neutrophils: Differential Involvement of Rho GTPases. Am. J. Pathol. 2002, 160, 2275–2283. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of Acute Inflammation in the Lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Serhan, C. Macrophage Proresolving Mediators-the When and Where. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariel, A.; Chiang, N.; Arita, M.; Petasis, N.A.; Serhan, C.N. Aspirin-Triggered Lipoxin A4 and B4 Analogs Block Extracellular Signal-Regulated Kinase-Dependent TNF-Alpha Secretion from Human T Cells. J. Immunol. 2003, 170, 6266–6272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tungen, J.E.; Gerstmann, L.; Vik, A.; De Matteis, R.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Kalesse, M.; Hansen, T.V. Resolving Inflammation: Synthesis, Configurational Assignment, and Biological Evaluations of RvD1n-3 DPA. Chemistry 2019, 25, 1476–1480. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.E.; Andreasen, S.O.; Christensen, J.P.; Thomsen, A.R. CD11b Expression as a Marker to Distinguish between Recently Activated Effector CD8(+) T Cells and Memory Cells. Int. Immunol. 2001, 13, 593–600. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of Resolvin D2 Receptor Mediating Resolution of Infections and Organ Protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 Receptor Axis in Infectious Inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef] [Green Version]
- Duffney, P.F.; Falsetta, M.L.; Rackow, A.R.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Key Roles for Lipid Mediators in the Adaptive Immune Response. J. Clin. Investig. 2018, 128, 2724–2731. [Google Scholar] [CrossRef]
- Kim, N.; Ramon, S.; Thatcher, T.H.; Woeller, C.F.; Sime, P.J.; Phipps, R.P. Specialized Proresolving Mediators (SPMs) Inhibit Human B-Cell IgE Production. Eur. J. Immunol. 2016, 46, 81–91. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [Green Version]
- Dartt, D.A.; Hodges, R.R.; Serhan, C.N. Immunoresolvent Resolvin D1 Maintains the Health of the Ocular Surface. Adv. Exp. Med. Biol. 2019, 1161, 13–25. [Google Scholar] [CrossRef]
- Spite, M.; Norling, L.V.; Summers, L.; Yang, R.; Cooper, D.; Petasis, N.A.; Flower, R.J.; Perretti, M.; Serhan, C.N. Resolvin D2 Is a Potent Regulator of Leukocytes and Controls Microbial Sepsis. Nature 2009, 461, 1287–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, F.; Roncucci, L. Chemerin/ChemR23 Axis in Inflammation Onset and Resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Haworth, O.; Cernadas, M.; Levy, B.D. NK Cells Are Effectors for Resolvin E1 in the Timely Resolution of Allergic Airway Inflammation. J. Immunol. 2011, 186, 6129–6135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Discovery of Specialized Pro-Resolving Mediators Marks the Dawn of Resolution Physiology and Pharmacology. Mol. Asp. Med. 2017, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saeki, K.; Yokomizo, T. Identification, Signaling, and Functions of LTB4 Receptors. Semin. Immunol. 2017, 33, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, A.; Zou, Y.; Ge, J. “Pro-Resolution” and Anti-Inflammation, a Role of RvE1 in Anti-Atherosclerosis and Plaque Stabilization. Med. Hypotheses 2008, 71, 252–255. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Correa, M.D.; López, M.R. Activación alternativa del macrófago: La diversidad en las respuestas de una célula de la inmunidad innata ante la complejidad de los eventos de su ambiente. Inmunologia 2007, 26, 73–86. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and Maresins: New pro-Resolving Families of Mediators in Acute Inflammation and Resolution Bioactive Metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.-M.; Chung, G.; Kim, Y.H.; Park, C.-K. The Role of Maresins in Inflammatory Pain: Function of Macrophages in Wound Regeneration. Int. J. Mol. Sci. 2019, 20, 5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häcker, H.; Karin, M. Regulation and Function of IKK and IKK-Related Kinases. Sci. STKE 2006, 2006, re13. [Google Scholar] [CrossRef]
- Bitto, A.; Minutoli, L.; David, A.; Irrera, N.; Rinaldi, M.; Venuti, F.S.; Squadrito, F.; Altavilla, D. Flavocoxid, a Dual Inhibitor of COX-2 and 5-LOX of Natural Origin, Attenuates the Inflammatory Response and Protects Mice from Sepsis. Crit. Care 2012, 16, R32. [Google Scholar] [CrossRef] [Green Version]
- Kohli, P.; Levy, B.D. Resolvins and Protectins: Mediating Solutions to Inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N. Novel Chemical Mediators in the Resolution of Inflammation: Resolvins and Protectins. Anesthesiol. Clin. 2006, 24, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.; Novgorodtseva, T.; Denisenko, Y.; Antonyuk, M.; Gvozdenko, T. Pro-Resolving Lipid Mediators in the Pathophysiology of Asthma. Medicina 2019, 55, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.; Xie, Y.-K.; Zhang, Z.-J.; Wang, Z.; Xu, Z.-Z.; Ji, R.-R. GPR37 Regulates Macrophage Phagocytosis and Resolution of Inflammatory Pain. J. Clin. Investig. 2018, 128, 3568–3582. [Google Scholar] [CrossRef] [Green Version]
- Freire, M.O.; Van Dyke, T.E. Natural Resolution of Inflammation. Periodontology 2000 2013, 63, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Totsch, S.K.; Sorge, R.E. Immune System Involvement in Specific Pain Conditions. Mol. Pain 2017, 13, 1744806917724559. [Google Scholar] [CrossRef] [Green Version]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-Immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and Analgesia: The Dual Effect of Nitric Oxide in the Nociceptive System. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.-R. Resolvins RvE1 and RvD1 Attenuate Inflammatory Pain via Central and Peripheral Actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herová, M.; Schmid, M.; Gemperle, C.; Hersberger, M. ChemR23, the Receptor for Chemerin and Resolvin E1, Is Expressed and Functional on M1 but Not on M2 Macrophages. J. Immunol. 2015, 194, 2330–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, J.; Woolf, C.J. The Neuropathic Pain Triad: Neurons, Immune Cells and Glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Bingham, B.; Ajit, S.K.; Blake, D.R.; Samad, T.A. The Molecular Basis of Pain and Its Clinical Implications in Rheumatology. Nat. Clin. Pract. Rheumatol. 2009, 5, 28–37. [Google Scholar] [CrossRef]
- Jara-Oseguera, A.; Simon, S.A.; Rosenbaum, T. TRPV1: On the Road to Pain Relief. Curr. Mol. Pharmacol. 2008, 1, 255–269. [Google Scholar] [CrossRef]
- Campbell, J.N.; Raja, S.N.; Meyer, R.A.; Mackinnon, S.E. Myelinated Afferents Signal the Hyperalgesia Associated with Nerve Injury. Pain 1988, 32, 89–94. [Google Scholar] [CrossRef]
- Schmidtko, A. Nitric Oxide-Mediated Pain Processing in the Spinal Cord. Handb. Exp. Pharmacol. 2015, 227, 103–117. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, C.-K.; Hwang, S.W. Biological Roles of Resolvins and Related Substances in the Resolution of Pain. Biomed. Res. Int. 2015, 2015, 830930. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J. New Pro-Resolving n-3 Mediators Bridge Resolution of Infectious Inflammation to Tissue Regeneration. Mol. Asp. Med. 2018, 64, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schwanke, R.C.; Marcon, R.; Bento, A.F.; Calixto, J.B. EPA- and DHA-Derived Resolvins’ Actions in Inflammatory Bowel Disease. Eur. J. Pharmacol. 2016, 785, 156–164. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Karamnov, S.; Choi, A.; Park, C.-K.; Xu, Z.-Z.; Ji, R.-R.; Zhu, M.; Petasis, N.A. Macrophage Proresolving Mediator Maresin 1 Stimulates Tissue Regeneration and Controls Pain. FASEB J. 2012, 26, 1755–1765. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.-Y.; Hsieh, C.-L.; Huang, C.-P.; Lin, Y.-W. Electroacupuncture Attenuates CFA-Induced Inflammatory Pain by Suppressing Nav1.8 through S100B, TRPV1, Opioid, and Adenosine Pathways in Mice. Sci. Rep. 2017, 7, 42531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.-Q.; Zhao, F.; Guan, S.-M.; Chen, J. Antisense-Mediated Knockdown of Na(V)1.8, but Not Na(V)1.9, Generates Inhibitory Effects on Complete Freund’s Adjuvant-Induced Inflammatory Pain in Rat. PLoS ONE 2011, 6, e19865. [Google Scholar] [CrossRef]
- Park, C.-K. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediat. Inflamm. 2015, 2015, 275126. [Google Scholar] [CrossRef] [Green Version]
- Park, C.-K.; Lü, N.; Xu, Z.-Z.; Liu, T.; Serhan, C.N.; Ji, R.-R. Resolving TRPV1- and TNF-α-Mediated Spinal Cord Synaptic Plasticity and Inflammatory Pain with Neuroprotectin D1. J. Neurosci. 2011, 31, 15072–15085. [Google Scholar] [CrossRef]
- Park, C.-K.; Xu, Z.-Z.; Liu, T.; Lü, N.; Serhan, C.N.; Ji, R.-R. Resolvin D2 Is a Potent Endogenous Inhibitor for Transient Receptor Potential Subtype V1/A1, Inflammatory Pain, and Spinal Cord Synaptic Plasticity in Mice: Distinct Roles of Resolvin D1, D2, and E1. J. Neurosci. 2011, 31, 18433–18438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Burston, J.J.; Li, L.; Ashraf, S.; Mapp, P.I.; Bennett, A.J.; Ravipati, S.; Pousinis, P.; Barrett, D.A.; Scammell, B.E.; et al. Targeting the D Series Resolvin Receptor System for the Treatment of Osteoarthritis Pain. Arthritis Rheumatol. 2017, 69, 996–1008. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Kim, Y.G.; Hwang, S.W. Resolvin D1 Attenuates Activation of Sensory Transient Receptor Potential Channels Leading to Multiple Anti-Nociception. Br. J. Pharmacol. 2010, 161, 707–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macpherson, L.J.; Xiao, B.; Kwan, K.Y.; Petrus, M.J.; Dubin, A.E.; Hwang, S.; Cravatt, B.; Corey, D.P.; Patapoutian, A. An Ion Channel Essential for Sensing Chemical Damage. J. Neurosci. 2007, 27, 11412–11415. [Google Scholar] [CrossRef]
- Sommer, C.; Birklein, F. Fighting off Pain with Resolvins. Nat. Med. 2010, 16, 518–520. [Google Scholar] [CrossRef]
- Arita, M.; Bianchini, F.; Aliberti, J.; Sher, A.; Chiang, N.; Hong, S.; Yang, R.; Petasis, N.A.; Serhan, C.N. Stereochemical Assignment, Antiinflammatory Properties, and Receptor for the Omega-3 Lipid Mediator Resolvin E1. J. Exp. Med. 2005, 201, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Meesawatsom, P.; Burston, J.; Hathway, G.; Bennett, A.; Chapman, V. Inhibitory Effects of Aspirin-Triggered Resolvin D1 on Spinal Nociceptive Processing in Rat Pain Models. J. Neuroinflamm. 2016, 13, 233. [Google Scholar] [CrossRef] [Green Version]
- Woolf, C.J.; Salter, M.W. Neuronal Plasticity: Increasing the Gain in Pain. Science 2000, 288, 1765–1768. [Google Scholar] [CrossRef]
- Liu, X.J.; Gingrich, J.R.; Vargas-Caballero, M.; Dong, Y.N.; Sengar, A.; Beggs, S.; Wang, S.-H.; Ding, H.K.; Frankland, P.W.; Salter, M.W. Treatment of Inflammatory and Neuropathic Pain by Uncoupling Src from the NMDA Receptor Complex. Nat. Med. 2008, 14, 1325–1332. [Google Scholar] [CrossRef] [Green Version]
- Ren, K.; Hylden, J.L.K.; Williams, G.M.; Ruda, M.A.; Dubner, R. The Effects of a Non-Competitive NMDA Receptor Antagonist, MK-801, on Behavioral Hyperalgesia and Dorsal Horn Neuronal Activity in Rats with Unilateral Inflammation. Pain 1992, 50, 331–344. [Google Scholar] [CrossRef]
- Quan-Xin, F.; Fan, F.; Xiang-Ying, F.; Shu-Jun, L.; Shi-Qi, W.; Zhao-Xu, L.; Xu-Jie, Z.; Qing-Chuan, Z.; Wei, W. Resolvin D1 Reverses Chronic Pancreatitis-Induced Mechanical Allodynia, Phosphorylation of NMDA Receptors, and Cytokines Expression in the Thoracic Spinal Dorsal Horn. BMC Gastroenterol. 2012, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Oehler, B.; Mohammadi, M.; Perpina Viciano, C.; Hackel, D.; Hoffmann, C.; Brack, A.; Rittner, H.L. Peripheral Interaction of Resolvin D1 and E1 with Opioid Receptor Antagonists for Antinociception in Inflammatory Pain in Rats. Front. Mol. Neurosci. 2017, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Yaksh, T.L. Substance P Release from Knee Joint Afferent Terminals: Modulation by Opioids. Brain Res. 1988, 458, 319–324. [Google Scholar] [CrossRef]
- Beaudry, H.; Dubois, D.; Gendron, L. Activation of Spinal Mu- and Delta-Opioid Receptors Potently Inhibits Substance P Release Induced by Peripheral Noxious Stimuli. J. Neurosci. 2011, 31, 13068–13077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khasabova, I.A.; Harding-Rose, C.; Simone, D.A.; Seybold, V.S. Differential Effects of CB1 and Opioid Agonists on Two Populations of Adult Rat Dorsal Root Ganglion Neurons. J. Neurosci. 2004, 24, 1744–1753. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.H.; Nishioka, H.; Wakabayashi, K.; Fujita, T.; Yonehara, N. Effect of Morphine on the Release of Excitatory Amino Acids in the Rat Hind Instep: Pain Is Modulated by the Interaction between the Peripheral Opioid and Glutamate Systems. Neuroscience 2006, 138, 1329–1339. [Google Scholar] [CrossRef]
- Celik, M.Ö.; Labuz, D.; Henning, K.; Busch-Dienstfertig, M.; Gaveriaux-Ruff, C.; Kieffer, B.L.; Zimmer, A.; Machelska, H. Leukocyte Opioid Receptors Mediate Analgesia via Ca(2+)-Regulated Release of Opioid Peptides. Brain Behav. Immun. 2016, 57, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.K.; Killam, K.F.; Chuang, L.F.; Kung, H.F.; Sheng, W.S.; Chao, C.C.; Yu, L.; Chuang, R.Y. Mu Opioid Receptor Gene Expression in Immune Cells. Biochem. Biophys. Res. Commun. 1995, 216, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Toskulkao, T.; Pornchai, R.; Akkarapatumwong, V.; Vatanatunyakum, S.; Govitrapong, P. Alteration of Lymphocyte Opioid Receptors in Methadone Maintenance Subjects. Neurochem. Int. 2010, 56, 285–290. [Google Scholar] [CrossRef]
- Machelska, H.; Celik, M.Ö. Opioid Receptors in Immune and Glial Cells-Implications for Pain Control. Front. Immunol. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Gu, Y.; Tao, X.; Serhan, C.N.; Ji, R.-R. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male but Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front. Pharmacol. 2019, 10, 745. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.-H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 Binds Human Phagocytes with Evidence for Proresolving Receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment That Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, L.; Caterina, M.J. Accelerating the Reversal of Inflammatory Pain with NPD1 and Its Receptor GPR37. J. Clin. Investig. 2018, 128, 3246–3249. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.; Donnelly, C.R.; Ji, R.-R. Regulation of Pain by Neuro-Immune Interactions between Macrophages and Nociceptor Sensory Neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pannell, M.; Labuz, D.; Celik, M.Ö.; Keye, J.; Batra, A.; Siegmund, B.; Machelska, H. Adoptive Transfer of M2 Macrophages Reduces Neuropathic Pain via Opioid Peptides. J. Neuroinflamm. 2016, 13, 262. [Google Scholar] [CrossRef]
- Wang, J.C.-F.; Strichartz, G.R. Prevention of Chronic Post-Thoracotomy Pain in Rats by Intrathecal Resolvin D1 and D2: Effectiveness of Perioperative and Delayed Drug Delivery. J. Pain 2017, 18, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamplona, F.A.; Ferreira, J.; de Lima, O.M.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-Inflammatory Lipoxin A4 Is an Endogenous Allosteric Enhancer of CB1 Cannabinoid Receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; He, S.; Hu, Y.; Zheng, H. Antagonism of Cannabinoid Receptor 1 Attenuates the Anti-Inflammatory Effects of Electroacupuncture in a Rodent Model of Migraine. Acupunct. Med. 2016, 34, 463–470. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Berta, T.; Ji, R.-R. Resolvin E1 Inhibits Neuropathic Pain and Spinal Cord Microglial Activation Following Peripheral Nerve Injury. J. Neuroimmune Pharmacol. 2013, 8, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernangómez, M.; Klusáková, I.; Joukal, M.; Hradilová-Svíženská, I.; Guaza, C.; Dubový, P. CD200R1 Agonist Attenuates Glial Activation, Inflammatory Reactions, and Hypersensitivity Immediately after Its Intrathecal Application in a Rat Neuropathic Pain Model. J. Neuroinflamm. 2016, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.-J.; Ji, R.-R. Activation of JNK Pathway in Persistent Pain. Neurosci. Lett. 2008, 437, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, M.; Inoue, K.; Salter, M.W. Neuropathic Pain and Spinal Microglia: A Big Problem from Molecules in “Small” Glia. Trends Neurosci. 2005, 28, 101–107. [Google Scholar] [CrossRef]
- Svensson, C.I.; Zattoni, M.; Serhan, C.N. Lipoxins and Aspirin-Triggered Lipoxin Inhibit Inflammatory Pain Processing. J. Exp. Med. 2007, 204, 245–252. [Google Scholar] [CrossRef]
- Miao, G.-S.; Liu, Z.-H.; Wei, S.-X.; Luo, J.-G.; Fu, Z.-J.; Sun, T. Lipoxin A4 Attenuates Radicular Pain Possibly by Inhibiting Spinal ERK, JNK and NF-ΚB/P65 and Cytokine Signals, but Not P38, in a Rat Model of Non-Compressive Lumbar Disc Herniation. Neuroscience 2015, 300, 10–18. [Google Scholar] [CrossRef]
- Liu, J.; Peng, L.; Li, J. The Lipoxin A4 Receptor Agonist BML-111 Alleviates Inflammatory Injury and Oxidative Stress in Spinal Cord Injury. Med. Sci. Monit. 2020, 26, e919883. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Suter, M.R. P38 MAPK, Microglial Signaling, and Neuropathic Pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, P.; Doran, P.; Brady, H.R.; McGinty, A. Suppressors of Cytokine Signalling (SOCS): Putative Modulators of Cytokine Bioactivity in Health and Disease. J. Nephrol. 2000, 13, 9–14. [Google Scholar]
- Hu, S.; Mao-Ying, Q.-L.; Wang, J.; Wang, Z.-F.; Mi, W.-L.; Wang, X.-W.; Jiang, J.-W.; Huang, Y.-L.; Wu, G.-C.; Wang, Y.-Q. Lipoxins and Aspirin-Triggered Lipoxin Alleviate Bone Cancer Pain in Association with Suppressing Expression of Spinal Proinflammatory Cytokines. J. Neuroinflamm. 2012, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-Y.; Liu, Z.-H.; Zhu, Q.; Wen, S.; Yang, C.-X.; Fu, Z.-J.; Sun, T. Resolvin D2 Relieving Radicular Pain Is Associated with Regulation of Inflammatory Mediators, Akt/GSK-3β Signal Pathway and GPR18. Neurochem. Res. 2018, 43, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-Z.; Liu, X.-J.; Berta, T.; Park, C.-K.; Lü, N.; Serhan, C.N.; Ji, R.-R. Neuroprotectin/Protectin D1 Protects against Neuropathic Pain in Mice after Nerve Trauma. Ann. Neurol. 2013, 74, 490–495. [Google Scholar] [CrossRef]
- Gao, J.; Tang, C.; Tai, L.W.; Ouyang, Y.; Li, N.; Hu, Z.; Chen, X. Pro-Resolving Mediator Maresin 1 Ameliorates Pain Hypersensitivity in a Rat Spinal Nerve Ligation Model of Neuropathic Pain. J. Pain Res. 2018, 11, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, M.; Takeuchi, T.; Koubayashi, E.; Harada, S.; Ota, K.; Kojima, Y.; Higuchi, K. Induction of Brain-Derived Neurotrophic Factor in Enteric Glial Cells Stimulated by Interleukin-1β via a c-Jun N-Terminal Kinase Pathway. J. Clin. Biochem. Nutr. 2020, 66, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.-B.; Zuo, X.-L.; Zhao, Q.-J.; Chen, F.-X.; Yang, J.; Dong, Y.-Y.; Wang, P.; Li, Y.-Q. Brain-Derived Neurotrophic Factor Contributes to Abdominal Pain in Irritable Bowel Syndrome. Gut 2012, 61, 685–694. [Google Scholar] [CrossRef]
- Reischer, G.; Heinke, B.; Sandkühler, J. Interferon-γ Facilitates the Synaptic Transmission between Primary Afferent C-Fibres and Lamina I Neurons in the Rat Spinal Dorsal Horn via Microglia Activation. Mol. Pain 2020, 16, 1744806920917249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, M.A.; Cho, I.-H.; Kim, M.S.; Lee, S.; Jo, E.-K.; Choi, S.-Y.; Park, K.; Kim, J.S.; Akira, S.; et al. A Critical Role of Toll-like Receptor 2 in Nerve Injury-Induced Spinal Cord Glial Cell Activation and Pain Hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.-Y.; Kawasaki, Y.; Tan, P.-H.; Wen, Y.-R.; Huang, J.; Ji, R.-R. Role of the CX3CR1/P38 MAPK Pathway in Spinal Microglia for the Development of Neuropathic Pain Following Nerve Injury-Induced Cleavage of Fractalkine. Brain Behav. Immun. 2007, 21, 642–651. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Liu, Z.J.; Miao, S.; Zou, L.B.; Cai, L.; Wu, P.; Ye, D.Y.; Wu, Q.; Li, H.H. Lipoxin A4 Ameliorates Cerebral Ischaemia/Reperfusion Injury through Upregulation of Nuclear Factor Erythroid 2-Related Factor 2. Neurol. Res. 2013, 35, 968–975. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, H.; Wang, Y.; Li, L.; Wu, J.; Wang, F.; Sun, S.; Yao, S.; Shang, Y. Aspirin-Triggered Lipoxin A₄ Attenuates Lipopolysaccharide-Induced Intracellular ROS in BV2 Microglia Cells by Inhibiting the Function of NADPH Oxidase. Neurochem. Res. 2012, 37, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Ohira, T.; Sun, Y.-P.; Elangovan, S.; Chiang, N.; Serhan, C.N. Resolvin E1 Selectively Interacts with Leukotriene B4 Receptor BLT1 and ChemR23 to Regulate Inflammation. J. Immunol. 2007, 178, 3912–3917. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-Q.; Zhang, H.-B.; Wang, J.; Xia, L.-J.; Zhang, W. Lipoxin A4 Ameliorates Ischemia/Reperfusion Induced Spinal Cord Injury in Rabbit Model. Int. J. Clin. Exp. Med. 2015, 8, 12826–12833. [Google Scholar]
- Leuti, A.; Maccarrone, M.; Chiurchiù, V. Proresolving Lipid Mediators: Endogenous Modulators of Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 8107265. [Google Scholar] [CrossRef]
- Tsujino, H.; Kondo, E.; Fukuoka, T.; Dai, Y.; Tokunaga, A.; Miki, K.; Yonenobu, K.; Ochi, T.; Noguchi, K. Activating Transcription Factor 3 (ATF3) Induction by Axotomy in Sensory and Motoneurons: A Novel Neuronal Marker of Nerve Injury. Mol. Cell. Neurosci. 2000, 15, 170–182. [Google Scholar] [CrossRef]
- Ohuchi, K.; Ono, Y.; Joho, M.; Tsuruma, K.; Ogami, S.; Yamane, S.; Funato, M.; Kaneko, H.; Nakamura, S.; Hara, H.; et al. A Docosahexaenoic Acid-Derived Pro-Resolving Agent, Maresin 1, Protects Motor Neuron Cells Death. Neurochem. Res. 2018, 43, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J. The Resolution Code of Acute Inflammation: Novel pro-Resolving Lipid Mediators in Resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paragomi, P.; Rahimian, R.; Kazemi, M.H.; Gharedaghi, M.H.; Khalifeh-Soltani, A.; Azary, S.; Javidan, A.N.; Moradi, K.; Sakuma, S.; Dehpour, A.R. Antinociceptive and Antidiarrheal Effects of Pioglitazone in a Rat Model of Diarrhoea-Predominant Irritable Bowel Syndrome: Role of Nitric Oxide. Clin. Exp. Pharmacol. Physiol. 2014, 41, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, A.P.; Batisti, A.P.; Benevides, M.L.A.C.S.; Turnes, B.L.; Martins, D.F.; Kanis, L.; Duarte, E.C.W.; Cavalheiro, A.J.; Bueno, P.C.P.; Seed, M.P.; et al. Hydroalcoholic Crude Extract of Casearia Sylvestris Sw. Reduces Chronic Post-Ischemic Pain by Activation of pro-Resolving Pathways. J. Ethnopharmacol. 2017, 204, 179–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmoaty, S.; Wigerblad, G.; Bas, D.B.; Codeluppi, S.; Fernandez-Zafra, T.; El-Awady, E.-S.; Moustafa, Y.; Abdelhamid, A.E.S.; Brodin, E.; Svensson, C.I. Spinal Actions of Lipoxin A4 and 17(R)-Resolvin D1 Attenuate Inflammation-Induced Mechanical Hypersensitivity and Spinal TNF Release. PLoS ONE 2013, 8, e75543. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Wu, X.; Wei, N.; Liu, X.; Zhou, Y.; Shang, C.; Duan, Y.; Dong, Y. Lipoxin A4 Protects against Spinal Cord Injury via Regulating Akt/Nuclear Factor (Erythroid-Derived 2)-like 2/Heme Oxygenase-1 Signaling. Biomed. Pharmacother. 2018, 97, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Li, Q.; Liu, S.B.; Mi, W.-L.; Hu, S.; Zhao, J.; Tian, Y.; Mao-Ying, Q.L.; Jiang, J.W.; Ma, H.J.; et al. Aspirin-Triggered Lipoxin A4 Attenuates Mechanical Allodynia in Association with Inhibiting Spinal JAK2/STAT3 Signaling in Neuropathic Pain in Rats. Neuroscience 2014, 273, 65–78. [Google Scholar] [CrossRef]
- Sun, T.; Yu, E.; Yu, L.; Luo, J.; Li, H.; Fu, Z. LipoxinA(4) Induced Antinociception and Decreased Expression of NF-ΚB and pro-Inflammatory Cytokines after Chronic Dorsal Root Ganglia Compression in Rats. Eur. J. Pain 2012, 16, 18–27. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Mao-Ying, Q.-L.; Liu, H.; Wang, Z.-F.; Zhang, M.-T.; Wang, J.; Li, Q.; Liu, S.-B.; Mi, W.-L.; et al. Early Single Aspirin-Triggered Lipoxin Blocked Morphine Anti-Nociception Tolerance through Inhibiting NALP1 Inflammasome: Involvement of PI3k/Akt Signaling Pathway. Brain Behav. Immun. 2015, 50, 63–77. [Google Scholar] [CrossRef]
- Huang, L.; Wang, C.-F.; Serhan, C.N.; Strichartz, G. Enduring Prevention and Transient Reduction of Postoperative Pain by Intrathecal Resolvin D1. Pain 2011, 152, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Miao, G.; Wang, J.; Yang, C.; Fu, Z.; Sun, T. Resolvin D1 Inhibits Mechanical Hypersensitivity in Sciatica by Modulating the Expression of Nuclear Factor-ΚB, Phospho-Extracellular Signal-Regulated Kinase, and Pro- and Antiinflammatory Cytokines in the Spinal Cord and Dorsal Root Ganglion. Anesthesiology 2016, 124, 934–944. [Google Scholar] [CrossRef]
- Zhang, L.; Terrando, N.; Xu, Z.-Z.; Bang, S.; Jordt, S.-E.; Maixner, W.; Serhan, C.N.; Ji, R.-R. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-Operative Pain after Bone Fracture in Mice. Front. Pharmacol. 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.P.; Sperotto, N.D.M.; Maciel, I.S.; Leite, C.E.; Souza, A.H.; Campos, M.M. Effects of D-Series Resolvins on Behavioral and Neurochemical Changes in a Fibromyalgia-like Model in Mice. Neuropharmacology 2014, 86, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.C.; Orlando, R.M.; Turchetti-Maia, R.M.; de Francischi, J.N. Comparative Effects of the Ω3 Polyunsaturated Fatty Acid Derivatives Resolvins E1 and D1 and Protectin DX in Models of Inflammation and Pain. J. Inflamm. Res. 2017, 10, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Barden, A.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Short-Term n-3 Fatty Acid Supplementation but Not Aspirin Increases Plasma Proresolving Mediators of Inflammation. J. Lipid Res. 2014, 55, 2401–2407. [Google Scholar] [CrossRef] [Green Version]
- Tjonahen, E.; Oh, S.F.; Siegelman, J.; Elangovan, S.; Percarpio, K.B.; Hong, S.; Arita, M.; Serhan, C.N. Resolvin E2: Identification and Anti-Inflammatory Actions: Pivotal Role of Human 5-Lipoxygenase in Resolvin E Series Biosynthesis. Chem. Biol. 2006, 13, 1193–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, Y.; Arita, M.; Iwamoto, R.; Urabe, D.; Todoroki, H.; Masuda, K.; Inoue, M.; Arai, H. Stereochemical Assignment and Anti-Inflammatory Properties of the Omega-3 Lipid Mediator Resolvin E3. J. Biochem. 2013, 153, 355–360. [Google Scholar] [CrossRef] [PubMed]
- A.T. Resolve SARL. A Multicenter, Double-Masked, Parallel-Group, Vehicle-Controlled Study to Assess the Efficacy and Safety of RX-10045 Nanomicellar Ophthalmic Solution for Treatment of Ocular Inflammation and Pain in Subjects Undergoing Cataract Surgery. 2019. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- Ramsden, C.E.; Faurot, K.R.; Zamora, D.; Palsson, O.S.; MacIntosh, B.A.; Gaylord, S.; Taha, A.Y.; Rapoport, S.I.; Hibbeln, J.R.; Davis, J.M.; et al. Targeted Alterations in Dietary N-3 and n-6 Fatty Acids Improve Life Functioning and Reduce Psychological Distress among Patients with Chronic Headache: A Secondary Analysis of a Randomized Trial. Pain 2015, 156, 587–596. [Google Scholar] [CrossRef]
- Tajmirriahi, M.; Sohelipour, M.; Basiri, K.; Shaygannejad, V.; Ghorbani, A.; Saadatnia, M. The Effects of Sodium Valproate with Fish Oil Supplementation or Alone in Migraine Prevention: A Randomized Single-Blind Clinical Trial. Iran. J. Neurol. 2012, 11, 21–24. [Google Scholar]
- Caturla, N.; Funes, L.; Pérez-Fons, L.; Micol, V. A Randomized, Double-Blinded, Placebo-Controlled Study of the Effect of a Combination of Lemon Verbena Extract and Fish Oil Omega-3 Fatty Acid on Joint Management. J. Altern. Complement. Med. 2011, 17, 1051–1063. [Google Scholar] [CrossRef]
- Tomer, A.; Kasey, S.; Connor, W.E.; Clark, S.; Harker, L.A.; Eckman, J.R. Reduction of Pain Episodes and Prothrombotic Activity in Sickle Cell Disease by Dietary N-3 Fatty Acids. Thromb. Haemost. 2001, 85, 966–974. [Google Scholar] [CrossRef]
- Durán, A.M.; Salto, L.M.; Câmara, J.; Basu, A.; Paquien, I.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De León, M. Effects of Omega-3 Polyunsaturated Fatty-Acid Supplementation on Neuropathic Pain Symptoms and Sphingosine Levels in Mexican-Americans with Type 2 Diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Barden, A.E.; Moghaddami, M.; Mas, E.; Phillips, M.; Cleland, L.G.; Mori, T.A. Specialised Pro-Resolving Mediators of Inflammation in Inflammatory Arthritis. Prostaglandins Leukot. Essent. Fatty Acids 2016, 107, 24–29. [Google Scholar] [CrossRef]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; DiGiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary Fish Oil and Olive Oil Supplementation in Patients with Rheumatoid Arthritis. Clinical and Immunologic Effects. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1990, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Geusens, P.; Wouters, C.; Nijs, J.; Jiang, Y.; Dequeker, J. Long-Term Effect of Omega-3 Fatty Acid Supplementation in Active Rheumatoid Arthritis. A 12-Month, Double-Blind, Controlled Study. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1994, 37, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Tulleken, J.E.; Limburg, P.C.; Muskiet, F.A.; van Rijswijk, M.H. Vitamin E Status during Dietary Fish Oil Supplementation in Rheumatoid Arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1990, 33, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, B.; Khan, F.; Kumar, P.; Pullar, T.; Belch, J.J.F. C-Reactive Protein: The Underlying Cause of Microvascular Dysfunction in Rheumatoid Arthritis. Rheumatology 2008, 47, 1780–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and Time-Dependent Increase in Circulating Anti-Inflammatory and pro-Resolving Lipid Mediators Following Eicosapentaenoic Acid Supplementation in Patients with Major Depressive Disorder and Chronic Inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2021, 164, 102219. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, S.M.N.S. Comparative Effectiveness of the Different Treatment Modalities for Management of Vaso-Occlusive Painful Crisis in Pediatric Sickle Cell Disease. 2021. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- Kenney, K. Targeted Alteration in Omega-3 and Omega-6 Fatty Acids for Post-Traumatic Headache (Nutrition for PTH). 2018. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- Universidade do Porto. Effects of an Anti-Inflammatory Nutritional Intervention in Disease Assessment Parameters, Inflammatory Markers, and Quality of Life of Patients with Fibromyalgia. 2020. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- Costenbader, K.H. Vitamin D and Fish Oil for Autoimmune Disease, Inflammation and Knee Pain. 2021. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- University of North Carolina. Chapel Hill Pilot, Double-Blind, Randomized Controlled, Multi-Center Study of the Effects of Fish Oil and Vitamin D in the Prevention of Chronic Pain Following Major Thermal Burn Injury. 2020. Available online: clinicaltrials.gov (accessed on 10 July 2021).
- Swisse Wellness Pty Ltd. A Randomised, Double-Blind, Placebo Controlled Study to Investigate the Effect on Knee Pain Reduction and Safety of Swisse High Strength Deep Sea Krill Oil (Superba BOOST) in Adults with Mild to Moderate Osteoarthritis of the Knee. 2020. Available online: clinicaltrials.gov (accessed on 10 July 2021).
| Authors (REF) | Pain-Related Conditions | Methodology | Results |
|---|---|---|---|
| Goldberg et al. [15] | Rheumatoid arthritis and joint pain | Meta-analysis with 17 randomized controlled clinical trials evaluating the pain-relieving effects of ω-3 PUFA in patients with rheumatoid arthritis or joint pain related to inflammatory bowel disease and dysmenorrhea. | Treatment with ω-3 PUFA for 3–4 months reduced patient-reported joint pain intensity (SMD: −0.26; 95% CI: −0.49 to −0.03, p = 0.03), minutes of morning stiffness (SMD: −0.43; 95% CI: −0.72 to −0.15, p = 0.003), number of painful and/or tender joints (SMD: −0.29; 95% CI: −0.48 to −0.10, p = 0.003), and NSAID use (SMD: −0.40; 95% CI: −0.72 to −0.08, p = 0.01). |
| Geusens et al. [173] | Rheumatoid arthritis | Randomized, double-blind controlled trial that assessed the long-term effects of supplementation with various doses of ω-3 PUFA in 90 patients with active rheumatoid arthritis. | There was a significant improvement in the patient’s global evaluation and the physician’s assessment of pain in patients treated with 2.6 mg/day of ω-3 (p < 0.05). |
| Tajmirriahi et al. [167] | Migraine | Randomized, single-blind clinical trial that evaluated the effect of dietary supplementation with fish oil for migraine prevention in 67 patients taking sodium valproate. | There was a significant decrease in frequency (mean baseline from 13.7 to 2.4; p = 0.044), and severity of migraines (mean baseline from 7.9 to 2.9; p = 0.046) in participants treated with sodium valproate and fish oil supplementation after the first month of treatment. |
| Tomer et al. [169] | Sickle cell disease | A double-blind clinical trial that assessed the effects of dietary ω-3 PUFA on the frequency of pain episodes in patients with sickle cell disease in comparison with controls on olive oil. | Treatment with dietary ω-3 PUFA for 1 year reduced the frequency of pain episodes (p < 0.01). |
| Durán et al. [170] | Diabetic neuropathy | Interventional single-group study to assess the efficacy of dietary ω-3 PUFA in 40 participants with type 2 diabetes. | There was a significant reduction in pain-related neuropathy symptoms after three months of treatment with ω-3 PUFA (change from baseline −2.1 (p = 0.014) and −9.2 (p = 0.002)). |
| Ramsden et al. [166] | Chronic headache | Randomized, parallel-group and 12-week trial designed to test the clinical effects of a diet high in ω-3 and low in ω-6 PUFA compared to a diet low in ω-6 PUFA in 67 subjects with chronic headaches. | There was reduction of pain frequency (p < 0.001), intensity (p < 0.001), and psychological distress (p = 0.022) in patients treated with a diet high in ω-3 and low in ω-6 PUFA. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Castillo, M.; Ortega, Á.; Cudris-Torres, L.; Duran, P.; Rojas, M.; Manzano, A.; Garrido, B.; Salazar, J.; Silva, A.; Rojas-Gomez, D.M.; et al. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? Int. J. Mol. Sci. 2021, 22, 10370. https://doi.org/10.3390/ijms221910370
Chávez-Castillo M, Ortega Á, Cudris-Torres L, Duran P, Rojas M, Manzano A, Garrido B, Salazar J, Silva A, Rojas-Gomez DM, et al. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? International Journal of Molecular Sciences. 2021; 22(19):10370. https://doi.org/10.3390/ijms221910370
Chicago/Turabian StyleChávez-Castillo, Mervin, Ángel Ortega, Lorena Cudris-Torres, Pablo Duran, Milagros Rojas, Alexander Manzano, Bermary Garrido, Juan Salazar, Aljadis Silva, Diana Marcela Rojas-Gomez, and et al. 2021. "Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy?" International Journal of Molecular Sciences 22, no. 19: 10370. https://doi.org/10.3390/ijms221910370








