Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence
Abstract
:1. Introduction
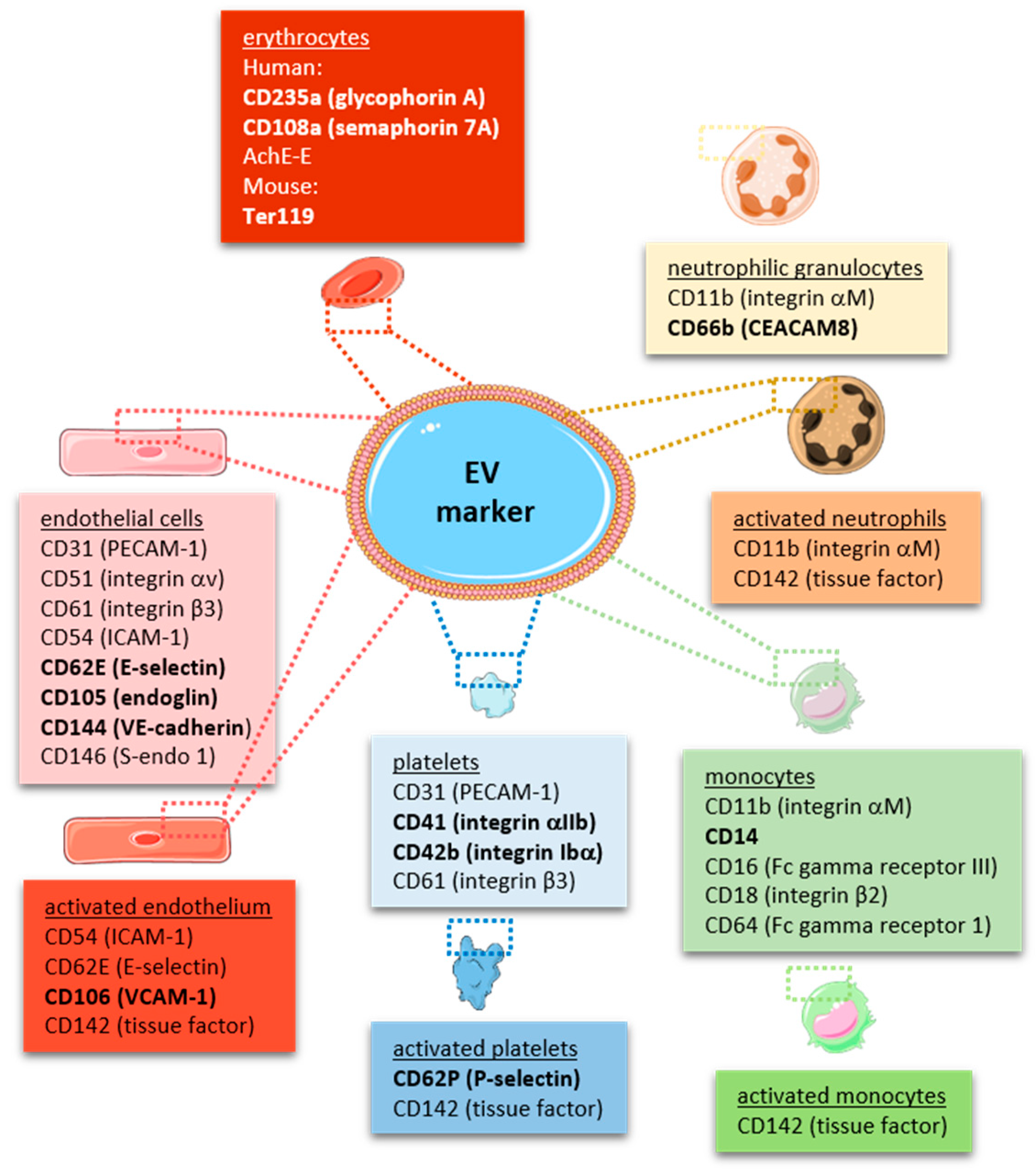
2. Extracellular Vesicles: Definition and Characterization
3. Mechanisms of EV Formation
3.1. General
3.2. Activation and Inflammation
3.3. Age and Senescence
3.4. Cell Death and Apoptosis
4. Fate and Effects of EVs on Recipient Cells
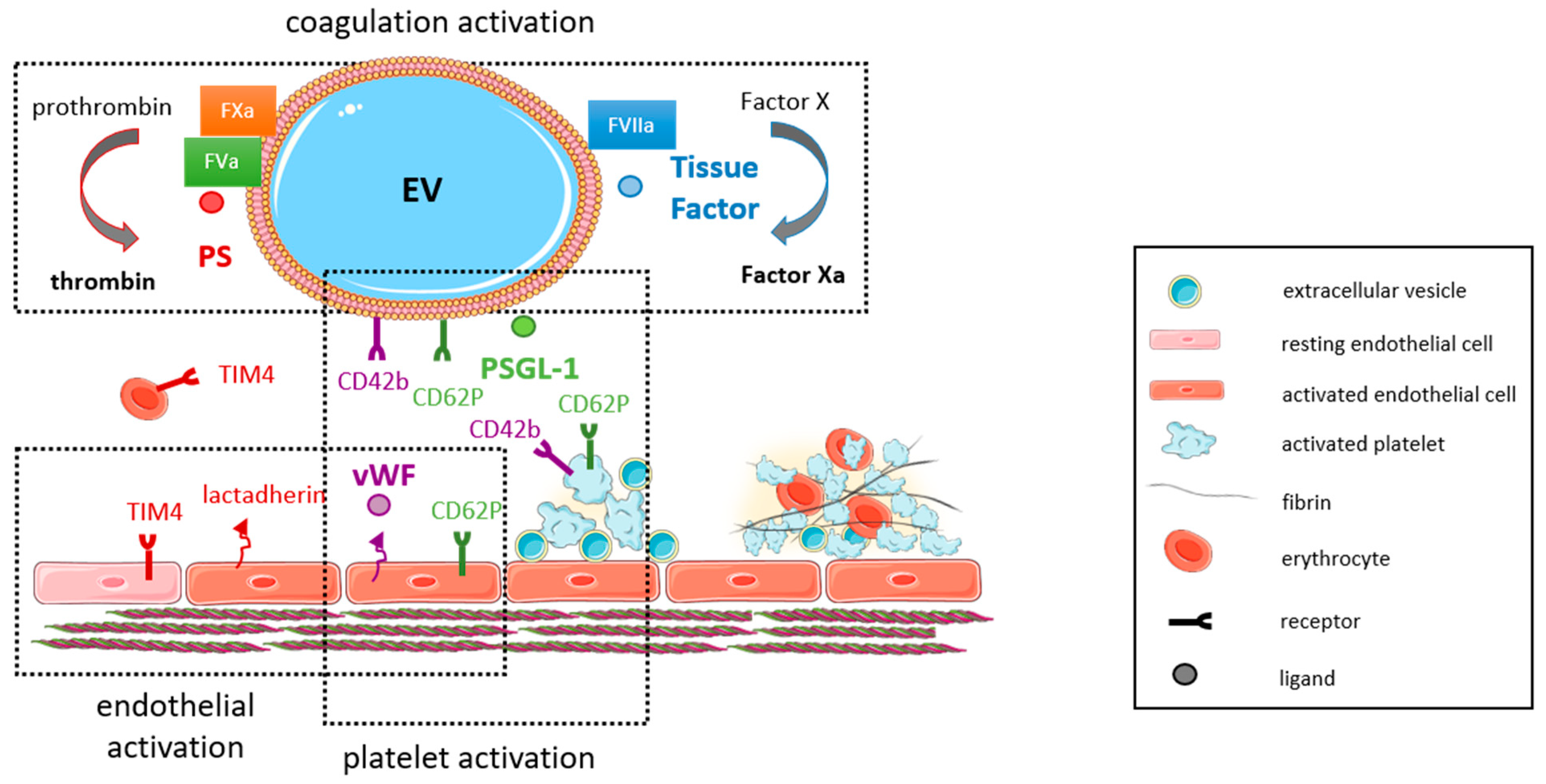
5. Extracellular Vesicles and Thrombosis
6. EVs and Thrombosis in the Arterial System: Clinical Evidence
7. EVs and Thrombosis in the Venous System: Findings in Human Studies
8. Extracellular Vesicles and Thrombosis Associated with COVID-19 Infection
9. Arterial and Venous Thrombosis: Findings in Mouse Models
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACS | acute coronary syndrome |
| ADP | adenosine diphosphate |
| AMI | acute myocardial infarction |
| ANG II | angiotensin II |
| Apo-EV | apoptotic cell-derived EV |
| CAD | coronary artery disease |
| CD | cluster of differentiation |
| CRP-XL | cross-linked CRP peptides |
| DVT | deep vein thrombosis |
| EV | extracellular vesicle |
| FVL | factor V Leiden |
| HAEC | human arterial endothelial cell |
| HBMEC | human brain microvascular endothelial cell |
| HUVEC | human umbilical vein endothelial cell |
| ICAM-1 | intracellular adhesion molecule-1 |
| IL | interleukin |
| ISEV | International Society for Extracellular Vesicles |
| IVC | inferior Vena cava |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MI | myocardial infarction |
| miR | microRNA |
| MP | microparticle |
| MV | microvesicle |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NO | nitric oxide |
| NTA | nanoparticle tracking analysis |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAR | protease-activated receptor |
| PE | pulmonary embolism |
| PMA | phorbol myristate acetate |
| PS | phosphatidylserine |
| PSGL-1 | P-selectin glycoprotein ligand-1 (CD162) |
| PCI | percutaneous coronary intervention |
| PTCA | percutaneous coronary angioplasty |
| RBC | red blood cell (erythrocyte) |
| ROS | reactive oxygen species |
| STEMI | ST-segment elevation myocardial infarction |
| TF | tissue factor |
| TFPI | tissue factor pathway inhibitor |
| THP-1 | human monocytic leukemia cell line |
| TIM4 | T-cell membrane 4 |
| TNFα | tumor necrosis factor-alpha |
| TRAP6 | thrombin receptor activator peptide-6 |
| uPAR | urokinase type plasminogen activator receptor |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VTE | venous thromboembolism |
| vWF | von Willebrand factor |
References
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Mathew, A.; Mason, A.B.; Teng, K. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins. J. Cell. Physiol. 1991, 147, 27–36. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Sims, P.J.; Faioni, E.; Wiedmer, T.; Shattil, S. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988, 263, 18205–18212. [Google Scholar] [CrossRef]
- Giesen, P.L.; Rauch, U.; Bohrmann, B.; Kling, D.; Roqué, M.; Fallon, J.T.; Badimon, J.J.; Himber, J.; Riederer, M.A.; Nemerson, Y. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. USA 1999, 96, 2311–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.L.; Holmgren, L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc. Natl. Acad. Sci. USA 2001, 98, 6407–6411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, F.; Yang, X.; Hoyer, F.F.; Paul, K.; Heiermann, N.; Becher, M.U.; Hussein, N.A.; Kebschull, M.; Bedorf, J.; Franklin, B.S. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1925–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.M.; Sadik, M.; Alaarg, A.; Smith, C.I.E.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. JTH 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lötvall, J.; Hill, A.F.; Hochberg, F.; Buzás, E.I.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Böing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Berckmans, R.J.; Lacroix, R.; Hau, C.M.; Sturk, A.; Nieuwland, R. Extracellular vesicles and coagulation in blood from healthy humans revisited. J. Extracell. Vesicles 2019, 8, 1688936. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.; Hole, P.; Carr, B.; Redman, C.W.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Shet, A.S.; Aras, O.; Gupta, K.; Hass, M.J.; Rausch, D.J.; Saba, N.; Koopmeiners, L.; Key, N.S.; Hebbel, R.P. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood 2003, 102, 2678–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, M.; Grassi, A.; Barbui, T. Platelet-derived microvesicles in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb. Haemost. 1996, 75, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Hayward, C.P.; Warkentin, T.E.; Horsewood, P.; Chorneyko, K.A.; Kelton, J.G. Morphological analysis of microparticle generation in heparin-induced thrombocytopenia. Blood 2000, 96, 188–194. [Google Scholar] [CrossRef]
- Tomer, A.; Harker, L.A.; Kasey, S.; Eckman, J.R. Thrombogenesis in sickle cell disease. J. Lab. Clin. Med. 2001, 137, 398–407. [Google Scholar] [CrossRef]
- Deng, F.; Wang, S.; Zhang, L. Endothelial Microparticles Act as Novel Diagnostic and Therapeutic Biomarkers of Diabetes and Its Complications: A Literature Review. BioMed Res. Int. 2016, 2016, 9802026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, M.C.; Andriantsitohaina, R. Extracellular Vesicles in Metabolic Syndrome. Circ. Res. 2017, 120, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Jy, W.; Horstman, L.L.; Janania, J.; Reyes, Y.; Kelley, R.E.; Ahn, Y.S. Elevated platelet microparticles in transient ischemic attacks, lacunar infarcts, and multiinfarct dementias. Thromb. Res. 1993, 72, 295–304. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2017, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Chen, J.; Yang, L.; Liu, J.; Zhang, X.; Zhang, Y.; Tu, Q.; Yin, D.; Lin, D.; Wong, P.-P.; et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat. Cell Biol. 2019, 21, 498–510. [Google Scholar] [CrossRef]
- Kay, J.G.; Grinstein, S. Phosphatidylserine-mediated cellular signaling. Adv. Exp. Med. Biol. 2013, 991, 177–193. [Google Scholar]
- Schutters, K.; Kusters, D.H.; Chatrou, M.L.; Montero-Melendez, T.; Donners, M.; Deckers, N.M.; Krysko, D.V.; Vandenabeele, P.; Perretti, M.; Schurgers, L.J.; et al. Cell surface-expressed phosphatidylserine as therapeutic target to enhance phagocytosis of apoptotic cells. Cell Death Differ. 2013, 20, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, D.E.; Exner, T.; Ma, D.D.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb. Haemost. 2010, 103, 1044–1052. [Google Scholar]
- Shi, J.; Pipe, S.W.; Rasmussen, J.T.; Heegaard, C.W.; Gilbert, G.E. Lactadherin blocks thrombosis and hemostasis in vivo: Correlation with platelet phosphatidylserine exposure. J. Thromb. Haemost. JTH 2008, 6, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid Hussein, M.N.; Meesters, E.W.; Osmanovic, N.; Romijn, F.P.; Nieuwland, R.; Sturk, A. Antigenic characterization of endothelial cell-derived microparticles and their detection ex vivo. J. Thromb. Haemost. JTH 2003, 1, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Abid Hussein, M.N.; Böing, A.N.; Biró, E.; Hoek, F.J.; Vogel, G.M.; Meuleman, D.G.; Sturk, A.; Nieuwland, R. Phospholipid composition of in vitro endothelial microparticles and their in vivo thrombogenic properties. Thromb. Res. 2008, 121, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Combes, V.; Simon, A.-C.; Grau, G.-E.; Arnoux, D.; Camoin, L.; Sabatier, F.; Mutin, M.; Sanmarco, M.; Sampol, J.; Dignat-George, F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Investig. 1999, 104, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, A.; Wilkinson, P.; Gui, M.; Gales, T.; Hu, E.; Edelberg, J. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J. Thromb. Haemost. JTH 2009, 7, 701–709. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003, 109, 175–180. [Google Scholar] [CrossRef]
- Holnthoner, W.; Bonstingl, C.; Hromada, C.; Muehleder, S.; Zipperle, J.; Stojkovic, S.; Redl, H.; Wojta, J.; Schöchl, H.; Grillari, J.; et al. Endothelial Cell-derived Extracellular Vesicles Size-dependently Exert Procoagulant Activity Detected by Thromboelastometry. Sci. Rep. 2017, 7, 3707. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, F.; Roux, V.; Anfosso, F.; Camoin, L.; Sampol, J.; Dignat-George, F. Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor–dependent procoagulant activity. Blood 2002, 99, 3962–3970. [Google Scholar] [CrossRef]
- Hunter, L.W.; Jayachandran, M.; Miller, V.M. Sex differences in the expression of cell adhesion molecules on microvesicles derived from cultured human brain microvascular endothelial cells treated with inflammatory and thrombotic stimuli. Biol. Sex Differ. 2019, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.K.; Hattori, R.; Esmon, C.T.; Sims, P.J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J. Biol. Chem. 1990, 265, 3809–3814. [Google Scholar] [CrossRef]
- Ponomareva, A.A.; Nevzorova, T.A.; Mordakhanova, E.R.; Andrianova, I.A.; Rauova, L.; Litvinov, R.I.; Weisel, J.W. Intracellular origin and ultrastructure of platelet-derived microparticles. J. Thromb. Haemost. JTH 2017, 15, 1655–1667. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, S.V.; Malinowski, K.; Golightly, M.; Jesty, J.; Goligorsky, M.S. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation 2002, 106, 2372–2378. [Google Scholar] [CrossRef]
- Martínez de Lizarrondo, S.; Roncal, C.; Calvayrac, O.; Rodríguez, C.; Varo, N.; Purroy, A.; Lorente, L.; Rodríguez, J.A.; Doeuvre, L.; Hervás-Stubbs, S.; et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1477–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoncini, S.; Njock, M.S.; Robert, S.; Camoin-Jau, L.; Sampol, J.; Harlé, J.R.; Nguyen, C.; Dignat-George, F.; Anfosso, F. TRAIL/Apo2L mediates the release of procoagulant endothelial microparticles induced by thrombin in vitro: A potential mechanism linking inflammation and coagulation. Circ. Res. 2009, 104, 943–951. [Google Scholar] [CrossRef]
- Bombeli, T.; Karsan, A.; Tait, J.F.; Harlan, J.M. Apoptotic vascular endothelial cells become procoagulant. Blood 1997, 89, 2429–2442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutlu, A.; Gyulkhandanyan, A.V.; Freedman, J.; Leytin, V. Concurrent and separate inside-out transition of platelet apoptosis and activation markers to the platelet surface. Br. J. Haematol. 2013, 163, 377–384. [Google Scholar] [CrossRef]
- Christersson, C.; Johnell, M.; Siegbahn, A. The influence of direct thrombin inhibitors on the formation of platelet-leukocyte aggregates and tissue factor expression. Thromb. Res. 2010, 126, e327–e333. [Google Scholar] [CrossRef] [PubMed]
- Aatonen, M.T.; Öhman, T.; Nyman, T.A.; Laitinen, S.; Grönholm, M.; Siljander, P.R.M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 2014, 3, 24692. [Google Scholar] [CrossRef]
- Siljander, P.; Carpen, O.; Lassila, R. Platelet-derived microparticles associate with fibrin during thrombosis. Blood 1996, 87, 4651–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aharon, A.; Tamari, T.; Brenner, B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 2008, 100, 878–885. [Google Scholar]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef] [PubMed]
- Butenas, S.; Bouchard, B.A.; Brummel-Ziedins, K.E.; Parhami-Seren, B.; Mann, K.G. Tissue factor activity in whole blood. Blood 2005, 105, 2764–2770. [Google Scholar] [CrossRef]
- Lee, S.K.; Yang, S.H.; Kwon, I.; Lee, O.H.; Heo, J.H. Role of tumour necrosis factor receptor-1 and nuclear factor-κB in production of TNF-α-induced pro-inflammatory microparticles in endothelial cells. Thromb. Haemost. 2014, 112, 580–588. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Brodsky, S.V.; Zhang, F.; Nasjletti, A.; Goligorsky, M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol.-Heart Circ. Physiol. 2004, 286, H1910–H1915. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Hu, X.X.; Lin, Z.B.; Chang, F.J.; Ou, Z.J.; Wang, Z.P.; Ou, J.S. Circulating microparticles from patients with valvular heart disease and cardiac surgery inhibit endothelium-dependent vasodilation. J. Thorac. Cardiovasc. Surg. 2015, 150, 666–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbanelli, L.; Buratta, S.; Sagini, K.; Tancini, B.; Emiliani, C. Extracellular Vesicles as New Players in Cellular Senescence. Int. J. Mol. Sci. 2016, 17, 1408. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [Green Version]
- Kadota, T.; Fujita, Y.; Yoshioka, Y.; Araya, J.; Kuwano, K.; Ochiya, T. Emerging role of extracellular vesicles as a senescence-associated secretory phenotype: Insights into the pathophysiology of lung diseases. Mol. Asp. Med. 2018, 60, 92–103. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misawa, T.; Tanaka, Y.; Okada, R.; Takahashi, A. Biology of extracellular vesicles secreted from senescent cells as senescence-associated secretory phenotype factors. Geriatr. Gerontol. Int. 2020, 20, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A. Age-related changes in plasma extracellular vesicle characteristics and internalization by leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef] [Green Version]
- Forest, A.; Pautas, E.; Ray, P.; Bonnet, D.; Verny, M.; Amabile, N.; Boulanger, C.; Riou, B.; Tedgui, A.; Mallat, Z.J.J. Circulating microparticles and procoagulant activity in elderly patients. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 414–420. [Google Scholar] [CrossRef]
- Li, M.; Liao, L.; Tian, W. Extracellular Vesicles Derived From Apoptotic Cells: An Essential Link between Death and Regeneration. Front. Cell Dev. Biol. 2020, 8, 573511. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, L.; Szeles, A.; Rajnavölgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, D.; Lee, H.; Menon, A.A.; Wu, J.; Hu, K.; Jin, Y. Macrophage-derived apoptotic bodies promote the proliferation of the recipient cells via shuttling microRNA-221/222. J. Leukoc. Biol. 2017, 101, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [Green Version]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef]
- Feigelson, S.W.; Grabovsky, V.; Shamri, R.; Levy, S.; Alon, R. The CD81 tetraspanin facilitates instantaneous leukocyte VLA-4 adhesion strengthening to vascular cell adhesion molecule 1 (VCAM-1) under shear flow. J. Biol. Chem. 2003, 278, 51203–51212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, E.L.; Ridger, V.; Ferraro, F.; Turmaine, M.; Saftig, P.; Cutler, D.F. CD63 is an essential cofactor to leukocyte recruitment by endothelial P-selectin. Blood 2011, 118, 4265–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, R.; Monjas, A.; Yánez-Mó, M.; Cardeñes, B.; Morlino, G.; Gilsanz, A.; Machado-Pineda, Y.; Lafuente, E.; Monk, P.; Sánchez-Madrid, F.; et al. Different states of integrin LFA-1 aggregation are controlled through its association with tetraspanin CD9. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853 Pt A, 2464–2480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasgupta, S.K.; Le, A.; Chavakis, T.; Rumbaut, R.E.; Thiagarajan, P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation 2012, 125, 1664–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Dasgupta, S.K.; Abdel-Monem, H.; Niravath, P.; Le, A.; Bellera, R.V.; Langlois, K.; Nagata, S.; Rumbaut, R.E.; Thiagarajan, P. Lactadherin and clearance of platelet-derived microvesicles. Blood 2009, 113, 1332–1339. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Zhao, W.-L.; Ye, Y.-Y.; Bai, X.-C.; Liu, R.-Q.; Chang, L.-F.; Zhou, Q.; Sui, S.-F. Cellular Internalization of Exosomes Occurs Through Phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Brioschi, M.; Wait, R.; Begum, S.; Gianazza, E.; Pirillo, A.; Mussoni, L.; Tremoli, E. Proteome of endothelial cell-derived procoagulant microparticles. Proteomics 2005, 5, 4443–4455. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Del Conde, I.; Shrimpton, C.N.; Thiagarajan, P.; Lopez, J.A. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood 2005, 106, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.M.; Rizzo, V. Microparticle-Induced Activation of the Vascular Endothelium Requires Caveolin-1/Caveolae. PLoS ONE 2016, 11, e0149272. [Google Scholar] [CrossRef] [Green Version]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suades, R.; Padro, T.; Vilahur, G.; Badimon, L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thromb. Haemost. 2012, 108, 1208–1219. [Google Scholar] [CrossRef]
- Reddy, E.C.; Rand, M.L. Procoagulant Phosphatidylserine-Exposing Platelets in vitro and in vivo. Front. Cardiovasc. Med. 2020, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Heemskerk, J.W.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thromb. Haemost. 2002, 88, 186–193. [Google Scholar]
- Yang, A.; Chen, F.; He, C.; Zhou, J.; Lu, Y.; Dai, J.; Birge, R.B.; Wu, Y. The Procoagulant Activity of Apoptotic Cells Is Mediated by Interaction with Factor XII. Front. Immunol. 2017, 8, 1188. [Google Scholar] [CrossRef] [Green Version]
- Sevinsky, J.R.; Rao, L.V.; Ruf, W. Ligand-induced protease receptor translocation into caveolae: A mechanism for regulating cell surface proteolysis of the tissue factor-dependent coagulation pathway. J. Cell Biol. 1996, 133, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupu, C.; Goodwin, C.A.; Westmuckett, A.D.; Emeis, J.J.; Scully, M.F.; Kakkar, V.V.; Lupu, F. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomains/caveolae. Regulatory mechanism(s) of the anticoagulant properties of the endothelium. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Biro, E.; Sturk-Maquelin, K.; Vogel, G.; Meuleman, D.; Smit, M.; Hack, C.; Sturk, A.; Nieuwland, R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J. Thromb. 2003, 1, 2561–2568. [Google Scholar] [CrossRef] [Green Version]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef]
- Reininger, A.J.; Heijnen, H.F.; Schumann, H.; Specht, H.M.; Schramm, W.; Ruggeri, Z.M. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 2006, 107, 3537–3545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, W.; Zhou, Y.; Hilton, T.; Zhao, Z.; Liu, W.; Wang, M.; Yeon, J.; Houck, K.; Thiagarajan, P.; et al. von Willebrand factor enhances microvesicle-induced vascular leakage and coagulopathy in mice with traumatic brain injury. Blood 2018, 132, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Lipets, E.N.; Antonova, O.A.; Shustova, O.N.; Losenkova, K.V.; Mazurov, A.V.; Ataullakhanov, F.I. Use of Thrombodynamics for revealing the participation of platelet, erythrocyte, endothelial, and monocyte microparticles in coagulation activation and propagation. PLoS ONE 2020, 15, e0227932. [Google Scholar] [CrossRef]
- Shustova, O.N.; Antonova, O.A.; Golubeva, N.V.; Khaspekova, S.G.; Yakushkin, V.V.; Aksuk, S.A.; Alchinova, I.B.; Karganov, M.Y.; Mazurov, A.V. Differential procoagulant activity of microparticles derived from monocytes, granulocytes, platelets and endothelial cells: Impact of active tissue factor. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2017, 28, 373–382. [Google Scholar] [CrossRef]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mellacheruvu, D.; Wright, Z.; Couzens, A.L.; Lambert, J.-P.; St-Denis, N.A.; Li, T.; Miteva, Y.V.; Hauri, S.; Sardiu, M.E.; Low, T.Y.; et al. The CRAPome: A contaminant repository for affinity purification–mass spectrometry data. Nat. Methods 2013, 10, 730–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinkhani, B.; Kuypers, S.; van den Akker, N.M.S.; Molin, D.G.M.; Michiels, L. Extracellular Vesicles Work as a Functional Inflammatory Mediator Between Vascular Endothelial Cells and Immune Cells. Front. Immunol. 2018, 9, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Deng, X.; Ma, R.; Dong, Z.; Wang, F.; Shi, J. The Exposure of Phosphatidylserine Influences Procoagulant Activity in Retinal Vein Occlusion by Microparticles, Blood Cells, and Endothelium. Oxidative Med. Cell. Longev. 2018, 2018, 3658476. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Zabeo, E.; Radu, C.M.; Spiezia, L.; Gavasso, S.; Fadin, M.; Woodhams, B.; Vettor, R.; Simioni, P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb. Haemost. 2015, 113, 85–96. [Google Scholar] [PubMed]
- Steppich, B.; Mattisek, C.; Sobczyk, D.; Kastrati, A.; Schömig, A.; Ott, I. Tissue factor pathway inhibitor on circulating microparticles in acute myocardial infarction. Thromb. Haemost. 2005, 93, 35–39. [Google Scholar] [CrossRef]
- Rothmeier, A.S.; Versteeg, H.H.; Ruf, W. Factor VIIa-induced interaction with integrin controls the release of tissue factor on extracellular vesicles from endothelial cells. J. Thromb. Haemost. 2019, 17, 627–634. [Google Scholar] [CrossRef]
- Macey, M.G.; Wolf, S.I.; Lawson, C. Microparticle formation after exposure of blood to activated endothelium under flow. Cytom. Part A 2010, 77A, 761–768. [Google Scholar] [CrossRef]
- Vallier, L.; Cointe, S.; Lacroix, R.; Bonifay, A.; Judicone, C.; Dignat-George, F.; Kwaan, H.C. Microparticles and Fibrinolysis. Semin. Thromb. Hemost. 2017, 43, 129–134. [Google Scholar] [CrossRef]
- Lacroix, R.; Plawinski, L.; Robert, S.; Doeuvre, L.; Sabatier, F.; de Lizarrondo, S.M.; Mezzapesa, A.; Anfosso, F.; Leroyer, A.S.; Poullin, P. Leukocyte-and endothelial-derived microparticles: A circulating source for fibrinolysis. Haematologica 2012, 97, 1864–1872. [Google Scholar] [CrossRef]
- Castaman, G.; Yu-Feng, L.; Battistin, E.; Rodeghiero, F. Characterization of a novel bleeding disorder with isolated prolonged bleeding time and deficiency of platelet microvesicle generation. Br. J. Haematol. 1997, 96, 458–463. [Google Scholar] [CrossRef]
- Gemmell, C.H.; Sefton, M.V.; Yeo, E.L. Platelet-derived microparticle formation involves glycoprotein IIb-IIIa. Inhibition by RGDS and a Glanzmann’s thrombasthenia defect. J. Biol. Chem. 1993, 268, 14586–14589. [Google Scholar] [CrossRef]
- Fujii, T.; Sakata, A.; Nishimura, S.; Eto, K.; Nagata, S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc. Natl. Acad. Sci. USA 2015, 112, 12800–12805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.; Gröger, M.; Rauscher, S.; Fendl, B.; Eichhorn, T.; Fischer, M.B.; Spittler, A.; Weber, V. Differential Interaction of Platelet-Derived Extracellular Vesicles with Leukocyte Subsets in Human Whole Blood. Sci. Rep. 2018, 8, 6598. [Google Scholar] [CrossRef] [PubMed]
- Flaumenhaft, R.; Dilks, J.R.; Richardson, J.; Alden, E.; Patel-Hett, S.R.; Battinelli, E.; Klement, G.L.; Sola-Visner, M.; Italiano, J.E., Jr. Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood 2009, 113, 1112–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gąsecka, A.; Rogula, S.; Eyileten, C.; Postuła, M.; Jaguszewski, M.J.; Kochman, J.; Mazurek, T.; Nieuwland, R.; Filipiak, K.J. Role of P2Y Receptors in Platelet Extracellular Vesicle Release. Int. J. Mol. Sci. 2020, 21, 6065. [Google Scholar] [CrossRef] [PubMed]
- Brisson, A.R.; Tan, S.; Linares, R.; Gounou, C.; Arraud, N. Extracellular vesicles from activated platelets: A semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets 2017, 28, 263–271. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Tait, J.F. Collagen-induced exposure of anionic phospholipid in platelets and platelet-derived microparticles. J. Biol. Chem. 1991, 266, 24302–24307. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef] [Green Version]
- Holme, P.A.; Orvim, U.; Hamers, M.J.; Solum, N.O.; Brosstad, F.R.; Barstad, R.M.; Sakariassen, K.S. Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with a severe stenosis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 646–653. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Goetzl, L.; Karliner, J.S.; Tang, N.; Pulliam, L. Human plasma platelet-derived exosomes: Effects of aspirin. FASEB J. 2016, 30, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Connor, D.E.; Ly, K.; Aslam, A.; Boland, J.; Low, J.; Jarvis, S.; Muller, D.W.; Joseph, J.E. Effects of antiplatelet therapy on platelet extracellular vesicle release and procoagulant activity in health and in cardiovascular disease. Platelets 2016, 27, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Franca, C.N.; Pinheiro, L.F.; Izar, M.C.; Brunialti, M.K.; Salomao, R.; Bianco, H.T.; Kasmas, S.H.; Barbosa, S.P.; de Nucci, G.; Fonseca, F.A. Endothelial progenitor cell mobilization and platelet microparticle release are influenced by clopidogrel plasma levels in stable coronary artery disease. Circ. J. 2012, 76, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behan, M.W.; Fox, S.C.; Heptinstall, S.; Storey, R.F. Inhibitory effects of P2Y12 receptor antagonists on TRAP-induced platelet aggregation, procoagulant activity, microparticle formation and intracellular calcium responses in patients with acute coronary syndromes. Platelets 2005, 16, 73–80. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; van der Pol, E.; Hajji, N.; Ćwiek, A.; Pluta, K.; Konwerski, M.; Filipiak, K.J. P2Y12 antagonist ticagrelor inhibits the release of procoagulant extracellular vesicles from activated platelets. Cardiol. J. 2019, 26, 782–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontiggia, L.; Steiner, B.; Ulrichts, H.; Deckmyn, H.; Forestier, M.; Beer, J.H. Platelet microparticle formation and thrombin generation under high shear are effectively suppressed by a monoclonal antibody against GPIba. Thromb. Haemost. 2006, 96, 774–780. [Google Scholar] [CrossRef]
- Shai, E.; Rosa, I.; Parguina, A.F.; Motahedeh, S.; Varon, D.; Garcia, A. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. J. Proteom. 2012, 76, 287–296. [Google Scholar] [CrossRef]
- Milioli, M.; Ibanez-Vea, M.; Sidoli, S.; Palmisano, G.; Careri, M.; Larsen, M.R. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J. Proteom. 2015, 121, 56–66. [Google Scholar] [CrossRef]
- Heemskerk, J.W.; Vuist, W.M.; Feijge, M.A.; Reutelingsperger, C.P.; Lindhout, T. Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets: Evidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood 1997, 90, 2615–2625. [Google Scholar] [CrossRef]
- Rand, M.L.; Wang, H.; Bang, K.W.; Packham, M.A.; Freedman, J. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J. Thromb. Haemost. JTH 2006, 4, 1621–1623. [Google Scholar] [CrossRef]
- Rank, A.; Nieuwland, R.; Crispin, A.; Grützner, S.; Iberer, M.; Toth, B.; Pihusch, R. Clearance of platelet microparticles in vivo. Platelets 2011, 22, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Sinauridze, E.I.; Kireev, D.A.; Popenko, N.Y.; Pichugin, A.V.; Panteleev, M.A.; Krymskaya, O.V.; Ataullakhanov, F.I. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb. Haemost. 2007, 97, 425–434. [Google Scholar] [PubMed]
- Van Der Meijden, P.E.; Van Schilfgaarde, M.; Van Oerle, R.; Renné, T.; ten Cate, H.; Spronk, H.M. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J. Thromb. Haemost. JTH 2012, 10, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, M.J.; Bradford, R.; Hobl, E.L.; Lin, F.C.; Jilma, B.; Key, N.S. Procoagulant microparticles promote coagulation in a factor XI-dependent manner in human endotoxemia. J. Thromb. Haemost. JTH 2016, 14, 1031–1042. [Google Scholar] [CrossRef]
- Aleman, M.M.; Gardiner, C.; Harrison, P.; Wolberg, A.S. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J. Thromb. Haemost. JTH 2011, 9, 2251–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, I.; Klocke, A.; Alex, M.; Kotzsch, M.; Luther, T.; Morgenstern, E.; Zieseniss, S.; Zahler, S.; Preissner, K.; Engelmann, B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003, 17, 476–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somajo, S.; Koshiar, R.L.; Norstrom, E.; Dahlback, B. Protein S and factor V in regulation of coagulation on platelet microparticles by activated protein C. Thromb. Res. 2014, 134, 144–152. [Google Scholar] [CrossRef]
- Nomura, S.; Tandon, N.N.; Nakamura, T.; Cone, J.; Fukuhara, S.; Kambayashi, J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis 2001, 158, 277–287. [Google Scholar] [CrossRef]
- Mause, S.F.; von Hundelshausen, P.; Zernecke, A.; Koenen, R.R.; Weber, C. Platelet microparticles: A transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Chimen, M.; Evryviadou, A.; Box, C.L.; Harrison, M.J.; Hazeldine, J.; Dib, L.H.; Kuravi, S.J.; Payne, H.; Price, J.M.J.; Kavanagh, D.; et al. Appropriation of GPIbα from platelet-derived extracellular vesicles supports monocyte recruitment in systemic inflammation. Haematologica 2020, 105, 1248–1261. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Gross, P.L.; Furie, B.C.; Merrill-Skoloff, G.; Chou, J.; Furie, B. Leukocyte-versus microparticle-mediated tissue factor transfer during arteriolar thrombus development. J. Leukoc. Biol. 2005, 78, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Tripisciano, C.; Weiss, R.; Karuthedom George, S.; Fischer, M.B.; Weber, V. Extracellular Vesicles Derived From Platelets, Red Blood Cells, and Monocyte-Like Cells Differ Regarding Their Ability to Induce Factor XII-Dependent Thrombin Generation. Front. Cell Dev. Biol. 2020, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Reilly, M.P.; Casasanto, P.; McKenzie, S.E.; Williams, K.J. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Maly, M.; Hrachovinova, I.; Tomasov, P.; Salaj, P.; Hajek, P.; Veselka, J. Patients with acute coronary syndromes have low tissue factor activity and microparticle count, but normal concentration of tissue factor antigen in platelet free plasma: A pilot study. Eur. J. Haematol. 2009, 82, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Keuren, J.F.; Jie, K.S.; Leers, M.P. Increased expression of TF on monocytes, but decreased numbers of TF bearing microparticles in blood from patients with acute myocardial infarction. Eur. J. Haematol. 2009, 83, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Pereira, B.; Averous, G.; Faure, A.; Jesel, L.; Germain, P.; Grunebaum, L.; Ohlmann, P.; Freyssinet, J.M.; Bareiss, P.; et al. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with ST-segment elevation myocardial infarction: Role of endothelial damage and leukocyte activation. Atherosclerosis 2009, 204, 636–641. [Google Scholar] [CrossRef]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef] [PubMed]
- Gamonet, C.; Desmarets, M.; Mourey, G.; Biichle, S.; Aupet, S.; Laheurte, C.; François, A.; Resch, E.; Bigey, F.; Binda, D.; et al. Processing methods and storage duration impact extracellular vesicle counts in red blood cell units. Blood Adv. 2020, 4, 5527–5539. [Google Scholar] [CrossRef] [PubMed]
- Jy, W.; Ricci, M.; Shariatmadar, S.; Gomez-Marin, O.; Horstman, L.H.; Ahn, Y.S. Microparticles in stored red blood cells as potential mediators of transfusion complications. Transfusion 2011, 51, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Koshiar, R.L.; Somajo, S.; Norström, E.; Dahlbäck, B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS ONE 2014, 9, e104200. [Google Scholar]
- Fischer, D.; Büssow, J.; Meybohm, P.; Weber, C.F.; Zacharowski, K.; Urbschat, A.; Müller, M.M.; Jennewein, C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017, 57, 2701–2711. [Google Scholar] [CrossRef]
- Peters, A.L.; Vlaar, A.P.J.; van Bruggen, R.; de Korte, D.; Meijers, J.C.M.; Nieuwland, R.; Juffermans, N.P. Transfusion of autologous extracellular vesicles from stored red blood cells does not affect coagulation in a model of human endotoxemia. Transfusion 2018, 58, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Cloos, A.S.; Ghodsi, M.; Stommen, A.; Vanderroost, J.; Dauguet, N.; Pollet, H.; D’Auria, L.; Mignolet, E.; Larondelle, Y.; Terrasi, R.; et al. Interplay Between Plasma Membrane Lipid Alteration, Oxidative Stress and Calcium-Based Mechanism for Extracellular Vesicle Biogenesis From Erythrocytes During Blood Storage. Front. Physiol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, K.A.; Mbagwu, S.; Hernández-Castañeda, M.A.; Adapa, S.R.; Walch, M.; Filgueira, L.; Falquet, L.; Jiang, R.H.Y.; Ghiran, I.; Mantel, P.Y. Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci. Rep. 2018, 8, 884. [Google Scholar] [CrossRef] [Green Version]
- Kozuma, Y.; Sawahata, Y.; Takei, Y.; Chiba, S.; Ninomiya, H. Procoagulant properties of microparticles released from red blood cells in paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 2011, 152, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, A.A.; Adly, A.A.; Ismail, E.A.; Habeeb, N.M.; Farouk, A. Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: Relation to cardiovascular complications. Platelets 2013, 24, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Suades, R.; Padró, T.; Vilahur, G.; Martin-Yuste, V.; Sabaté, M.; Sans-Roselló, J.; Sionis, A.; Badimon, L. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J. Thromb. Haemost. JTH 2015, 13, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Noubouossie, D.F.; Henderson, M.W.; Mooberry, M.; Ilich, A.; Ellsworth, P.; Piegore, M.; Skinner, S.C.; Pawlinski, R.; Welsby, I.; Renné, T.; et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood 2020, 135, 755–765. [Google Scholar] [CrossRef]
- Ueba, T.; Haze, T.; Sugiyama, M.; Higuchi, M.; Asayama, H.; Karitani, Y.; Nishikawa, T.; Yamashita, K.; Nagami, S.; Nakayama, T.; et al. Level, distribution and correlates of platelet-derived microparticles in healthy individuals with special reference to the metabolic syndrome. Thromb. Haemost. 2008, 100, 280–285. [Google Scholar] [PubMed]
- Ueba, T.; Nomura, S.; Inami, N.; Nishikawa, T.; Kajiwara, M.; Iwata, R.; Yamashita, K. Plasma level of platelet-derived microparticles is associated with coronary heart disease risk score in healthy men. J. Atheroscler. Thromb. 2010, 17, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Bi, Y.; Yu, M.; Li, T.; Tong, D.; Yang, X.; Zhang, C.; Guo, L.; Wang, C.; Kou, Y.; et al. Phosphatidylserine-exposing blood cells and microparticles induce procoagulant activity in non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 258, 138–143. [Google Scholar] [CrossRef]
- Wang, L.; Bi, Y.; Cao, M.; Ma, R.; Wu, X.; Zhang, Y.; Ding, W.; Liu, Y.; Yu, Q.; Zhang, Y.; et al. Microparticles and blood cells induce procoagulant activity via phosphatidylserine exposure in NSTEMI patients following stent implantation. Int. J. Cardiol. 2016, 223, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, X.; Si, Y.; Yao, Z.; Dong, Z.; Novakovic, V.A.; Guo, L.; Tong, D.; Chen, H.; Bi, Y.; et al. Increased blood cell phosphatidylserine exposure and circulating microparticles contribute to procoagulant activity after carotid artery stenting. J. Neurosurg. 2017, 127, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Han, X.; Li, Y.; Cheng, W.; Wang, L.; Lu, Z.; Yang, J.; Zhao, M. The Level of Circulating Microparticles in Patients with Coronary Heart Disease: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2020, 13, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, L.M.; Porto, I.; Di Vito, L.; De Maria, G.L.; Leone, A.M.; Tinelli, G.; Tritarelli, A.; Di Rocco, G.; Snider, F.; Capogrossi, M.C.; et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 2012, 76, 2174–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Zhao, W.B.; Chen, Y.; Hu, H.Y. Higher Plasma Concentrations of Platelet Microparticles in Patients With Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2016, 32, 1325.e1–1325.e10. [Google Scholar] [CrossRef]
- Suades, R.; Padró, T.; Crespo, J.; Ramaiola, I.; Martin-Yuste, V.; Sabaté, M.; Sans-Roselló, J.; Sionis, A.; Badimon, L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016, 202, 378–387. [Google Scholar] [CrossRef]
- Nylander, S.; Femia, E.A.; Scavone, M.; Berntsson, P.; Asztély, A.K.; Nelander, K.; Löfgren, L.; Nilsson, R.G.; Cattaneo, M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to P2Y12 antagonism. J. Thromb. Haemost. JTH 2013, 11, 1867–1876. [Google Scholar] [CrossRef]
- Morel, O.; Hugel, B.; Jesel, L.; Mallat, Z.; Lanza, F.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Cazenave, J.P.; Tedgui, A.; et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J. Thromb. Haemost. JTH 2004, 2, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Christersson, C.; Thulin, Å.; Siegbahn, A. Microparticles during long-term follow-up after acute myocardial infarction. Association to atherosclerotic burden and risk of cardiovascular events. Thromb. Haemost. 2017, 117, 1571–1581. [Google Scholar] [PubMed]
- Gasecka, A.; Nieuwland, R.; Budnik, M.; Dignat-George, F.; Eyileten, C.; Harrison, P.; Lacroix, R.; Leroyer, A.; Opolski, G.; Pluta, K.; et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J. Thromb. Haemost. JTH 2020, 18, 609–623. [Google Scholar] [CrossRef] [Green Version]
- Haller, P.M.; Stojkovic, S.; Piackova, E.; Andric, T.; Wisgrill, L.; Spittler, A.; Wojta, J.; Huber, K.; Jäger, B. The association of P2Y(12) inhibitors with pro-coagulatory extracellular vesicles and microRNAs in stable coronary artery disease. Platelets 2020, 31, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Spaulding, C.; Picard, F.; Schaison, F.; Melle, J.; Weber, S.; Fontenay-Roupie, M. Flow cytometry detection of platelet procoagulation activity and microparticles in patients with unstable angina treated by percutaneous coronary angioplasty and stent implantation. Thromb. Haemost. 2001, 86, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B., Jr.; Szuk, T.; Debreceni, I.B.; Kappelmayer, J. Platelet-derived microparticle levels are significantly elevated in patients treated by elective stenting compared to subjects with diagnostic catheterization alone. Platelets 2010, 21, 147–151. [Google Scholar] [CrossRef]
- Mallat, Z.; Hugel, B.; Ohan, J.; Lesèche, G.; Freyssinet, J.M.; Tedgui, A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: A role for apoptosis in plaque thrombogenicity. Circulation 1999, 99, 348–353. [Google Scholar] [CrossRef] [Green Version]
- Leroyer, A.S.; Isobe, H.; Lesèche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef] [Green Version]
- Min, P.K.; Kim, J.Y.; Chung, K.H.; Lee, B.K.; Cho, M.; Lee, D.L.; Hong, S.Y.; Choi, E.Y.; Yoon, Y.W.; Hong, B.K.; et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2013, 227, 323–328. [Google Scholar] [CrossRef]
- Porto, I.; Biasucci, L.M.; De Maria, G.L.; Leone, A.M.; Niccoli, G.; Burzotta, F.; Trani, C.; Tritarelli, A.; Vergallo, R.; Liuzzo, G.; et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 2012, 33, 2928–2938. [Google Scholar] [CrossRef] [Green Version]
- Bernal-Mizrachi, L.; Jy, W.; Fierro, C.; Macdonough, R.; Velazques, H.A.; Purow, J.; Jimenez, J.J.; Horstman, L.L.; Ferreira, A.; de Marchena, E.; et al. Endothelial microparticles correlate with high-risk angiographic lesions in acute coronary syndromes. Int. J. Cardiol. 2004, 97, 439–446. [Google Scholar] [CrossRef]
- Vélez, P.; Parguiña, A.F.; Ocaranza-Sánchez, R.; Grigorian-Shamagian, L.; Rosa, I.; Alonso-Orgaz, S.; de la Cuesta, F.; Guitián, E.; Moreu, J.; Barderas, M.G.; et al. Identification of a circulating microvesicle protein network involved in ST-elevation myocardial infarction. Thromb. Haemost. 2014, 112, 716–726. [Google Scholar] [CrossRef]
- Stubbs, J.D.; Lekutis, C.; Singer, K.L.; Bui, A.; Yuzuki, D.; Srinivasan, U.; Parry, G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc. Natl. Acad. Sci. USA 1990, 87, 8417–8421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinning, J.M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef] [Green Version]
- Empana, J.P.; Boulanger, C.M.; Tafflet, M.; Renard, J.M.; Leroyer, A.S.; Varenne, O.; Prugger, C.; Silvain, J.; Tedgui, A.; Cariou, A.; et al. Microparticles and sudden cardiac death due to coronary occlusion. The TIDE (Thrombus and Inflammation in sudden DEath) study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 28–36. [Google Scholar] [CrossRef]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Shantsila, E.; Tapp, L.D.; López-Cuenca, A.; Romero, A.I.; Hernández-Romero, D.; Orenes-Piñero, E.; Manzano-Fernández, S.; Valdés, M.; Marín, F.; et al. Small-size circulating microparticles in acute coronary syndromes: Relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 2013, 227, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Chu, K.; Lee, S.T.; Park, H.K.; Bahn, J.J.; Kim, D.H.; Kim, J.H.; Kim, M.; Kun Lee, S.; Roh, J.K. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann. Neurol. 2009, 66, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kou, Y.; Zou, L.; Liu, R.; Zhao, X.; Wang, Y.; Zhang, C.; Dong, Z.; Kou, J.; Bi, Y.; Fu, L.; et al. Intravascular cells and circulating microparticles induce procoagulant activity via phosphatidylserine exposure in heart failure. J. Thromb. Thrombolysis 2019, 48, 187–194. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Laake, K.; Myhre, P.; Bratseth, V.; Arnesen, H.; Solheim, S.; Badimon, L.; Seljeflot, I. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS ONE 2017, 12, e0172558. [Google Scholar]
- Chiva-Blanch, G.; Bratseth, V.; Ritschel, V.; Andersen, G.; Halvorsen, S.; Eritsland, J.; Arnesen, H.; Badimon, L.; Seljeflot, I. Monocyte-derived circulating microparticles (CD14(+), CD14(+)/CD11b(+) and CD14(+)/CD142(+)) are related to long-term prognosis for cardiovascular mortality in STEMI patients. Int. J. Cardiol. 2017, 227, 876–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimbene, A.; Hernandez, R.; George, J.K.; Parker, A.; Bergeron, A.L.; Pradhan, S.; Vijayan, K.V.; Civitello, A.; Simpson, L.; Nawrot, M.; et al. Association between cell-derived microparticles and adverse events in patients with nonpulsatile left ventricular assist devices. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2014, 33, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, L.; Ederhy, S.; Di Angelantonio, E.; Toti, F.; Zobairi, F.; Dufaitre, G.; Meuleman, C.; Mallat, Z.; Boccara, F.; Tedgui, A.; et al. Factors influencing the level of circulating procoagulant microparticles in acute pulmonary embolism. Arch. Cardiovasc. Dis. 2010, 103, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, L.; Ederhy, S.; Di Angelantonio, E.; Toti, F.; Zobairi, F.; Dufaitre, G.; Meuleman, C.; Mallat, Z.; Boccara, F.; Tedgui, A.; et al. Circulating procoagulant microparticles in acute pulmonary embolism: A case-control study. Int. J. Cardiol. 2010, 145, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Ye, C.; Huang, Y.; Liu, L.; Wang, S. Circulating tissue factor positive microparticles in patients with acute recurrent deep venous thrombosis. Thromb. Res. 2012, 130, 253–258. [Google Scholar] [CrossRef]
- Thaler, J.; Koppensteiner, R.; Pabinger, I.; Ay, C.; Gremmel, T. Microparticle-associated tissue factor activity in patients with acute unprovoked deep vein thrombosis and during the course of one year. Thromb. Res. 2014, 134, 1093–1096. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Radu, C.M.; Gavasso, S.; Zerbinati, P.; Woodhams, B.; Simioni, P. Circulating microparticles in carriers of prothrombin G20210A mutation. Thromb. Haemost. 2014, 112, 432–437. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bon, M.; Gavasso, S.; Zerbinati, P.; Woodhams, B.; Tormene, D.; Prandoni, P.; Simioni, P. Circulating microparticles in carriers of factor V Leiden with and without a history of venous thrombosis. Thromb. Haemost. 2012, 108, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Enjeti, A.K.; Lincz, L.F.; Scorgie, F.E.; Seldon, M. Circulating microparticles are elevated in carriers of factor V Leiden. Thromb. Res. 2010, 126, 250–253. [Google Scholar] [CrossRef]
- Rectenwald, J.E.; Myers, D.D., Jr.; Hawley, A.E.; Longo, C.; Henke, P.K.; Guire, K.E.; Schmaier, A.H.; Wakefield, T.W. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. A pilot study. Thromb. Haemost. 2005, 94, 1312–1317. [Google Scholar] [CrossRef]
- Bucciarelli, P.; Martinelli, I.; Artoni, A.; Passamonti, S.M.; Previtali, E.; Merati, G.; Tripodi, A.; Mannucci, P.M. Circulating microparticles and risk of venous thromboembolism. Thromb. Res. 2012, 129, 591–597. [Google Scholar] [CrossRef]
- Jamaly, S.; Basavaraj, M.G.; Starikova, I.; Olsen, R.; Braekkan, S.K.; Hansen, J.B. Elevated plasma levels of P-selectin glycoprotein ligand-1-positive microvesicles in patients with unprovoked venous thromboembolism. J. Thromb. Haemost. JTH 2018, 16, 1546–1554. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Heresi, G.A.; Velasquez, H.; Jy, W.; Jimenez, J.J.; Ahn, E.; Horstman, L.L.; Soriano, A.O.; Zambrano, J.P.; Ahn, Y.S.J.J. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J. Am. Coll. Cardiol. 2005, 45, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Freyssinet, J.M.; Sailer, T.; Vormittag, R.; Pabinger, I. Circulating procoagulant microparticles in patients with venous thromboembolism. Thromb. Res. 2009, 123, 724–726. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer 2007, 110, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bulato, C.; Castelli, M.; Gavasso, S.; Simioni, P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb. Res. 2011, 127, 473–477. [Google Scholar] [CrossRef]
- Hernández, C.; Orbe, J.; Roncal, C.; Alvarez-Hernandez, M.; Martinez de Lizarrondo, S.; Alves, M.T.; García Mata, J.; Páramo, J.A. Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb. Haemost. 2013, 110, 598–608. [Google Scholar] [CrossRef]
- Lacroix, R.; Vallier, L.; Bonifay, A.; Simoncini, S.; Mege, D.; Aubert, M.; Panicot-Dubois, L.; Dubois, C.; Dignat-George, F. Microvesicles and Cancer Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 593–603. [Google Scholar] [CrossRef]
- Hisada, Y.; Mackman, N. Cancer cell-derived tissue factor-positive extracellular vesicles: Biomarkers of thrombosis and survival. Curr. Opin. Hematol. 2019, 26, 349–356. [Google Scholar] [CrossRef]
- van Es, N.; Hisada, Y.; Di Nisio, M.; Cesarman, G.; Kleinjan, A.; Mahé, I.; Otten, H.M.; Kamphuisen, P.W.; Berckmans, R.J.; Büller, H.R.; et al. Extracellular vesicles exposing tissue factor for the prediction of venous thromboembolism in patients with cancer: A prospective cohort study. Thromb. Res. 2018, 166, 54–59. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Connors, J.M.; Warkentin, T.E.; Thachil, J.; Levi, M. The unique characteristics of COVID-19 coagulopathy. Crit. Care (Lond. Engl.) 2020, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate with SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Tanriverdi, K.; Somasundaran, M.; Liu, P.; Soofi, S.; Bhandari, R.; Godwin, M.; Parsi, K.M.; et al. SARS-CoV-2 Initiates Programmed Cell Death in Platelets. Circ. Res. 2021. [Google Scholar] [CrossRef]
- Liu, F.; Han, K.; Blair, R.; Kenst, K.; Qin, Z.; Upcin, B.; Wörsdörfer, P.; Midkiff, C.C.; Mudd, J.; Belyaeva, E.; et al. SARS-CoV-2 Infects Endothelial Cells In Vivo and In Vitro. Front. Cell. Infect. Microbiol. 2021, 11, 701278. [Google Scholar] [CrossRef]
- Guervilly, C.; Bonifay, A.; Burtey, S.; Sabatier, F.; Cauchois, R.; Abdili, E.; Arnaud, L.; Lano, G.; Pietri, L.; Robert, T.; et al. Dissemination of extreme levels of extracellular vesicles: Tissue factor activity in patients with severe COVID-19. Blood Adv. 2021, 5, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Raineri, D.; Rolla, R.; Giordano, M.; Puricelli, C.; Vilardo, B.; Manfredi, M.; Cantaluppi, V.; Sainaghi, P.P.; Castello, L.; et al. Circulating Platelet-Derived Extracellular Vesicles Are a Hallmark of SARS-CoV-2 Infection. Cells 2021, 10, 85. [Google Scholar] [CrossRef]
- Balbi, C.; Burrello, J.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Pianezzi, E.; Burrello, A.; Caporali, E.; Grazioli, L.G.; Martinetti, G.; et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine 2021, 67, 103369. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients With COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated With Severity and Mortality-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Cook, C.; Kumar, A.; Spikes, L.; Chalise, P.; Dhillon, N.K. Extracellular vesicle-mediated endothelial apoptosis and EV-associated proteins correlate with COVID-19 disease severity. J. Extracell. Vesicles 2021, 10, e12117. [Google Scholar] [CrossRef]
- Inal, J. COVID-19 comorbidities, associated procoagulant extracellular vesicles and venous thromboembolisms: A possible link with ethnicity? Br. J. Haematol. 2020, 190, e218–e220. [Google Scholar] [CrossRef]
- Mackman, N.; Grover, S.P.; Antoniak, S. Tissue factor expression, extracellular vesicles and thrombosis after infection with the respiratory viruses influenza A virus and coronavirus. J. Thromb. Haemost. JTH 2021. [Google Scholar] [CrossRef]
- Georgescu, A.; Simionescu, M. Extracellular Vesicles: Versatile Nanomediators, Potential Biomarkers and Therapeutic Agents in Atherosclerosis and COVID-19-Related Thrombosis. Int. J. Mol. Sci. 2021, 22, 5967. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol. Med. 2004, 10, 171–178. [Google Scholar] [CrossRef]
- Ghosh, A.; Li, W.; Febbraio, M.; Espinola, R.G.; McCrae, K.R.; Cockrell, E.; Silverstein, R.L. Platelet CD36 mediates interactions with endothelial cell–derived microparticles and contributes to thrombosis in mice. J. Clin. Investig. 2008, 118, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Keuren, J.F.; Magdeleyns, E.J.; Bennaghmouch, A.; Bevers, E.M.; Curvers, J.; Lindhout, T. Microparticles adhere to collagen type I, fibrinogen, von Willebrand factor and surface immobilised platelets at physiological shear rates. Br. J. Haematol. 2007, 138, 527–533. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Kleiman, K.; Guo, C.; Eitzman, D.T. Hematopoietic Deficiency of miR-223 Attenuates Thrombosis in Response to Photochemical Injury in Mice. Sci. Rep. 2017, 7, 1606. [Google Scholar] [CrossRef]
- Brandt, M.; Giokoglu, E.; Garlapati, V.; Bochenek, M.L.; Molitor, M.; Hobohm, L.; Schönfelder, T.; Münzel, T.; Kossmann, S.; Karbach, S.H.; et al. Pulmonary Arterial Hypertension and Endothelial Dysfunction Is Linked to NADPH Oxidase-Derived Superoxide Formation in Venous Thrombosis and Pulmonary Embolism in Mice. Oxidative Med. Cell. Longev. 2018, 2018, 1860513. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Bauer, T.; Gogiraju, R.; Nadir, Y.; Mann, A.; Schönfelder, T.; Hünig, L.; Brenner, B.; Münzel, T.; Wenzel, P.; et al. The endothelial tumor suppressor p53 is essential for venous thrombus formation in aged mice. Blood Adv. 2018, 2, 1300–1314. [Google Scholar] [CrossRef]
- Ramacciotti, E.; Hawley, A.E.; Farris, D.M.; Ballard, N.E.; Wrobleski, S.K.; Myers, D.D., Jr.; Henke, P.K.; Wakefield, T.W. Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis. Thromb. Haemost. 2009, 101, 748–754. [Google Scholar]
- Myers, D.D., Jr.; Rectenwald, J.E.; Bedard, P.W.; Kaila, N.; Shaw, G.D.; Schaub, R.G.; Farris, D.M.; Hawley, A.E.; Wrobleski, S.K.; Henke, P.K.; et al. Decreased venous thrombosis with an oral inhibitor of P selectin. J. Vasc. Surg. 2005, 42, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Myers, D.D.; Hawley, A.E.; Farris, D.M.; Wrobleski, S.K.; Thanaporn, P.; Schaub, R.G.; Wagner, D.D.; Kumar, A.; Wakefield, T.W. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J. Vasc. Surg. 2003, 38, 1075–1089. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; Apta, B.H.R.; Bonna, A.M.; Harper, M.T. Platelet P-selectin triggers rapid surface exposure of tissue factor in monocytes. Sci. Rep. 2019, 9, 13397. [Google Scholar] [CrossRef]
- Sasano, T.; Cho, M.S.; Rodriguez-Aguayo, C.; Bayraktar, E.; Taki, M.; Afshar-Kharghan, V.; Sood, A.K. Role of tissue-factor bearing extracellular vesicles released from ovarian cancer cells in platelet aggregation in vitro and venous thrombosis in mice. Thromb. Update 2021, 2, 100020. [Google Scholar] [CrossRef]
- Wang, J.G.; Geddings, J.E.; Aleman, M.M.; Cardenas, J.C.; Chantrathammachart, P.; Williams, J.C.; Kirchhofer, D.; Bogdanov, V.Y.; Bach, R.R.; Rak, J.; et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012, 119, 5543–5552. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.A.; Obi, A.T.; Myers, D.D., Jr.; Wrobleski, S.K.; Henke, P.K.; Mackman, N.; Wakefield, T.W. Critical review of mouse models of venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Hisada, Y.; Ay, C.; Auriemma, A.C.; Cooley, B.C.; Mackman, N. Human pancreatic tumors grown in mice release tissue factor-positive microvesicles that increase venous clot size. J. Thromb. Haemost. JTH 2017, 15, 2208–2217. [Google Scholar] [CrossRef] [Green Version]
- Thomas, G.M.; Panicot-Dubois, L.; Lacroix, R.; Dignat-George, F.; Lombardo, D.; Dubois, C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J. Exp. Med. 2009, 206, 1913–1927. [Google Scholar] [CrossRef] [Green Version]
- Davila, M.; Amirkhosravi, A.; Coll, E.; Desai, H.; Robles, L.; Colon, J.; Baker, C.H.; Francis, J.L. Tissue factor-bearing microparticles derived from tumor cells: Impact on coagulation activation. J. Thromb. Haemost. JTH 2008, 6, 1517–1524. [Google Scholar] [CrossRef]
- Thomas, G.M.; Brill, A.; Mezouar, S.; Crescence, L.; Gallant, M.; Dubois, C.; Wagner, D.D. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J. Thromb. Haemost. JTH 2015, 13, 1310–1319. [Google Scholar] [CrossRef]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. JTH 2016, 14, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Mezouar, S.; Darbousset, R.; Dignat-George, F.; Panicot-Dubois, L.; Dubois, C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int. J. Cancer 2015, 136, 462–475. [Google Scholar] [CrossRef] [Green Version]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-activated platelet-derived exosomes regulate endothelial cell expression of ICAM-1 via microRNA-223 during the thrombosis-inflammation response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlowski, C.L.; Li, W.; Sun, M.; Ravichandran, K.; Hickman, D.; Kos, C.; Kaur, G.; Sen Gupta, A. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials 2017, 128, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Disharoon, D.; Marr, D.W.M.; Neeves, K.B. Engineered microparticles and nanoparticles for fibrinolysis. J. Thromb. Haemost. JTH 2019, 17, 2004–2015. [Google Scholar] [CrossRef]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Stimulus | Cell Type | Concentration/Incubation * | Readout |
|---|---|---|---|
| Endothelial Cells | |||
| Proinflammatory stimuli | |||
| IL1α | HUVEC | 5 ng/mL 0–72 h | Annexin V binding; TF and endothelial antigen expression; procoagulant effects in vivo [35,36] |
| IL1β | HUVEC | 10 U/mL 24 h | EV release and marker antigen expression [37] |
| LPS | HAEC | 10 ng/mL 24 h | EV release; PS and annexin V expression [38] |
| PMA | HUVEC | 100 ng/mL 24 h | EV release [37] |
| TNFα | Renal and brain microvascular and coronary artery macrovascular ECs | 10 ng/mL 24 h | Marker antigen expression and annexin V binding [39] |
| TNFα | HUVEC | 10 ng/mL 24 h | TF release by small and large EVs [40] |
| TNFα | HUVEC | 1–100 ng/mL 6 h (blebbing) 24 h (vesiculation) | EV release and marker antigen expression; procoagulant activity in vitro [37] |
| TNFα | HUVEC | 100 ng/mL 48 h | MP release [41] |
| TNFα | HBMEC | 20 ng/mL 20 h | MP release [42] |
| TNFα | HMEC-1 | 100 ng/mL 48 h | MP release [43] |
| Procoagulant stimuli | |||
| Ca2+ ionophore A23187 | HUVEC | 100 µmol/L 10 min | EV release [37] |
| Complement C5b-9 | HUVEC | 2 µg in 125 µL 10 min | EV release; prothrombinase and thrombomodulin activity [44] |
| PAI-1 | HUVEC | 1–10 ng/mL 1–3 h | Procoagulant, uPAR and integrin αvβ3 expression, thrombin generation [44,45,46] |
| Thrombin | HUVEC | 0.1–10 U/mL 24–48 h | Marker antigen expression; procoagulant MP release [37,47,48] |
| Thrombin | HBMEC | 2 U/mL 20 h | Procoagulant MP release [42] |
| Proapoptotic stimuli | |||
| Serum and growth factor deprivation | Renal and brain microvascular ECs; coronary artery macrovascular ECs | N/A 24 h | Marker antigen expression and annexin V binding [39] |
| Staurosporine | HUVEC | 100 nmol/L 2–16 h | Apoptotic MP/PS exposure; annexin V binding; TF expression; factor Xa activation [49] |
| Platelets | |||
| Ca2+ ionophore A23187 | Human platelets | 10 µM 15 min | CD62P and PS exposure, EV quantity and quality [45,46] |
| ABT-737 | Human platelets | 30 µM 90 min | CD62P and PS exposure [50] |
| ADP | Human platelets | 60 µM 30 min | EV quantity and quality [45] |
| ADP | Human platelets | 20 µM 1 h | EV quantity and quality [51] |
| Collagen | Human platelets | 10 µg/mL 30 min | EV generation [45,52] |
| Complement C5b-9 | Human platelets | 2 µg in 125 µL 10 min | EV release, factor V binding [7] |
| CRP-XL | Human platelets | 1 µg/mL 30 min | EV generation [45,52] |
| LPS | Human platelets | 100 ng/mL 3 h | EV quantity and quality [45] |
| Thrombin | Human platelets | 1 U/mL 15 min | CD62P and PS exposure [50] |
| Thrombin | Human platelets | 1 U/mL 30 min | EV generation [45,52] |
| Thrombin and CaCl2 | Human platelets | 0.25 U/mL 5 min | EV quantity and quality [53] |
| TRAP6 | Human platelets | 60 µM 30 min | EV quantity and quality [45] |
| Monocytes | |||
| Ca2+ ionophore A23187 | THP-1 | 10 mM 5 min | TF+ MP release [37,54] |
| LPS | THP-1 | 5 µg/mL 4 h | MP release [55] |
| LPS | THP-1 | 1 µg/mL 1 h (RT) | MP release [56] |
| LPS | THP-1 | 1 µg/mL 5 h (RT) | TF+ MP release [54] |
| EV Type | Model | Species | Main Findings |
|---|---|---|---|
| Arterial thrombosis | |||
| MPs | Laser-induced endothelial injury of arterioles in cremaster muscle | C57BL/6J, B6.Cg-SelPltm1Fur and C57BL/6J-Selptm1Bay mice | Interaction of TF-positive EVs with thrombi occurs through the interaction of PSGL-1 expressed on microparticles and P-selectin expressed on platelets [94] |
| MPs | FeCl3-induced injury of carotid artery | C57BL/6J and CD36−/− mice | PS-exposing MPs released from activated platelets bind to CD36 receptor on platelets which results in enhanced platelet activation and thrombus formation [227] |
| MPs | Wire-induced endothelial denudation of carotid artery | Swiss mice | MPs isolated from stored murine platelet concentrates adhere on the vascular wall through an integrin aIIbβ3-mediated mechanism [94] |
| EVs | Rose Bengal-induced carotid artery injury | C57BL/6J and miR-223−/− mice | Platelet-derived EVs accelerate arterial thrombus formation by transferring miR-223 and downregulating IGFR-1 on endothelial cells [229] |
| Tumor exosomes | Tandem stenosis at the carotid artery | C57BL/6J WT and apoE−/− mice | Elevated plasma exosomal miR-223, miR-339 and miR-21 levels in mice following induction of atherothrombosis [246] |
| Venous thrombosis | |||
| MPs | IVC ligation | C57BL/6J, CD62E−/−, CD62P−/− and Delta Cytoplasmic Tail mice | MPs derived from platelets and leukocytes correlate with venous thrombus weight MP re-injection leads to higher thrombus weight than IVC alone, TF on MPs correlates to MP numbers [232] |
| MPs | IVC ligation | C57BL/6, CD62P−/− and CD62E−/− mice | An association between platelet-MPs and venous thrombus weight was observed in mice lacking P- or E-selectin [233] |
| MPs | IVC ligation | C57BL/6J, CD62E−/−, CD62P−/− and Delta Cytoplasmic Tail mice | High circulating levels of soluble P-selectin and leucocyte MPs are associated with increased venous thrombosis. Antibodies directed against PSGL-1 decreased thrombus mass [234] |
| MPs | IVC ligation/stasis | Wistar Hsd/Cpb; WU rats | Human cell-derived microparticles promote venous thrombus formation in a tissue factor-dependent manner [93] |
| MPs | IVC ligation/stasis | Rats | MPs from IL1α-stimulated endothelial MPs promote venous thrombus formation, and thrombus weight is significantly reduced by anti-TF, but not by anti-Factor XII antibodies [36] |
| TF+ tumor EVs | IVC ligation/stenosis | Nude mice | EVs from TF-overexpressing human ovarian cancer cells enhance murine venous thrombus formation [236] |
| TF+ tumor MPs | IVC ligation/stenosis | Nude mice | Thrombus weight does not differ between human pancreatic tumor-bearing and control mice, whereas injection of TF+ MPs dose-dependently increases venous thrombosis [237] |
| TF+ tumor MPs | IVC ligation | Nude mice | Venous clots are larger in mice bearing tumors compared to controls [239] |
| Tumor MPs | Laser and FeCl3 injury, cremaster and mesentery | C57BL/6J mice | Mice with tumors have larger thrombi, and infusion of a blocking P-selectin antibody abolishes the thrombotic state observed after injection of MPs or in mice developing a tumor [240] |
| Tumor MPs | IVC ligation/particle flow restriction | C57BL/6J mice | Larger venous thrombi in tumor-bearing mice or mice injected with high TF tumor MPs having more pronounced effects than low TF tumor MPs [240] |
| TF+ tumor MPs | Femoral and IVC ligation | C57BL/6J and PAR4−/− mice | TF-positive MPs obtained from human pancreatic adenocarcinoma expressing low or high TF levels enhance venous thrombosis in two mouse models. Reduced thrombosis in PAR4−/− mice and in mice treated with clopidogrel suggested role of platelets [243] |
| TF+ tumor MPs | Laser injury cremaster muscle | C57BL/6J mice | Inhibition of platelet activation reduces integrin αvβ1 and αvβ3 dependent accumulation of cancer cell MPs and thrombosis in human pancreatic tumor-bearing mice [244] |
| Tumor exosomes | Rose Bengal venous thrombosis | BALB/c mice | Intravenous administration of 4T1-derived exosomes enhances NET formation and venous thrombosis in mice treated with G-CSF [245] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zifkos, K.; Dubois, C.; Schäfer, K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 9317. https://doi.org/10.3390/ijms22179317
Zifkos K, Dubois C, Schäfer K. Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence. International Journal of Molecular Sciences. 2021; 22(17):9317. https://doi.org/10.3390/ijms22179317
Chicago/Turabian StyleZifkos, Konstantinos, Christophe Dubois, and Katrin Schäfer. 2021. "Extracellular Vesicles and Thrombosis: Update on the Clinical and Experimental Evidence" International Journal of Molecular Sciences 22, no. 17: 9317. https://doi.org/10.3390/ijms22179317






