Effect of Argon-Based Atmospheric Pressure Plasma Treatment on Hard Tissue Formation on Titanium Surface
Abstract
:1. Introduction
2. Results
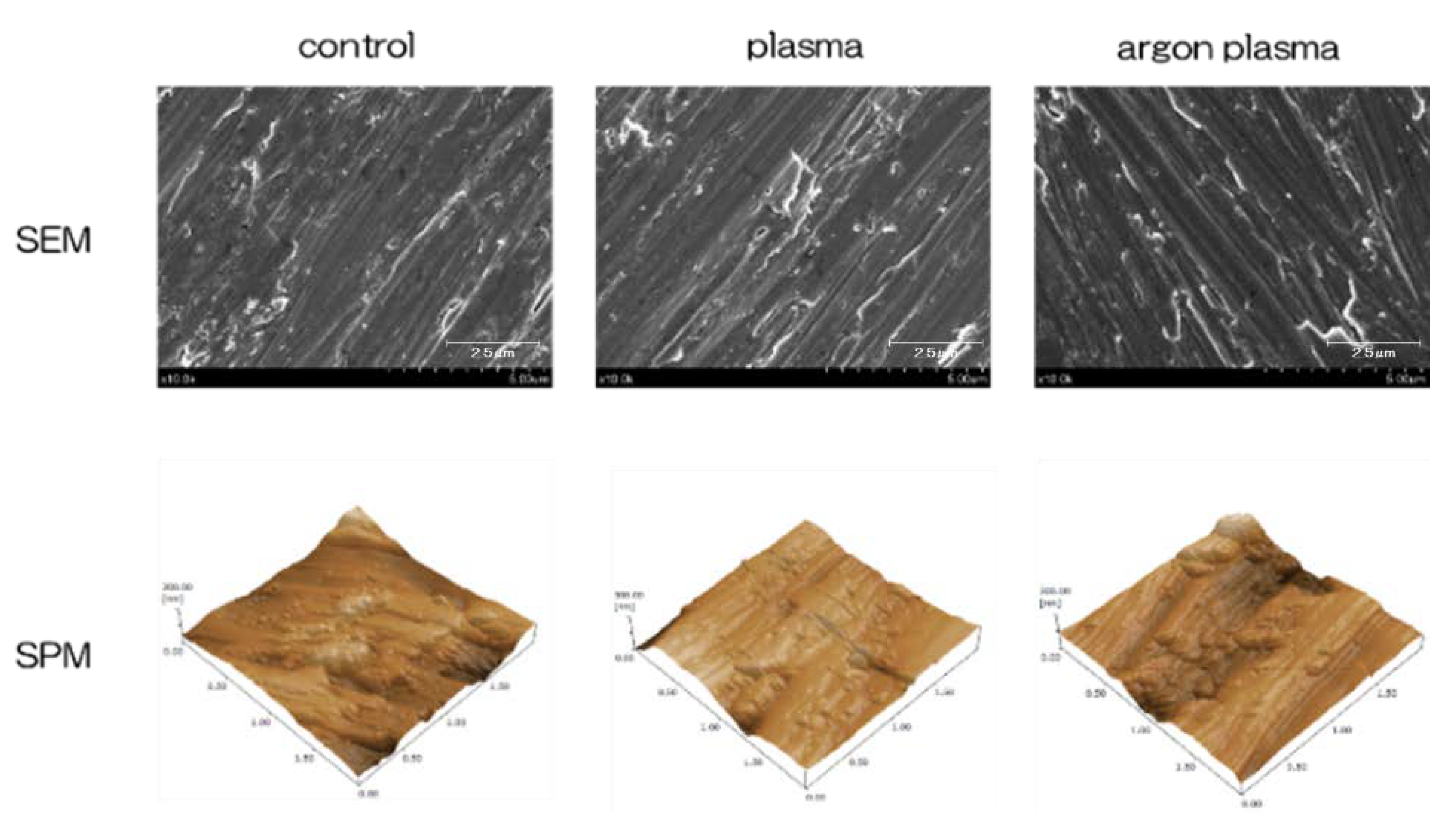
2.1. Surface Characterization
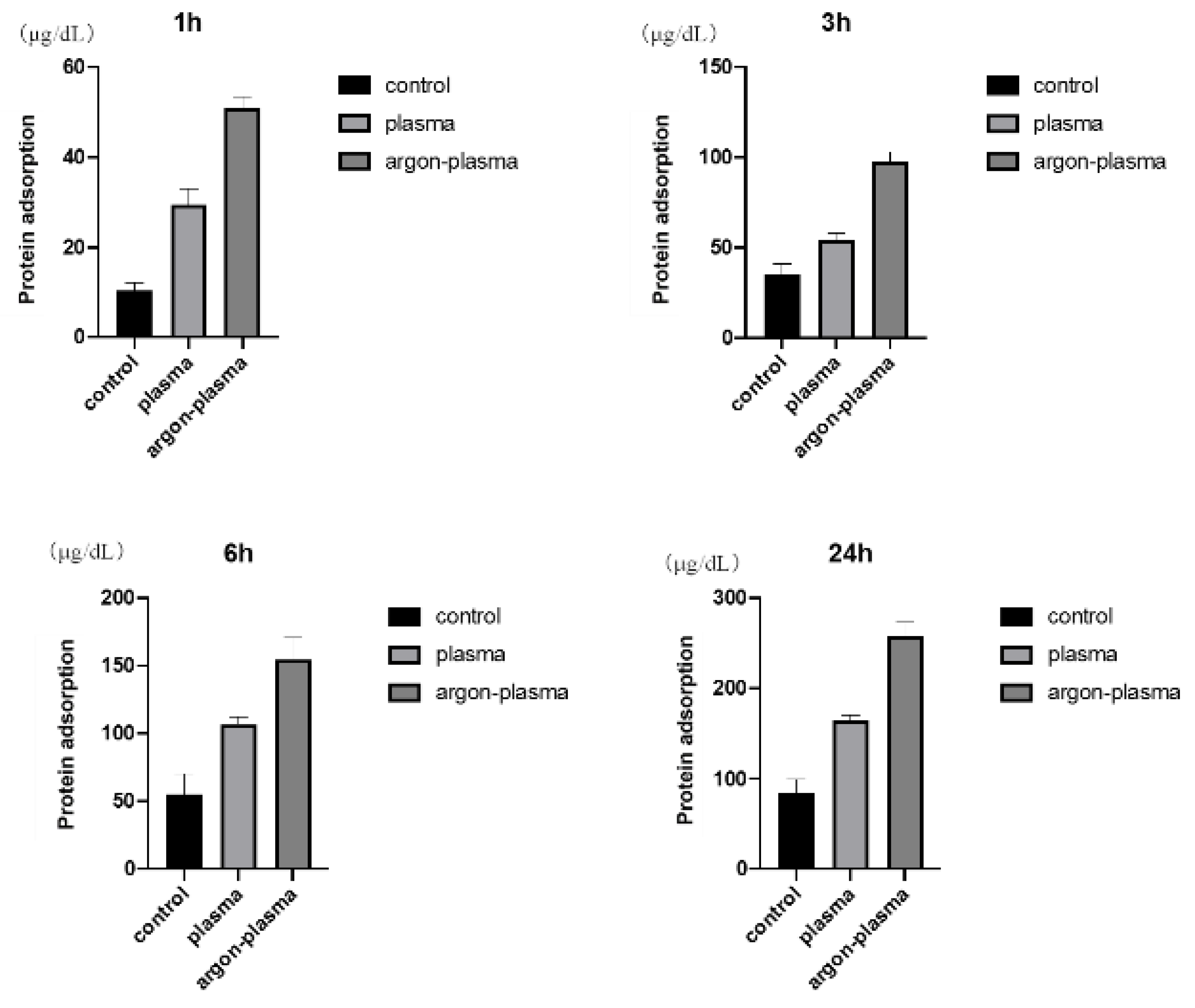
2.2. Evaluation of Protein Adsorption on the Titanium Surface
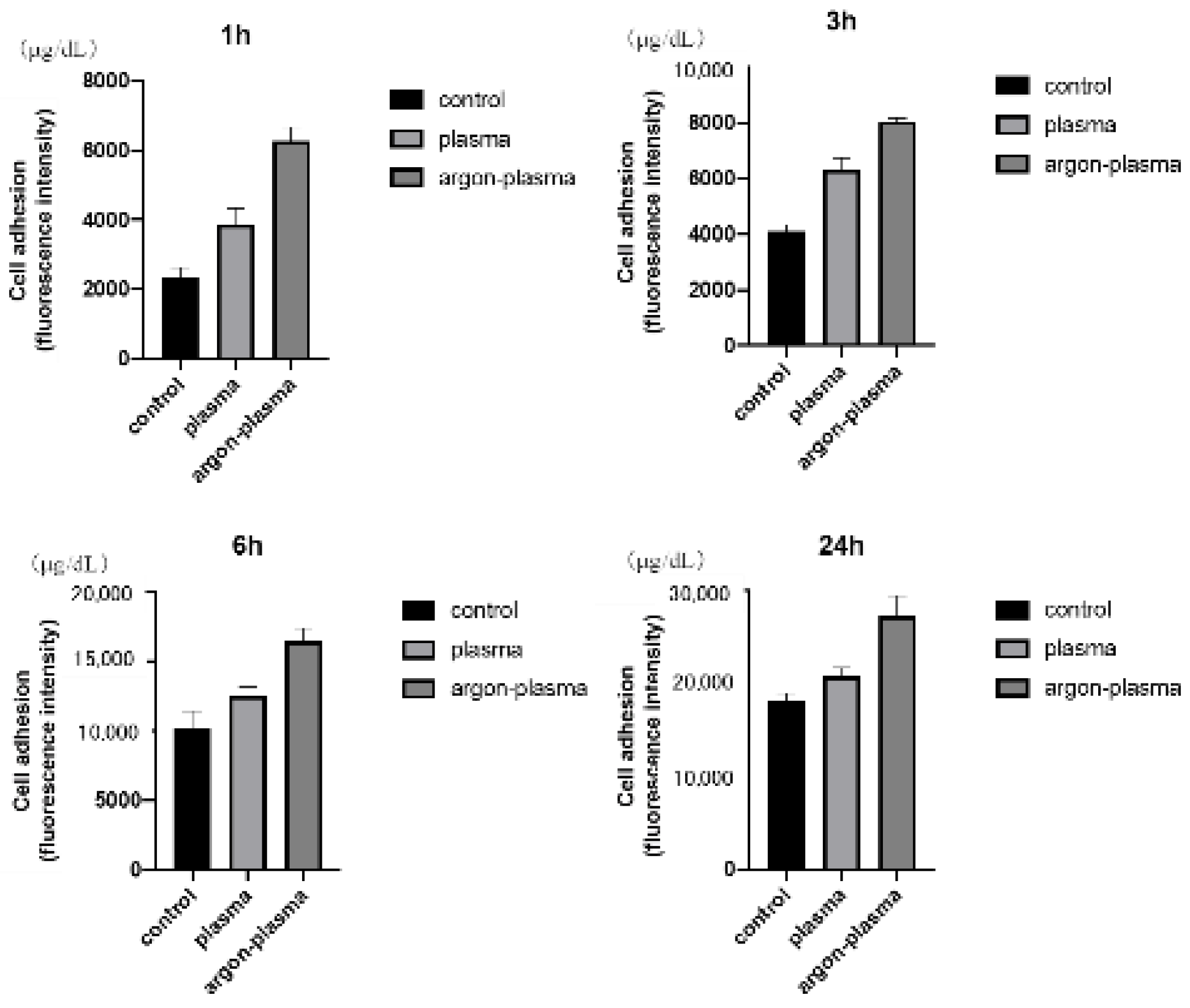
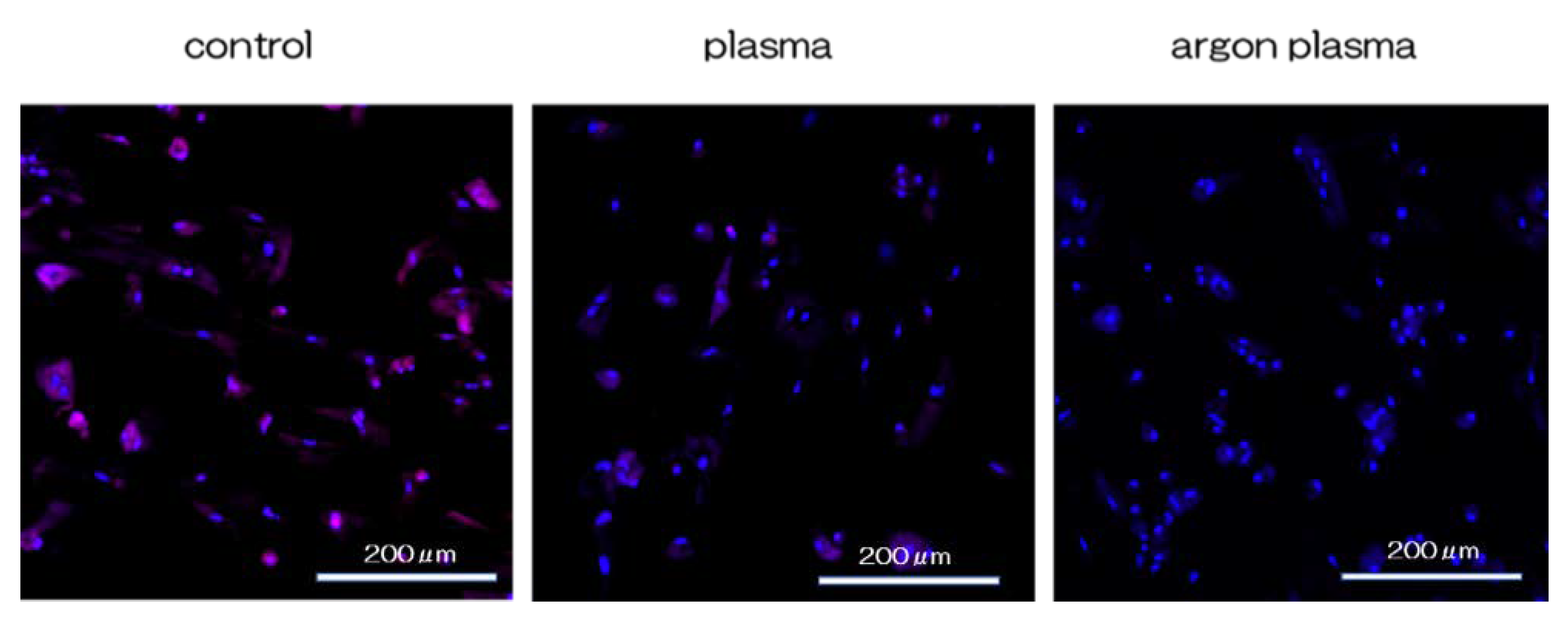
2.3. Effects of the Titanium Surface on Cell Adhesion and Morphology in RBMCs
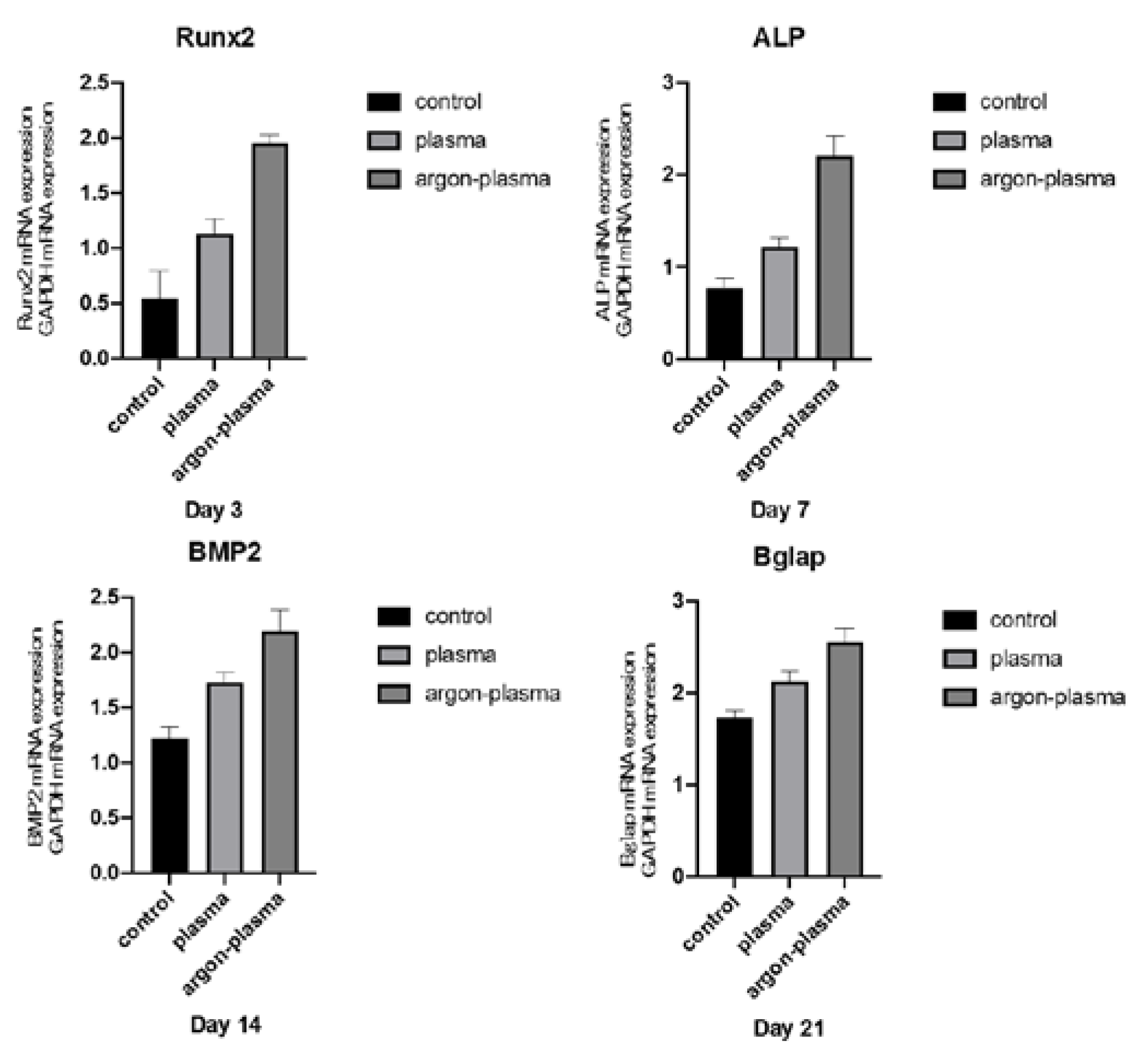
2.4. Argon Plasma Treatment-Induced Bone Differentiation on the Titanium Surface In Vitro
2.5. Cell Intracellular ROS Level of RBMCs on Titanium Surface
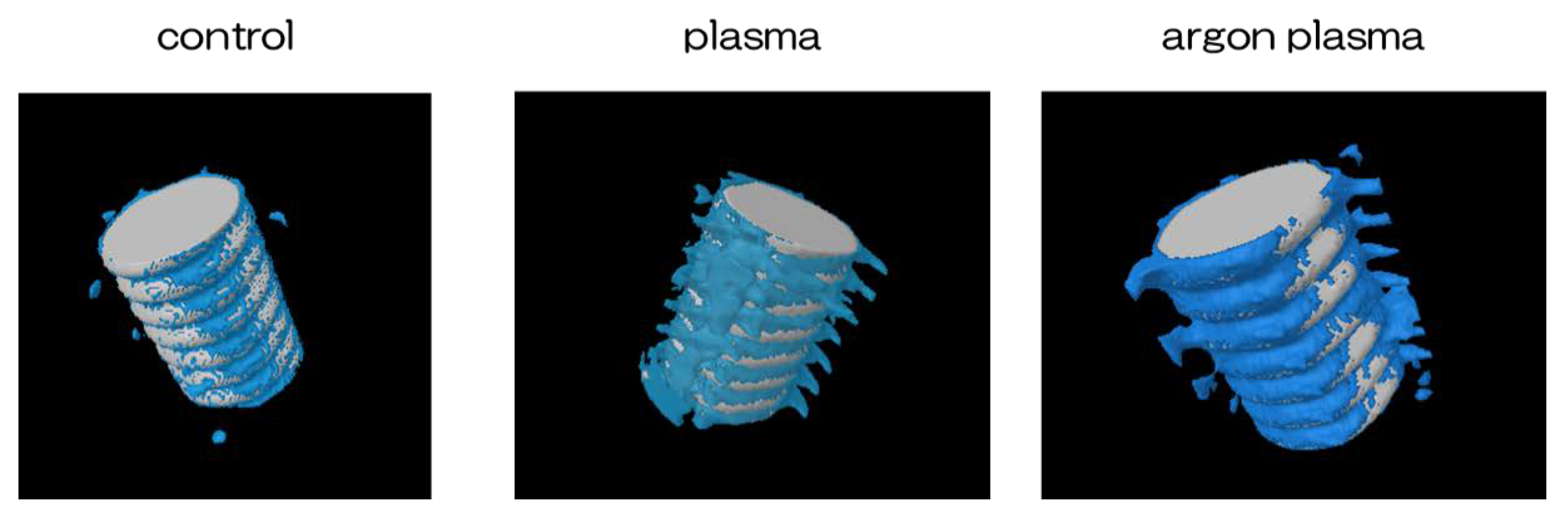
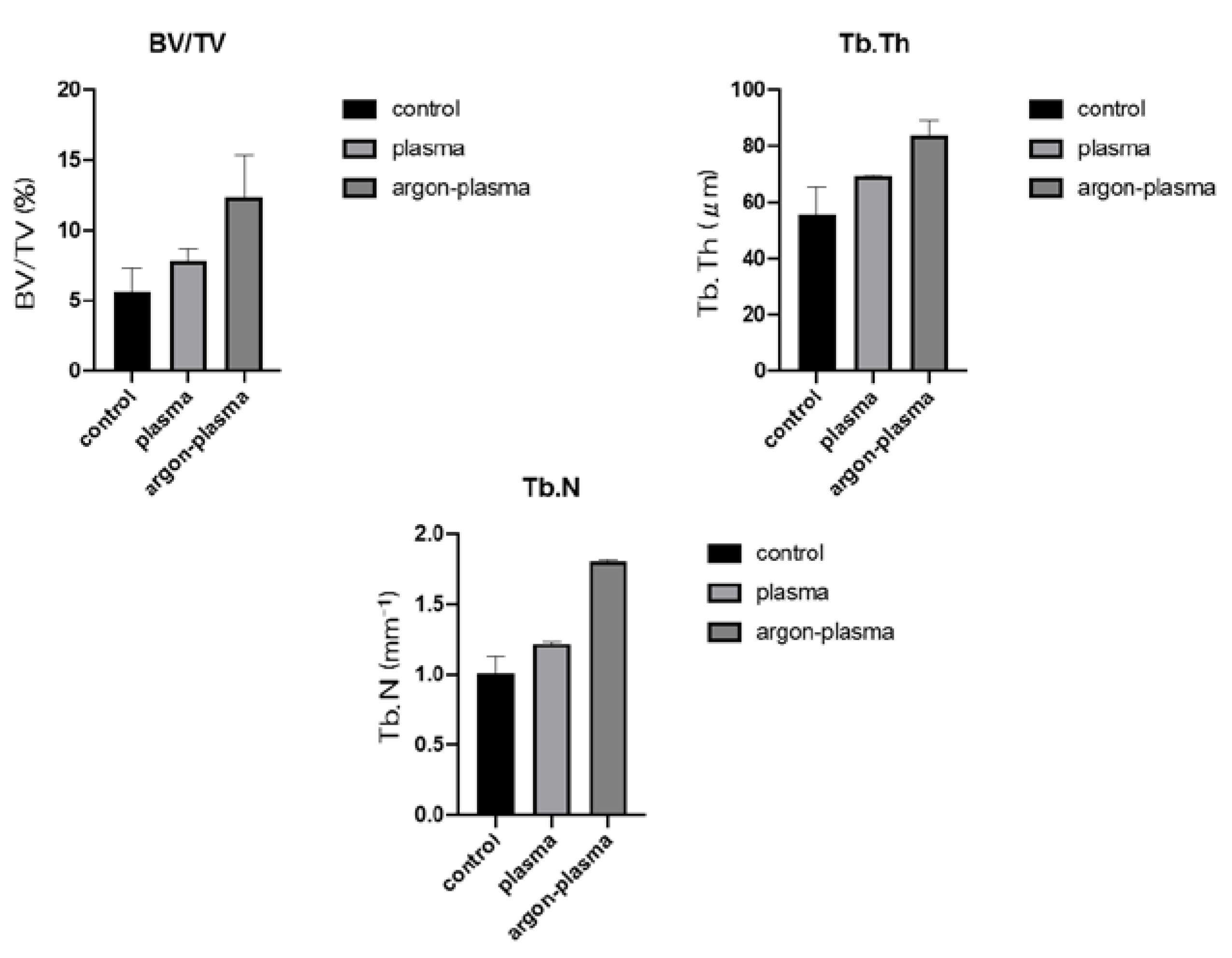
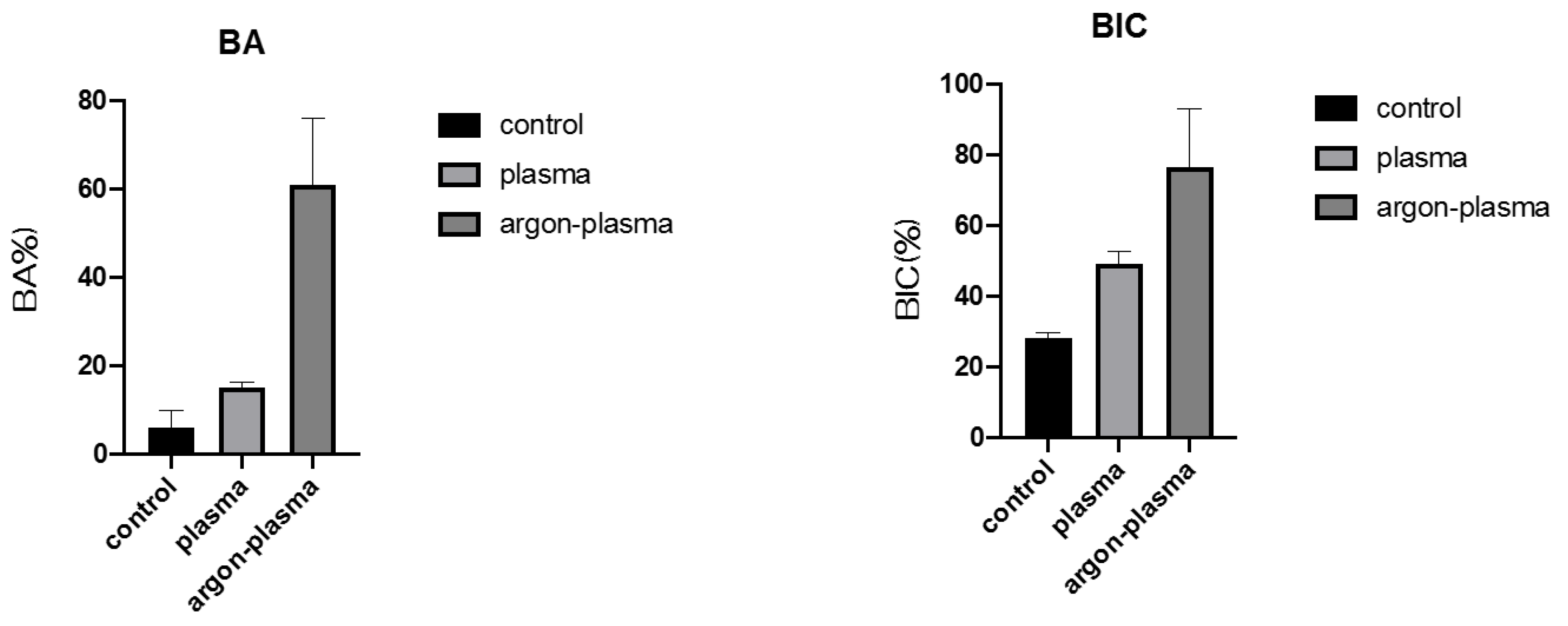
2.6. Evaluation of the Amount of New Bone Formation in the Tissue Surrounding the Titanium Implant Placement In Vivo
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Characterization of Materials
4.3. Protein Adsorption
4.4. Cell Culture
4.5. Cell Adhesion
4.6. qRT-PCR, Alkaline Phosphatase Activity, DNA Content, and Mineralization Determination
4.7. Cell Intracellular ROS Level and Mitochondrial Membrane Potential Change Detection of RBMCs
4.8. Animal Model and Surgical Procedures
4.9. Morphological Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Moradian-Oldak, J.; Wen, H.B.; Schneider, G.B.; Stanford, C.M. Tissue engineering strategies for the future generation of dental implants. Periodontology 2000 2006, 41, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Romeo, E.; Storelli, S. Systematic review of the survival rate and the biological, technical, and aesthetic complications of fixed dental prostheses with cantilevers on implants reported in longitudinal studies with a mean of 5 years follow-up. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 39–49. [Google Scholar] [CrossRef]
- Vandrovcová, M.; Bačáková, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Cooper, L.F.; Masuda, T.; Yliheikkilä, P.K.; Felton, D.A. Generalizations regarding the process and phenomenon of osseointegration. Part II. In vitro studies. Int. J. Oral Maxillofac. Implant. 1998, 13, 163–174. [Google Scholar]
- Ogawa, T.; Nishimura, I. Different bone integration profiles of turned and acid-etched implants associated with modulated expression of extracellular matrix genes. Int. J. Oral Maxillofac. Implant. 2003, 18, 200–210. [Google Scholar]
- Ogawa, T.; Ozawa, S.; Shih, J.H.; Ryu, K.H.; Sukotjo, C.; Yang, J.M.; Nishimura, I. Biomechanical evaluation of osseous implants having different surface topographies in rats. J. Dent. Res. 2000, 79, 1857–1863. [Google Scholar] [CrossRef]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Saruwatari, L.; Nakamura, H.K.; Yang, J.M.; Ogawa, T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. A 2005, 72, 296–305. [Google Scholar] [CrossRef]
- Murai, K.; Takeshita, F.; Ayukawa, Y.; Kiyoshima, T.; Suetsugu, T.; Tanaka, T. Light and electron microscopic studies of bone-titanium interface in the tibiae of young and mature rats. J. Biomed. Mater. Res. 1996, 30, 523–533. [Google Scholar] [CrossRef]
- Joos, U.; Meyer, U. New paradigm in implant osseointegration. Head Face Med. 2006, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Schwartz, Z.; Olivares-Navarrete, R.; Boyan, B.D.; Tannenbaum, R. Enhancement of surface wettability via the modification of microtextured titanium implant surfaces with polyelectrolytes. Langmuir 2011, 27, 5976–5985. [Google Scholar] [CrossRef] [Green Version]
- Elias, C.N.; Oshida, Y.; Lima, J.H.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Scheideler, L.; Rehbein, D.; Axmann, D.; Geis-Gerstorfer, J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials 2004, 25, 1429–1438. [Google Scholar] [CrossRef]
- Kloss, F.R.; Steinmüller-Nethl, D.; Stigler, R.G.; Ennemoser, T.; Rasse, M.; Hächl, O. In vivo investigation on connective tissue healing to polished surfaces with different surface wettability. Clin. Oral Implant. Res. 2011, 22, 699–705. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K.K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Prosthodont. Res. 2012, 56, 170–177. [Google Scholar] [CrossRef]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale features of a titanium surface: Effects of deposition time in NaOH solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surfaces with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Rat endothelial cell attachment, behavior and gene expression on NaOH-treated titanium surfaces. J. Oral Tissue Eng. 2013, 11, 189–200. [Google Scholar]
- Hara, Y.; Komasa, S.; Yoshimine, S.; Nishizaki, H.; Okazaki, J. Effect of nano modified titanium surface on adsorption of rat periodontal ligament cells. J. Osaka Dent. Univ. 2018, 52, 37–44. [Google Scholar]
- Kusumoto, T.; Yin, D.; Zhang, H.; Chen, L.; Nishizaki, H.; Komasa, Y.; Okazaki, J.; Komasa, S. Evaluation of the osteointegration of a novel alkali-treated implant system in vivo. J. Hard Tissue Biol. 2017, 26, 355–360. [Google Scholar] [CrossRef]
- Terada, C.; Komasa, S.; Kusumoto, T.; Kawazoe, T.; Okazaki, J. Effect of amelogenin coating of a nano-modified titanium surface on bioactivity. Int. J. Mol. Sci. 2018, 19, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR induced by alkali treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef] [Green Version]
- Komasa, S.; Nishizaki, M.; Zhang, H.; Takao, S.; Yin, D.; Terada, C.; Kobayashi, Y.; Kusumoto, T.; Yoshimine, S.; Nishizaki, H.; et al. Osseointegration of alkali-modified NANOZR implants: An in vivo study. Int. J. Mol. Sci. 2019, 20, 842. [Google Scholar] [CrossRef] [Green Version]
- Komasa, S.; Nishizaki, M.; Kusumoto, T.; Terada, C.; Derong, Y.; Kawamoto, A.; Yamamoto, S.; Yoshimine, S.; Nishizaki, H.; Shimizu, H.; et al. Osteogenesis-related gene expression on alkali-modified NANOZR and titanium surfaces with nanonetwork structures. J. Bio-Integr. 2017, 7, 87–94. [Google Scholar]
- Zeng, Y.; Yang, Y.; Chen, L.; Yin, D.; Zhang, H.; Tashiro, Y.; Inui, S.; Kusumoto, T.; Nishizaki, H.; Sekino, T.; et al. Optimized surface characteristics and enhanced in vivo osseointegration of alkali-treated titanium with nanonetwork structures. Int. J. Mol. Sci. 2019, 20, 1127. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Schröder, K.; Finke, B.; Ohl, A.; Lüthen, F.; Bergemann, C.; Nebe, B.; Rychly, J.; Walschus, U.; Schlosser, M.; Liefeith, K.; et al. Capability of differently charged plasma polymer coatings for control of tissue interactions with titanium surfaces. J. Adhes. Sci. Technol. 2010, 24, 1191–1205. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone–Titanium integration profile with uv-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.W. Purification of water with near—UV illuminated suspensions of titanium dioxide. Water Res. 1990, 24, 653–660. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Hatoko, M.; Komasa, S.; Zhang, H.; Sekino, T.; Okazaki, J. UV treatment improves the biocompatibility and antibacterial properties of crystallized nanostructured titanium surface. Int. J. Mol. Sci. 2019, 20, 5991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ujino, D.; Nishizaki, H.; Higuchi, S.; Komasa, S.; Okazaki, J. Effect of plasma treatment of titanium surface on biocompatibility. Appl. Sci. 2019, 9, 2257. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Tashiro, Y.; Komasa, S.; Miyake, A.; Komasa, Y.; Okazaki, J. Effects of surface modification on adsorption behavior of cell and protein on titanium surface by using quartz crystal microbalance system. Materials 2020, 14, 97. [Google Scholar] [CrossRef]
- Takao, S.; Komasa, S.; Agariguchi, A.; Kusumoto, T.; Pezzotti, G.; Okazaki, J. Effects of plasma treatment on the bioactivity of alkali-treated ceria-stabilised zirconia/alumina nanocomposite (NANOZR). Int. J. Mol. Sci. 2020, 21, 7476. [Google Scholar] [CrossRef] [PubMed]
- Komasa, S.; Takao, S.; Yang, Y.; Zeng, Y.; Li, M.; Yan, S.; Zhang, H.; Komasa, C.; Kobayashi, Y.; Nishizaki, H.; et al. Effects of UV treatment on ceria-stabilized zirconia/alumina nanocomposite (NANOZR). Materials 2020, 13, 2772. [Google Scholar] [CrossRef]
- Piattelli, A.; Corigliano, M.; Scarano, A.; Costigliola, G.; Paolantonio, M. Immediate loading of titanium plasma-sprayed implants: An histologic analysis in monkeys. J. Periodontol. 1998, 69, 321–327. [Google Scholar] [CrossRef]
- Ong, J.L.; Carnes, D.L.; Bessho, K. Evaluation of titanium plasma-sprayed and plasma-sprayed hydroxyapatite implants in vivo. Biomaterials 2004, 25, 4601–4606. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhao, H.; Liu, Y.; Liu, J.; Bai, N. Bioactive effects of low-temperature argon–Oxygen plasma on a titanium implant surface. ACS Omega 2020, 5, 3996–4003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canullo, L.; Genova, T.; Wang, H.L.; Carossa, S.; Mussano, F. Plasma of argon increases cell attachment and bacterial decontamination on different implant surfaces. Int. J. Oral Maxillofac. Implant. 2017, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, P.G.; Giro, G.; Teixeira, H.S.; Marin, C.; Witek, L.; Thompson, V.P.; Tovar, N.; Silva, N.R. Argon-based atmospheric pressure plasma enhances early bone response to rough titanium surfaces. J. Biomed. Mater. Res. A 2012, 100, 1901–1906. [Google Scholar] [CrossRef]
- Zeng, Y.; Komasa, S.; Nishida, H.; Agariguchi, A.; Sekino, T.; Okazaki, J. Enhanced osseointegration and bio-decontamination of nanostructured titanium based on non-thermal atmospheric pressure plasma. Int. J. Mol. Sci. 2020, 21, 3533. [Google Scholar] [CrossRef]
- Hettlich, H.J.; Otterbach, F.; Mittermayer, C.; Kaufmann, R.; Klee, D. Plasma-induced surface modifications on silicone intraocular lenses: Chemical analysis and in vitro characterization. Biomaterials 1991, 12, 521–524. [Google Scholar] [CrossRef]
- Yeung, K.W.K.; Chan, R.Y.L.; Lam, K.O.; Wu, S.L.; Liu, X.M.; Chung, C.Y.; Chu, P.K.; Lu, W.W.; Chan, D.; Luk, K.D.K.; et al. In vitro and in vivo characterization of novel plasma treated nickel titanium shape memory alloy for orthopedic implantation. Surf. Coat. Technol. 2007, 202, 1247–1251. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schröder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef]
- Gomathi, N.; Neogi, S. Surface modification of polypropylene using argon plasma: Statistical optimization of the process variables. Appl. Surf. Sci. 2009, 255, 7590–7600. [Google Scholar] [CrossRef]
- Jee, H.J.; Kim, H.J.; Kim, A.J.; Bae, Y.S.; Bae, S.S.; Yun, J. UV light induces premature senescence in Akt1-null mouse embryonic fibroblasts by increasing intracellular levels of ROS. Biochem. Biophys. Res. Commun. 2009, 383, 358–362. [Google Scholar]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1257. [Google Scholar]
- Tang, K.; Zhan, J.C.; Yang, H.R.; Huang, W.D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef]
- Zhao, L.; Mei, S.; Chu, P.K.; Zhang, Y.; Wu, Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials 2010, 31, 5072–5082. [Google Scholar] [CrossRef]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef]
- Guo, J.; Padilla, R.J.; Ambrose, W.; De Kok, I.J.; Cooper, L.F. The effect of hydrofluoric acid treatment of TiO2 grit blasted titanium implants on adherent osteoblast gene expression in vitro and in vivo. Biomaterials 2007, 28, 5418–5425. [Google Scholar] [CrossRef]
- Isa, Z.M.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C.M. Effects of fluoride-modified titanium surfaces on osteoblast proliferation and gene expression. Int. J. Oral Maxillofac. Implant. 2006, 21, 203–211. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komasa, S.; Kusumoto, T.; Hayashi, R.; Takao, S.; Li, M.; Yan, S.; Zeng, Y.; Yang, Y.; Hu, H.; Kobayashi, Y.; et al. Effect of Argon-Based Atmospheric Pressure Plasma Treatment on Hard Tissue Formation on Titanium Surface. Int. J. Mol. Sci. 2021, 22, 7617. https://doi.org/10.3390/ijms22147617
Komasa S, Kusumoto T, Hayashi R, Takao S, Li M, Yan S, Zeng Y, Yang Y, Hu H, Kobayashi Y, et al. Effect of Argon-Based Atmospheric Pressure Plasma Treatment on Hard Tissue Formation on Titanium Surface. International Journal of Molecular Sciences. 2021; 22(14):7617. https://doi.org/10.3390/ijms22147617
Chicago/Turabian StyleKomasa, Satoshi, Tetsuji Kusumoto, Rina Hayashi, Seiji Takao, Min Li, Sifan Yan, Yuhao Zeng, Yuanyuan Yang, Hui Hu, Yasuyuki Kobayashi, and et al. 2021. "Effect of Argon-Based Atmospheric Pressure Plasma Treatment on Hard Tissue Formation on Titanium Surface" International Journal of Molecular Sciences 22, no. 14: 7617. https://doi.org/10.3390/ijms22147617





