Role of Dendritic Cell in Diabetic Nephropathy
Abstract
:1. Introduction
2. Sources and Functions of Kidney Dendritic Cells
2.1. General Dendritic Cells
2.2. Kidney Dendritic Cells-Types
2.3. Kidney Dendritic Cells-Roles
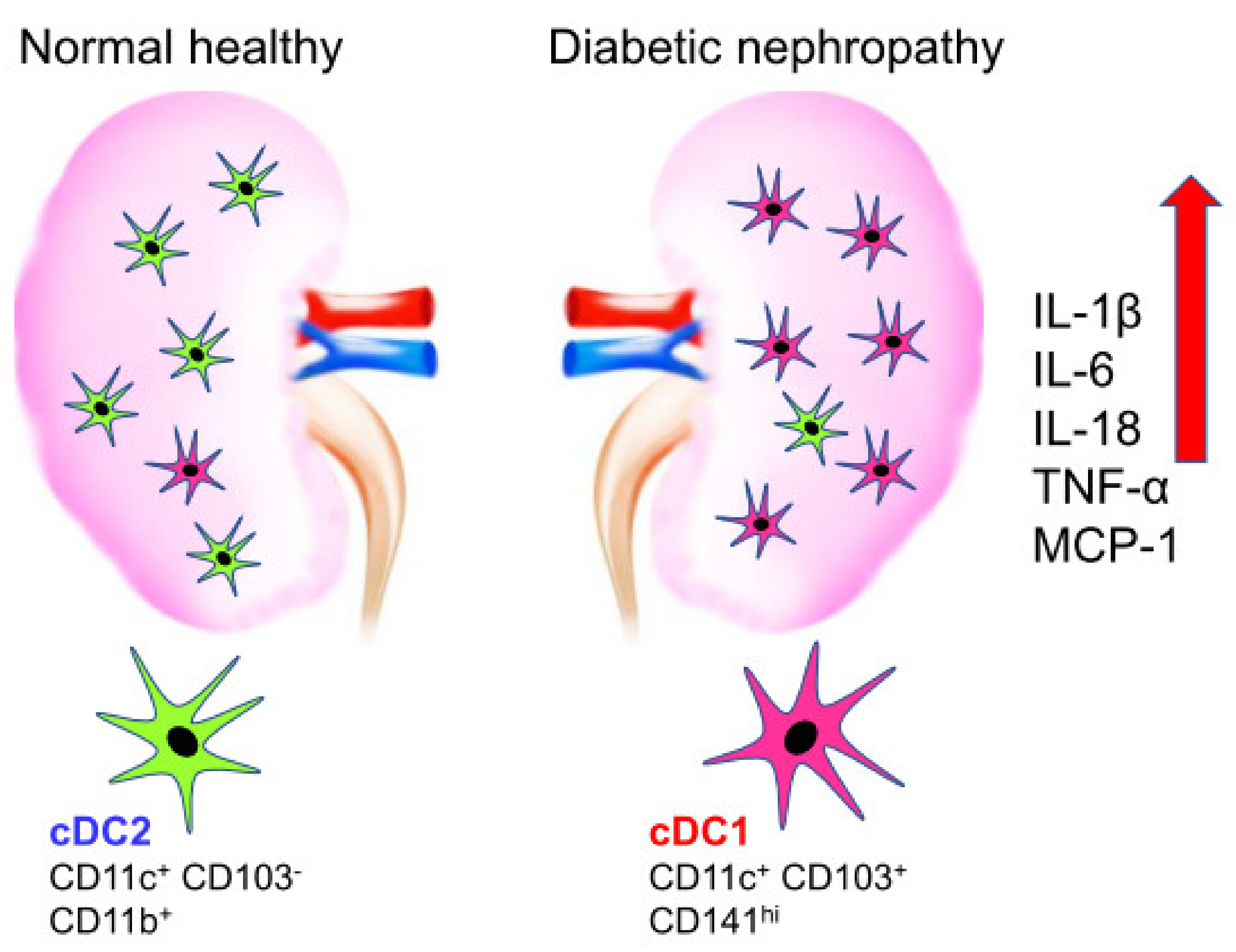
3. Pathologic Roles of Kidney Dendritic Cells in Diabetic Nephropathy
4. Mechanisms of Kidney Dendritic Cells Activation in Diabetic Nephropathy
5. Interventions of Dendritic Cell Function in Diabetic Nephropathy
5.1. Mesenchymal Stem Cells Transplantation
5.2. Fms-Like Tyrosine Kinase 3 Ligand Inhibition
5.3. Proinflammatory Cytokine Inhibition
5.4. Toll-Like Receptors Ligand’s Inhibition
5.5. Proteasome Inhibition
5.6. Scavenger Receptors Inhibition
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | Advanced Glycation End-product |
| AN | Adriamycin Nephropathy |
| Batf3 | Basic leucine zipper transcription factor ATF-like 3 |
| BDCA | Blood Dendritic Cell Antigen |
| CCR | C-C Chemokine Receptor |
| CD- | Cluster of Differentiation- |
| cDC | conventional Dendritic Cell |
| CKD | Chronic Kidney Disease |
| CLEC4A4 | C-type Lectin Domain Family 4 Member A4 |
| CLEC9A | C-type Lectin Domain Family 9 Member A |
| CSF-1 | Colony Stimulating Factor-1 |
| CTLs | Cytotoxic T cells |
| CX3CR | CX3C-Chemokine Receptor |
| DAMPs | Damage-Associated Molecular Patterns |
| DC | Dendritic Cell |
| DCIR2 | Dendritic Cell Inhibitory Receptor 2 |
| DM | Diabetes Mellitus |
| DN | Diabetic Nephropathy |
| Flt3 | Fms-Like Tyrosine Kinase 3 |
| FSGS | Focal Segmental Glomerulosclerosis |
| HMGB-1 | High-Mobility Group Protein Box-1 |
| IL- | Interleukin- |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MHC | Major Histocompatibility Complex |
| MSC | Mesenchymal Stem Cell |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PDCA-1 | Plasmacytoid Dendritic Cell Antigen-1 |
| TGF-β | Transforming Growth Factor-beta |
| TLR | Toll-Like Receptor |
| TNF-α | Tumor Necrosis Factor-alpha |
| XCR1 | XC-Chemokine Receptor 1 |
References
- Chen, Y.; Lee, K.; Ni, Z.; He, J.C. Diabetic Kidney Disease: Challenges, Advances, and Opportunities. Kidney Dis. 2020, 6, 215–225. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Schena, F.P.; Gesualdo, L. Pathogenetic Mechanisms of Diabetic Nephropathy. J. Am. Soc. Nephrol. 2005, 16 (Suppl. S1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Coates, P.T.H.; Colvin, B.L.; Ranganathan, A.; Duncan, F.; Lan, Y.; Shufesky, W.J.; Zahorchak, A.F.; Morelli, A.E.; Thomson, A.W. CCR and CC chemokine expression in relation to Flt3 ligand-induced renal dendritic cell mobilization. Kidney Int. 2004, 66, 1907–1917. [Google Scholar] [CrossRef] [Green Version]
- Sakai, N.; Wada, T.; Furuichi, K.; Iwata, Y.; Yoshimoto, K.; Kitagawa, K.; Kokubo, S.; Kobayashi, M.; Hara, A.; Yamahana, J.; et al. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am. J. Kidney Dis. 2005, 45, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.F.; Milena, F.J.; Mora, C.; León, C.; García, J. Renal Pro-Inflammatory Cytokine Gene Expression in Diabetic Nephropathy: Effect of Angiotensin-Converting Enzyme Inhibition and Pentoxifylline Administration. Am. J. Nephrol. 2006, 26, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ma, B.; Dong, B.; Zhao, P.; Tai, N.; Chen, L.; Wong, F.S.; Wen, L. Cellular and humoral immune responses in the early stages of diabetic nephropathy in NOD mice. J. Autoimmun. 2009, 32, 85–93. [Google Scholar] [CrossRef]
- Lin, M.; Yiu, W.H.; Wu, H.J.; Chan, L.Y.; Leung, J.C.; Au, W.S.; Chan, K.W.; Lai, K.N.; Tang, S.C. Toll-Like Receptor 4 Promotes Tubular Inflammation in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2011, 23, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.-Y.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G.; Lim, S.J.; Lee, S.-H. Aberrant Recruitment and Activation of T Cells in Diabetic Nephropathy. Am. J. Nephrol. 2012, 35, 164–174. [Google Scholar] [CrossRef]
- Verzola, D.; Cappuccino, L.; D’Amato, E.; Villaggio, B.; Gianiorio, F.; Mij, M.; Simonato, A.; Viazzi, F.; Salvidio, G.; Garibotto, G. Enhanced glomerular Toll-like receptor 4 expression and signaling in patients with type 2 diabetic nephropathy and microalbuminuria. Kidney Int. 2014, 86, 1229–1243. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.; Bock, F.; Dong, W.; Wang, H.; Kopf, S.; Kohli, S.; Al-Dabet, M.M.; Ranjan, S.; Wolter, J.; Wacker, C.; et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 2015, 87, 74–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.; Ping, F.; Mu, W.; Hill, P.; Atkins, R.C.; Chadban, S.J. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology 2006, 11, 226–231. [Google Scholar] [CrossRef]
- Klessens, C.Q.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Rabelink, T.; Bajema, I.M.; Ijpelaar, D. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2016, 32, 1322–1329. [Google Scholar] [CrossRef]
- Lampropoulou, I.T.; Stangou, Μ.; Sarafidis, P.; Gouliovaki, A.; Giamalis, P.; Tsouchnikas, I.; Didangelos, T.; Papagianni, A. TNF-α pathway and T-cell immunity are activated early during the development of diabetic nephropathy in Type II Diabetes Mellitus. Clin. Immunol. 2020, 215, 108423. [Google Scholar] [CrossRef]
- Lu, H.; Yao, K.; Huang, D.; Sun, A.; Zou, Y.; Qian, J.; Ge, J. High glucose induces upregulation of scavenger receptors and promotes maturation of dendritic cells. Cardiovasc. Diabetol. 2013, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Yang, T.; Wang, M. Dioscin attenuates oxLDL uptake and the inflammatory reaction of dendritic cells under high glucose conditions by blocking p38 MAPK. Mol. Med. Rep. 2020, 21, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Pichler, R.; Afkarian, M.; Dieter, B.; Tuttle, K. Immunity and inflammation in diabetic kidney disease: Translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Ren. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heymann, F.; Meyer-Schwesinger, C.; Hamilton-Williams, E.; Hammerich, L.; Panzer, U.; Kaden, S.; Quaggin, S.E.; Floege, J.; Gröne, H.-J.; Kurts, C. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Investig. 2009, 119, 1286–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, C.; Cao, X. Dendritic cells in the regulation of immunity and inflammation. Semin. Immunol. 2018, 35, 3–11. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 2009, 10, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Güttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Jongbloed, S.L.; Kassianos, A.; McDonald, K.J.; Clark, G.; Ju, X.; Angel, C.; Chen, C.-J.J.; Dunbar, R.; Wadley, R.B.; Jeet, V.; et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010, 207, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.-N.; Malinarich, F.; Malleret, B.; et al. Human Tissues Contain CD141hi Cross-Presenting Dendritic Cells with Functional Homology to Mouse CD103+ Nonlymphoid Dendritic Cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassianos, A.; Wang, X.; Sampangi, S.; Afrin, S.; Wilkinson, R.; Healy, H. Fractalkine–CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro–activated proximal tubular epithelial cells. Kidney Int. 2015, 87, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-Y.; Wang, C.-Y.; Lin, C.-C.; Chu, C.-L. A recently described type 2 conventional dendritic cell (cDC2) subset mediates inflammation. Cell. Mol. Immunol. 2020, 17, 1215–1217. [Google Scholar] [CrossRef]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef] [Green Version]
- Colonna, M.; Trinchieri, G.; Liu, Y.J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004, 5, 1219–1226. [Google Scholar] [CrossRef]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.J.; Rees, A.J.; Griffin, M.; Hughes, J.; Kurts, C.; Duffield, J. The Renal Mononuclear Phagocytic System. J. Am. Soc. Nephrol. 2011, 23, 194–203. [Google Scholar] [CrossRef]
- Rogers, N.M.; Ferenbach, D.; Isenberg, J.S.; Thomson, A.W.; Hughes, J. Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef]
- Woltman, A.; de Fijter, J.; Zuidwijk, K.; Vlug, A.; Bajema, I.; van der Kooij, S.; van Ham, V.; van Kooten, C. Quantification of dendritic cell subsets in human renal tissue under normal and pathological conditions. Kidney Int. 2007, 71, 1001–1008. [Google Scholar] [CrossRef] [Green Version]
- Kitching, A.R.; Ooi, J.D. Renal Dendritic Cells: The Long and Winding Road. J. Am. Soc. Nephrol. 2017, 29, 4–7. [Google Scholar] [CrossRef]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Lu, J.; Li, Q.; Wang, C.; Wang, X.M.; Lee, V.; Wang, C.; Nguyen, H.; Zheng, G.; Zhao, Y.; et al. CD103+ Dendritic Cells Elicit CD8+ T Cell Responses to Accelerate Kidney Injury in Adriamycin Nephropathy. J. Am. Soc. Nephrol. 2015, 27, 1344–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassianos, A.; Wang, X.; Sampangi, S.; Muczynski, K.; Healy, H.; Wilkinson, R. Increased tubulointerstitial recruitment of human CD141hi CLEC9A+ and CD1c+ myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am. J. Physiol.-Ren. Physiol. 2013, 305, F1391–F1401. [Google Scholar] [CrossRef] [Green Version]
- Steinman, R.M.; Idoyaga, J. Features of the dendritic cell lineage. Immunol. Rev. 2010, 234, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, L.; Sung, S.S.; Vergis, A.L.; Rosin, D.L.; Rose, C.E., Jr.; Lobo, P.I.; Okusa, M.D. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacs-Kornek, V.; Burgdorf, S.; Diehl, L.; Specht, S.; Kornek, M.; Kurts, C. The kidney-renal lymph node-system contributes to cross-tolerance against innocuous circulating antigen. J. Immunol. 2008, 180, 706–715. [Google Scholar] [CrossRef] [Green Version]
- Gottschalk, C.; Damuzzo, V.; Gotot, J.; Kroczek, R.A.; Yagita, H.; Murphy, K.M.; Knolle, P.A.; Ludwig-Portugall, I.; Kurts, C. Batf3-Dependent Dendritic Cells in the Renal Lymph Node Induce Tolerance against Circulating Antigens. J. Am. Soc. Nephrol. 2013, 24, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Scholz, J.; Lukacs-Kornek, V.; Engel, D.R.; Specht, S.; Kiss, E.; Eitner, F.; Floege, J.; Groene, H.-J.; Kurts, C. Renal Dendritic Cells Stimulate IL-10 Production and Attenuate Nephrotoxic Nephritis. J. Am. Soc. Nephrol. 2008, 19, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Tittel, A.P.; Heuser, C.; Ohliger, C.; Knolle, P.A.; Engel, D.R.; Kurts, C. Kidney Dendritic Cells Induce Innate Immunity against Bacterial Pyelonephritis. J. Am. Soc. Nephrol. 2011, 22, 1435–1441. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Muller, D.N.; Shagdarsuren, E.; Park, J.-K.; Dechend, R.; Mervaala, E.; Hampich, F.; Fiebeler, A.; Ju, X.; Finckenberg, P.; Theuer, J.; et al. Immunosuppressive Treatment Protects Against Angiotensin II-Induced Renal Damage. Am. J. Pathol. 2002, 161, 1679–1693. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.W.; Li, C.; Ahn, K.O.; Kim, J.; Moon, I.S.; Ahn, C.; Lee, J.R.; Yang, C.W. Cyclosporine-Induced Renal Injury Induces Toll-like Receptor and Maturation of Dendritic cells. Transplantation 2005, 80, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhou, T.; Sun, G.; Wang, W.; Zhang, Y.; Zhang, Y.; Hao, L.; Chen, N. Valsartan inhibited the accumulation of dendritic cells in rat fibrotic renal tissue. Cell. Mol. Immunol. 2006, 3, 213–220. [Google Scholar] [PubMed]
- Barbaro, N.; Foss, J.D.; Kryshtal, D.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Chen, T.; Wang, C.; Zhang, Z.; Wang, X.M.; Li, Q.; Lee, V.W.S.; Wang, Y.M.; Zheng, G.; Alexander, S.I.; et al. Flt3 inhibition alleviates chronic kidney disease by suppressing CD103+ dendritic cell-mediated T cell activation. Nephrol. Dial. Transplant. 2018, 34, 1853–1863. [Google Scholar] [CrossRef]
- Wardowska, A.; Komorniczak, M.; Bullo-Piontecka, B.; Dȩbska-Ślizień, M.A.; Pikuła, M. Transcriptomic and Epigenetic Alterations in Dendritic Cells Correspond With Chronic Kidney Disease in Lupus Nephritis. Front. Immunol. 2019, 10, 2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochheiser, K.; Heuser, C.; Krause, T.A.; Teteris, S.; Ilias, A.; Weisheit, C.; Hoss, F.; Tittel, A.P.; Knolle, P.A.; Panzer, U.; et al. Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J. Clin. Investig. 2013, 123, 4242–4254. [Google Scholar] [CrossRef]
- Tu, Y.; Jia, R.; Ding, G.; Chen, L. Effect of atorvastatin on dendritic cells of tubulointerstitium in diabetic rats. BMB Rep. 2010, 43, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, C.; Wen, X.; Chen, Y.; Mao, R.; Cui, D.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; et al. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103+DCs-mediated CD8+T cell responses. J. Cell. Mol. Med. 2020, 24, 5817–5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, C.L.; Sharp, P.S.; North, M.E.; Rainbow, S.J.; Knight, S.C. Advanced glycation end products modulate the maturation and function of peripheral blood dendritic cells. Diabetes 2004, 53, 1452–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, J.; Jia, Q.; Liang, C.; Luo, Y.; Huang, N.; Sun, A.; Wang, K.; Zou, Y.; Chen, H. Advanced Glycosylation End Products Might Promote Atherosclerosis Through Inducing the Immune Maturation of Dendritic Cells. Arter. Thromb. Vasc. Biol. 2005, 25, 2157–2163. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, R.; Yan, S.F.; Herold, K.; Clynes, R.; Schmidt, A.M. Receptor for advanced glycation end products: Fundamental roles in the inflammatory response: Winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann. N. Y. Acad. Sci. 2008, 1126, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrada, A.A.; Contreras, F.J.; Marini, N.P.; Amador, C.; Gonzalez, P.A.; Cortés, C.M.; Riedel, C.; Carvajal, C.; Figueroa, F.E.; Michea, L.F.; et al. Aldosterone Promotes Autoimmune Damage by Enhancing Th17-Mediated Immunity. J. Immunol. 2009, 184, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Chen, C.; Liu, Y.; Tian, C.; Li, H.H. Angiotensin II Regulates Dendritic Cells through Activation of NF-κB/p65, ERK1/2 and STAT1 Pathways. Cell. Physiol. Biochem. 2017, 42, 1550–1558. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, D.; Miyazaki, M.; Naka, R.; Koji, T.; Yagame, M.; Jinde, K.; Endoh, M.; Nomoto, Y.; Sakai, H. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 1995, 44, 1233–1238. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora-Fernández, C. The Role of Inflammatory Cytokines in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Macconi, D.; Chiabrando, C.; Schiarea, S.; Aiello, S.; Cassis, L.; Gagliardini, E.; Noris, M.; Buelli, S.; Zoja, C.; Corna, D.; et al. Proteasomal Processing of Albumin by Renal Dendritic Cells Generates Antigenic Peptides. J. Am. Soc. Nephrol. 2008, 20, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Kuppner, M.C.; Gastpar, R.; Gelwer, S.; Nössner, E.; Ochmann, O.; Scharner, A.; Issels, R.D. The role of heat shock protein (hsp70) in dendritic cell maturation: Hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur. J. Immunol. 2001, 31, 1602–1609. [Google Scholar] [CrossRef]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Säemann, M.D.; Weichhart, T.; Zeyda, M.; Staffler, G.; Schunn, M.; Stuhlmeier, K.M.; Sobanov, Y.; Stulnig, T.; Akira, S.; Von Gabain, A.; et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4–dependent mechanism. J. Clin. Investig. 2005, 115, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, D.; Wang, S.; Wang, M.; Lu, W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-κB signaling pathway. Mol. Med. Rep. 2018, 18, 3625–3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, B.; Li, Y.; Liu, Y.; Xu, Z. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J. Cell. Physiol. 2019, 234, 21249–21259. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Joffre, O.P.; Keller, A.M.; Rogers, N.C.; Martínez, D.; Hernanz-Falcón, P.; Rosewell, I.; Reis e Sousa, C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature 2009, 458, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-G.; Czabotar, P.E.; Policheni, A.N.; Caminschi, I.; Wan, S.S.; Kitsoulis, S.; Tullett, K.M.; Robin, A.Y.; Brammananth, R.; van Delft, M.F.; et al. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity 2012, 36, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Iyoda, T.; Shimoyama, S.; Liu, K.; Omatsu, Y.; Akiyama, Y.; Maeda, Y.; Takahara, K.; Steinman, R.M.; Inaba, K. The CD8+ Dendritic Cell Subset Selectively Endocytoses Dying Cells in Culture and In Vivo. J. Exp. Med. 2002, 195, 1289–1302. [Google Scholar] [CrossRef]
- Schulz, O.; Reis e Sousa, C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology 2002, 107, 183–189. [Google Scholar] [CrossRef]
- Djouad, F.; Charbonnier, L.-M.; Bouffi, C.; Louis-Plence, P.; Bony, C.; Apparailly, F.; Cantos, C.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cells Inhibit the Differentiation of Dendritic Cells Through an Interleukin-6-Dependent Mechanism. Stem Cells 2007, 25, 2025–2032. [Google Scholar] [CrossRef]
- Ramasamy, R.; Fazekasova, H.; Lam, E.; Soeiro, I.; Lombardi, G.; Dazzi, F. Mesenchymal Stem Cells Inhibit Dendritic Cell Differentiation and Function by Preventing Entry Into the Cell Cycle. Transplantation 2007, 83, 71–76. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, Z.; Zhang, R.; Yan, K.; Chen, L.; Chen, F.; Huang, W.; Lv, B.; Sun, C.; Jiang, X. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochem. Biophys. Res. Commun. 2014, 450, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, C.; Cao, F.; Lui, H.; Xia, B.; Wang, L. Bone marrow mesenchymal stem cells inhibit dendritic cells differentiation and maturation by microRNA-23b. Biosci. Rep. 2017, 37, 20160436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Chen, X.; Shao, H.; Bai, L.; Li, X.; Zhang, X. Mesenchymal Stem Cells Inhibited Dendritic Cells Via the Regulation of STAT1 and STAT6 Phosphorylation in Experimental Autoimmune Uveitis. Curr. Mol. Med. 2018, 17, 478–487. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liao, G.; Zhang, J.; Chen, Y.; Li, L.; Li, L.; Liu, F.; Chen, B.; Guo, G.; et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int. J. Mol. Med. 2018, 41, 2629–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Yuan, Y.; Liao, G.; Li, L.; Liu, J.; Chen, Y.; Zhang, J.; Cheng, J.; Lu, Y. Mesenchymal stem cells ameliorate hyperglycemia-induced endothelial injury through modulation of mitophagy. Cell Death Dis. 2018, 9, 837. [Google Scholar] [CrossRef]
- Kingston, D.; Schmid, M.A.; Onai, N.; Obata-Onai, A.; Baumjohann, D.; Manz, M. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood 2009, 114, 835–843. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, R.; Huang, B.; Hou, J.; Yin, J.; Zhao, T.; Zhou, L.; Wu, R.; Qian, Y.; Wang, F. Tumor necrosis factor-α blockade ameliorates diabetic nephropathy in rats. Clin. Kidney J. 2021, 14, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Balasubramaniam, G.; Almond, M.; Dasgupta, B. Improved renal function in diabetic patients with acute gout treated with anakinra. Kidney Int. 2015, 88, 195–196. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, R.; Chen, J.; Shi, M.; Li, W.; Zhang, X. High Mobility Group Box1 Inhibitor Glycyrrhizic Acid Attenuates Kidney Injury in Streptozotocin-Induced Diabetic Rats. Kidney Blood Press. Res. 2017, 42, 894–904. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, J.; Kwan, T.; Stribos, E.G.D.; Messchendorp, A.L.; Loh, Y.W.; Wang, X.; Paul, M.; Cunningham, E.C.; Habib, M.; et al. Blockade of HMGB1 Attenuates Diabetic Nephropathy in Mice. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Gramm, C.; Rothstein, L.; Clark, K.; Stein, R.; Dick, L.; Hwang, D.; Goldberg, A.L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994, 78, 761–771. [Google Scholar] [CrossRef]
- Luo, Z.-F.; Qi, W.; Feng, B.; Mu, J.; Zeng, W.; Guo, Y.-H.; Pang, Q.; Ye, Z.-L.; Liu, L.; Yuan, F.-H. Prevention of diabetic nephropathy in rats through enhanced renal antioxidative capacity by inhibition of the proteasome. Life Sci. 2011, 88, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, C.; Nan, Q.; Gao, C.; Feng, H.; Gou, F.; Chen, G.; Zhang, Z.; Yan, P.; Peng, J.; et al. The proteasome inhibitor, MG132, attenuates diabetic nephropathy by inhibiting SnoN degradation in vivo and in vitro. Biomed. Res. Int. 2014, 2014, 684765. [Google Scholar] [CrossRef]
- Zeng, W.; Qi, W.; Mu, J.; Wei, Y.; Yang, L.-L.; Zhang, Q.; Wu, Q.; Tang, J.-Y.; Feng, B. MG132 protects against renal dysfunction by regulating Akt-mediated inflammation in diabetic nephropathy. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
| Models | Results | Reference |
|---|---|---|
| Non-obese diabetic mice | CD11c+ DCs have close contact with CD4+ and CD8+ T cells in the glomeruli. | [7] |
| NOH transgenic mice | DCs in renal lymph nodes constitutively cross-present ovalbumin and activate CD8+ T cells | [18] |
| Human cord blood derived DCs | Angiotensin II promotes the maturation and infiltration in the renal interstitial of DCs via TNF-α leading to albuminuria and renal fibrosis. | [45] |
| Rat remnant kidneys induced by subtotal nephrectomy | The accumulation extent of DCs is associated with the loss of renal function and the progression of tubulointerstitial fibrosis. | [47] |
| Streptozotocin-induced diabetic rat (1) | CD1a+ CD80+ DCs accumulation in the renal tissue and the extent of DCs accumulation are closely correlated with the degree of renal tubulointerstitial injury. | [52] |
| Streptozotocin-induced diabetic rat (2) | CD11c+ CD103+ DC subsets increase, express more costimulatory molecules, and enhance capacity of priming CD8+ T cell responses | [53] |
| Rat proximal tubular cells | Albumin is a source of antigenic peptides and trigger CD8+ T cells after being transferred to and processed by DCs through a proteasome-dependent pathway. | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, M.; Lee, H.-Y.; Park, H.-Y.; Jhun, H.; Kim, S. Role of Dendritic Cell in Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7554. https://doi.org/10.3390/ijms22147554
Kim H, Kim M, Lee H-Y, Park H-Y, Jhun H, Kim S. Role of Dendritic Cell in Diabetic Nephropathy. International Journal of Molecular Sciences. 2021; 22(14):7554. https://doi.org/10.3390/ijms22147554
Chicago/Turabian StyleKim, Hyunwoo, Miyeon Kim, Hwa-Young Lee, Ho-Young Park, Hyunjhung Jhun, and Soohyun Kim. 2021. "Role of Dendritic Cell in Diabetic Nephropathy" International Journal of Molecular Sciences 22, no. 14: 7554. https://doi.org/10.3390/ijms22147554






