Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves
Abstract
:1. Introduction
2. Results
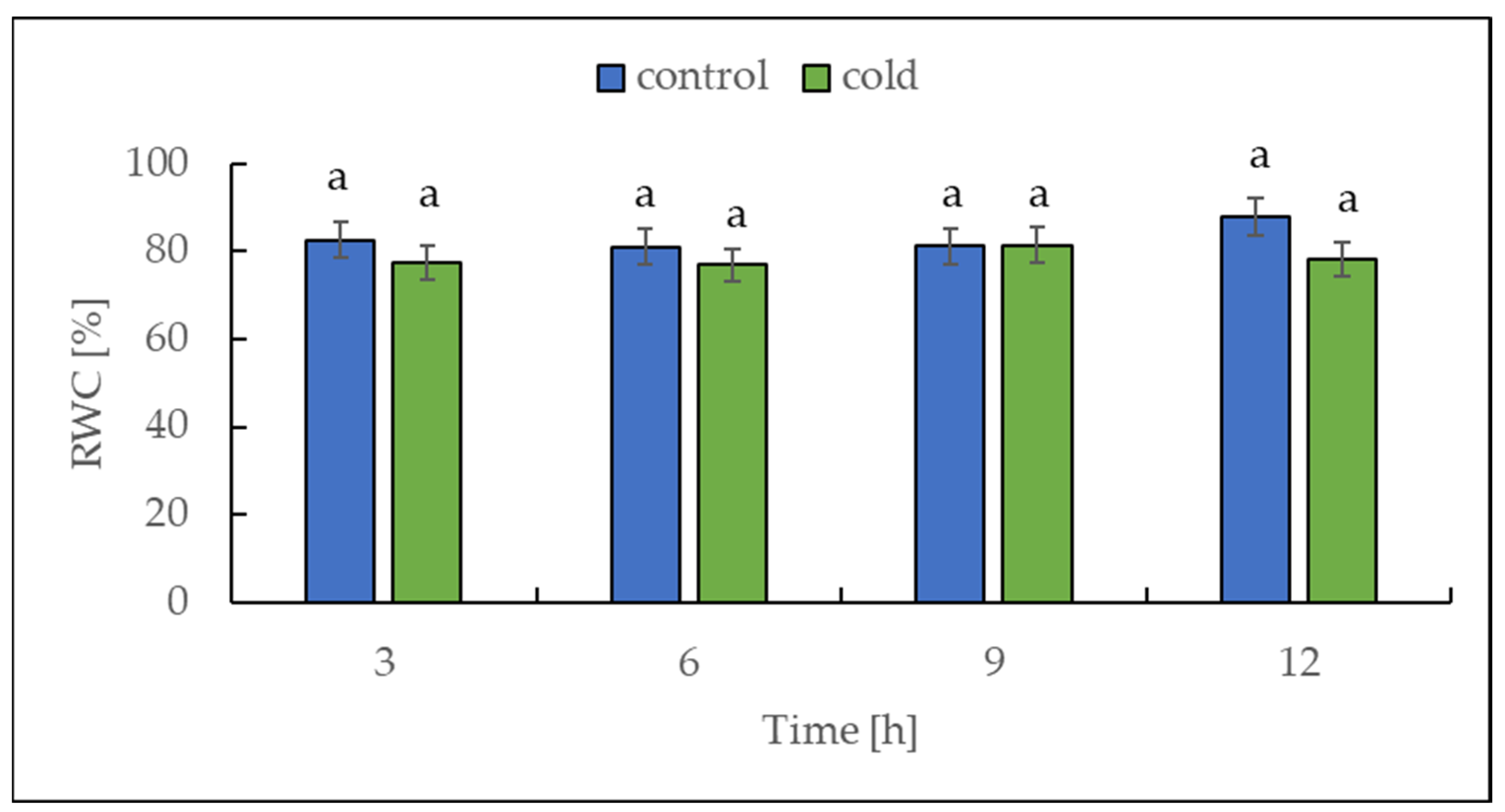
2.1. The Relative Water Content under Cold Treatment
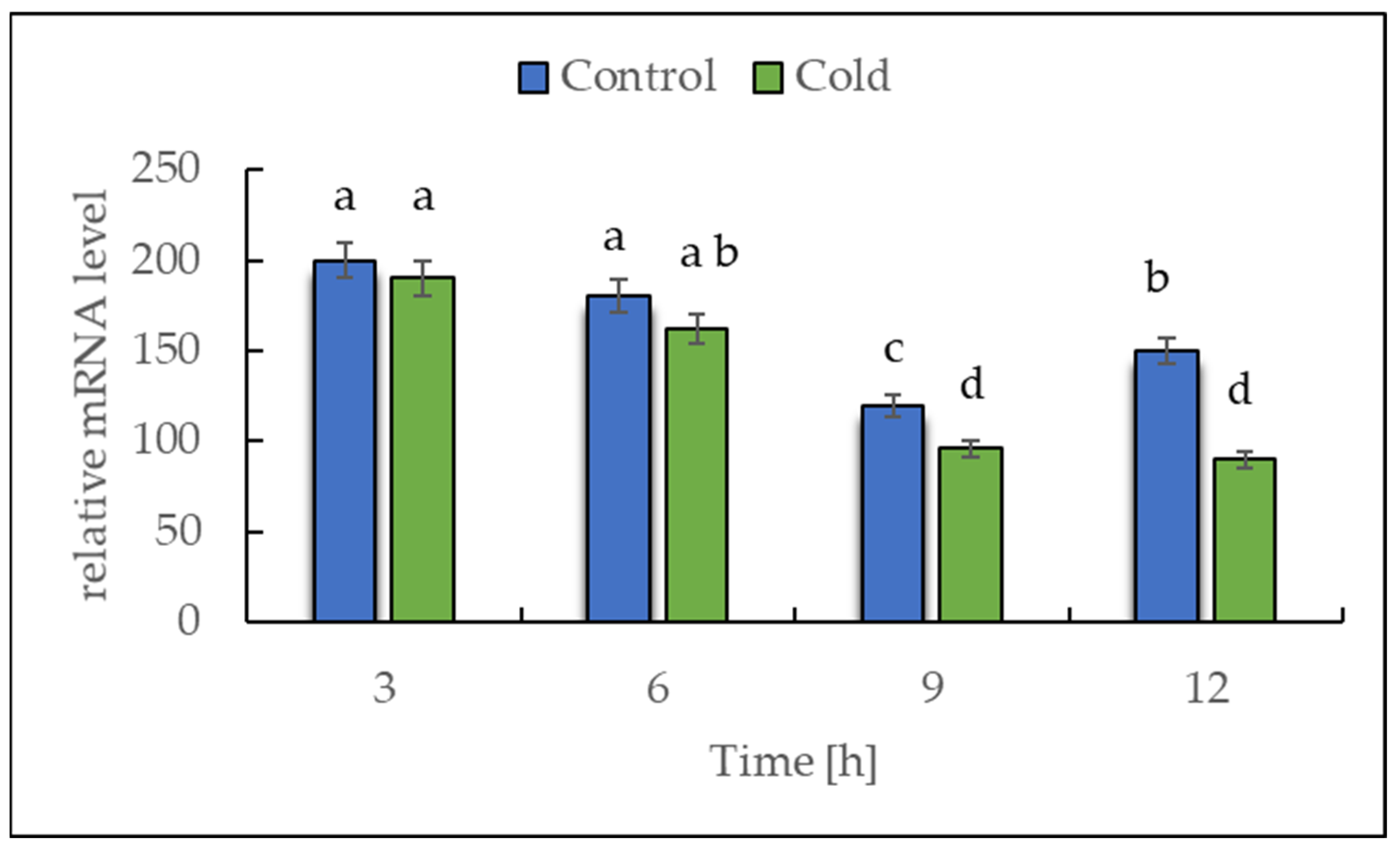
2.2. Expression of NADH Dehydrogenase in Cold-Stressed Plants
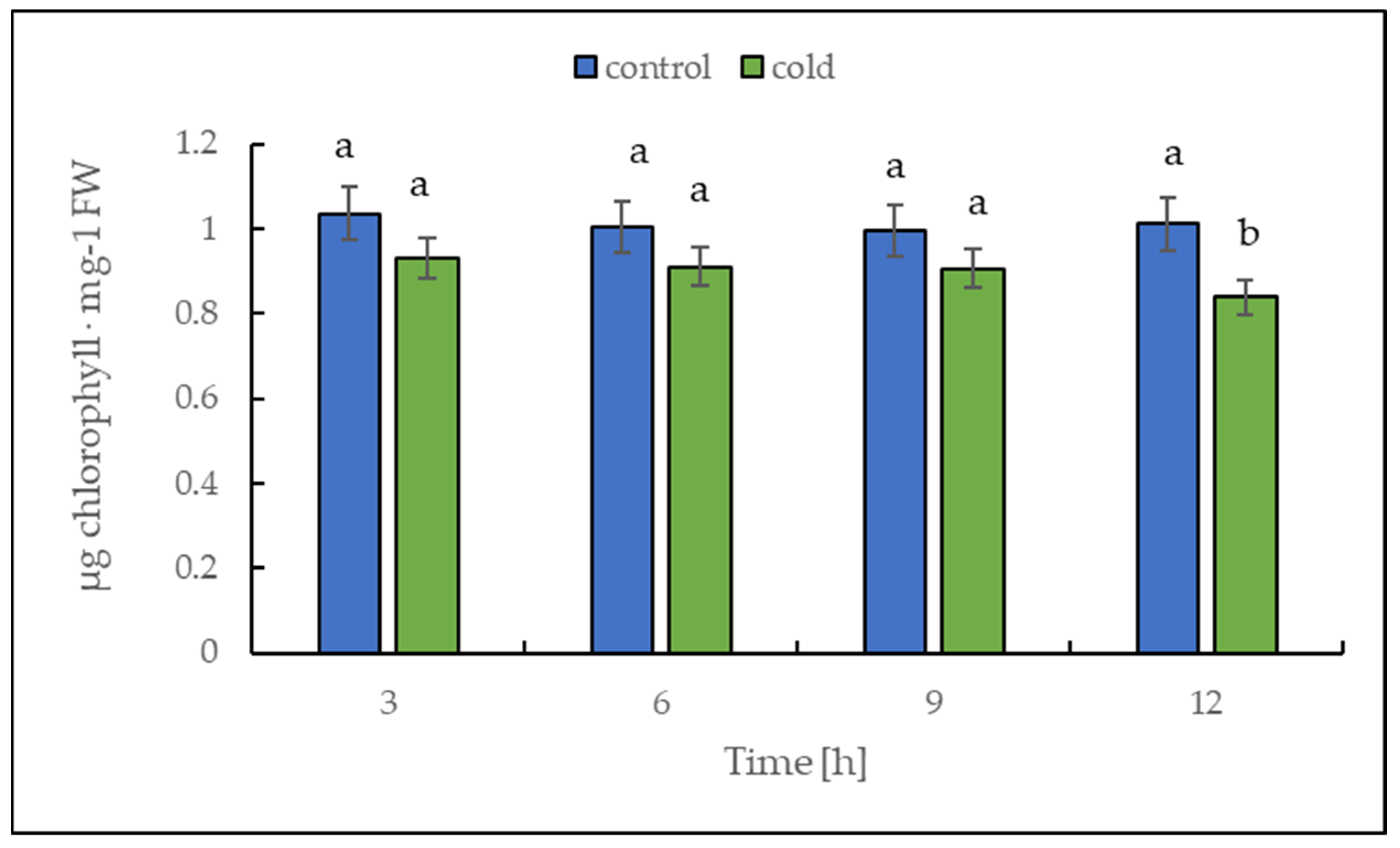
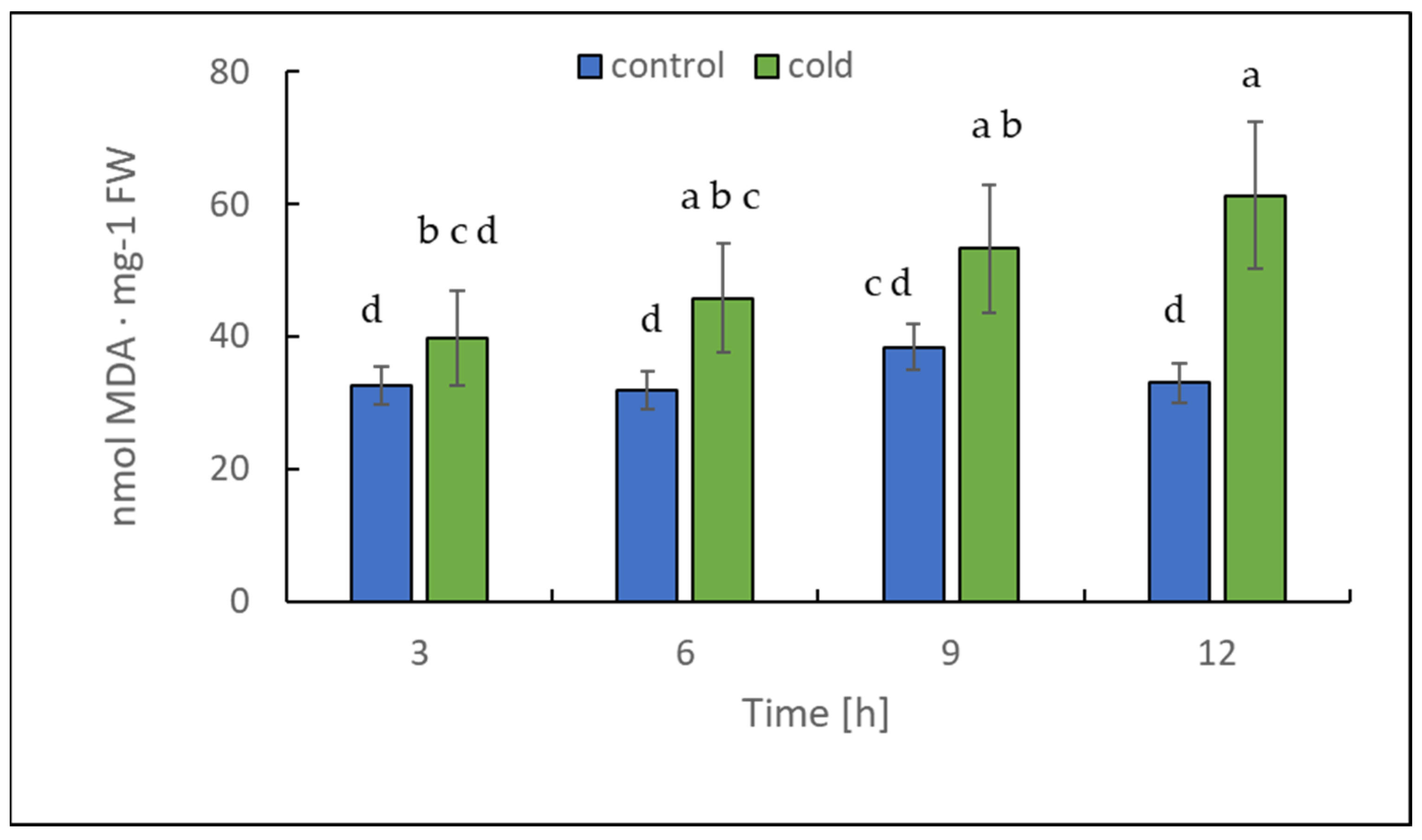
2.3. Malondialdehyde and Chlorophyll Content under Cold Treatment
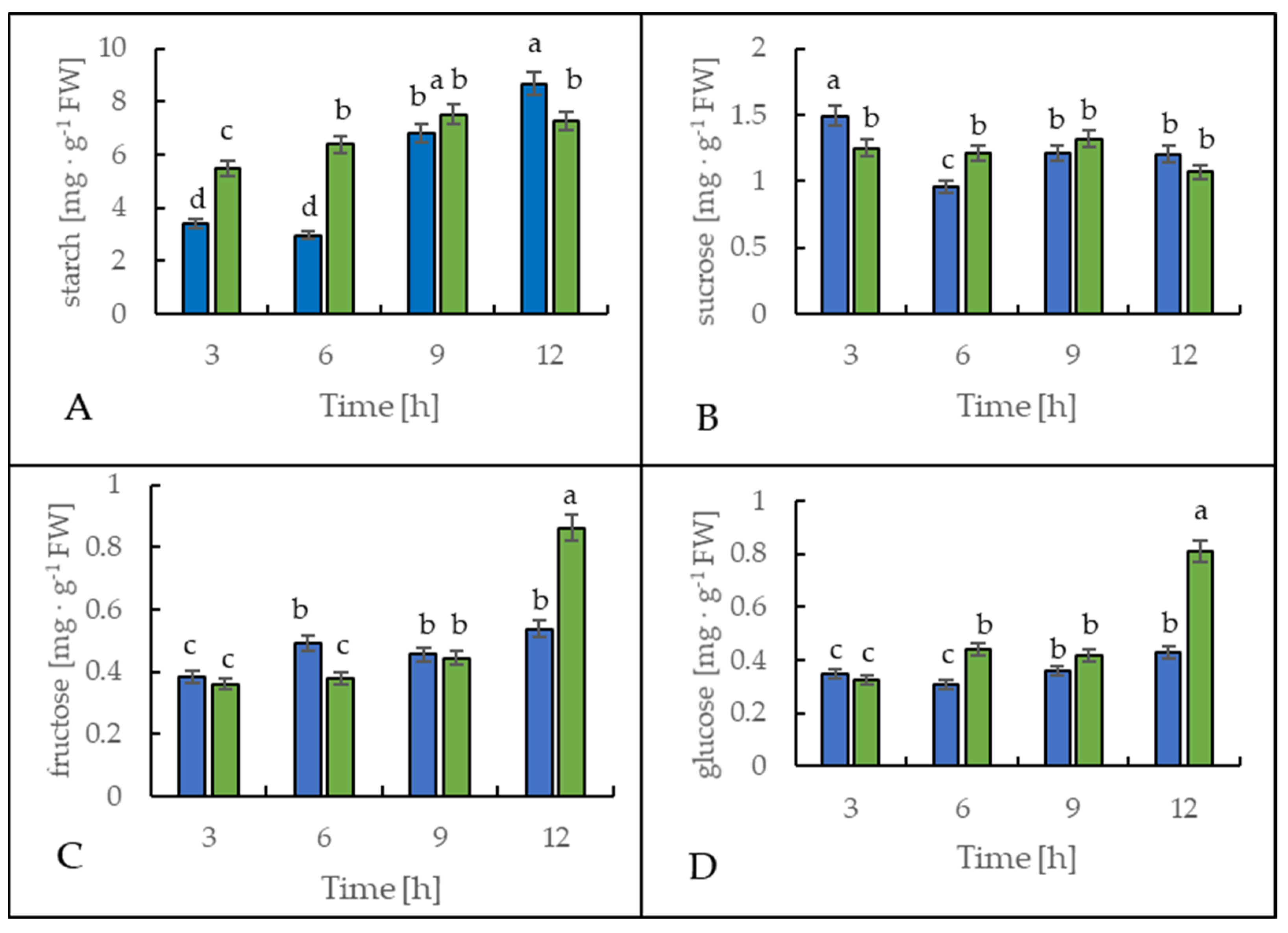
2.4. Soluble Sugar Content under Cold Treatment
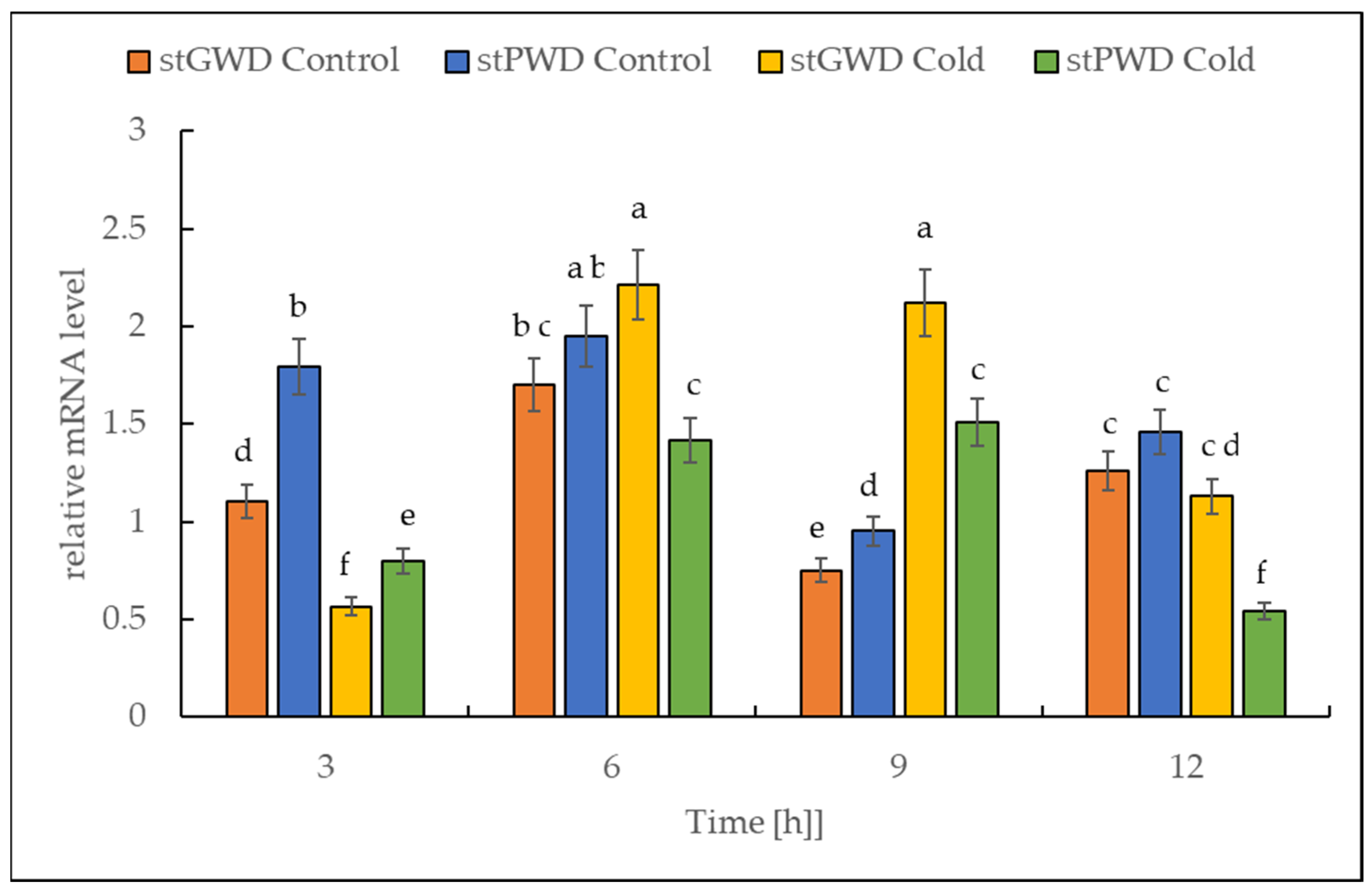
2.5. Expression of Both StGWD and StPWD under Cold Stress
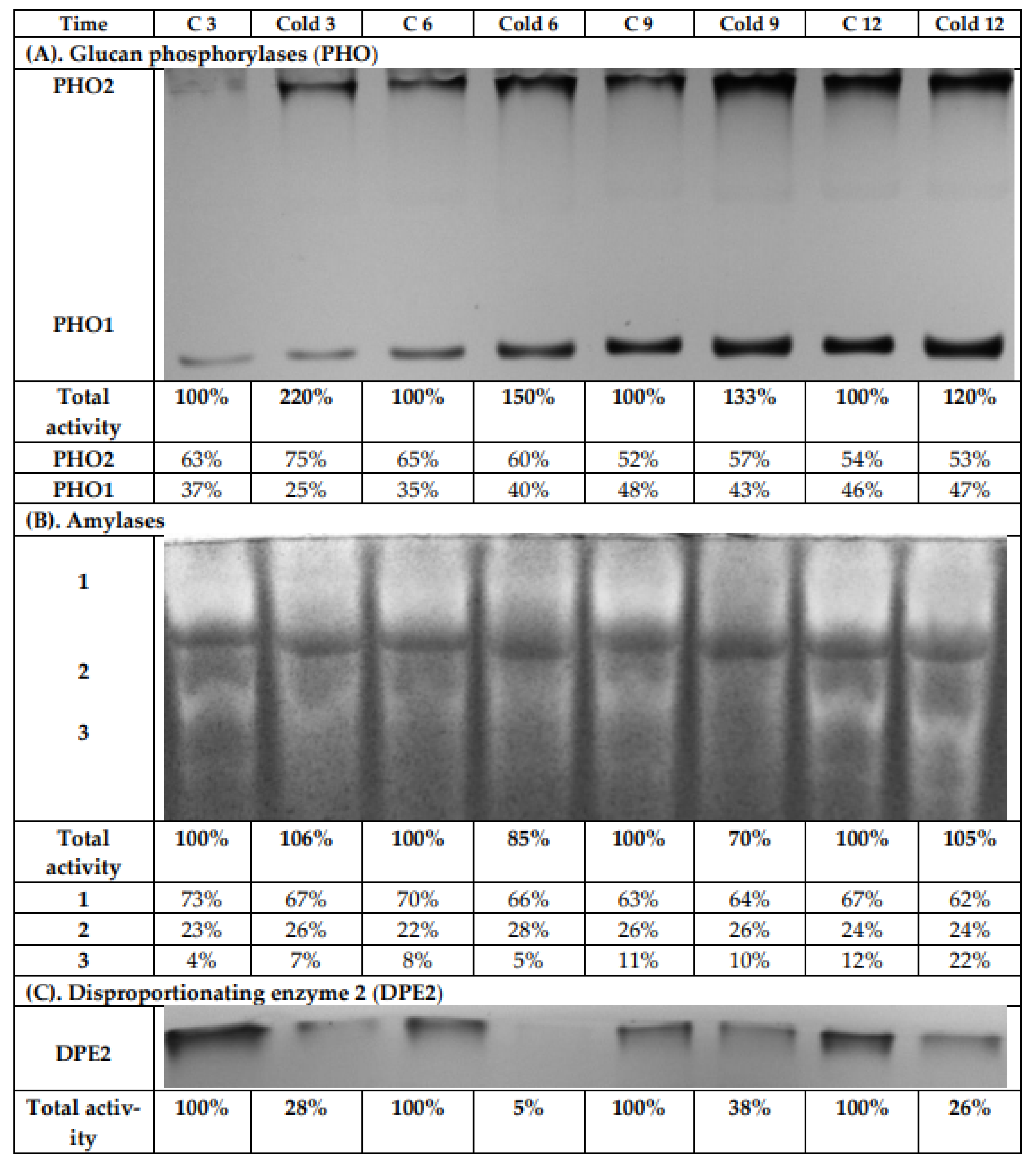
2.6. Glucan Phosphorylases, Amylases, and the Disproportionating Enzyme 2 Activities under Cold Treatment
2.7. The Acid Invertase Activity under Cold Treatment
3. Discussion
4. Materials and Methods
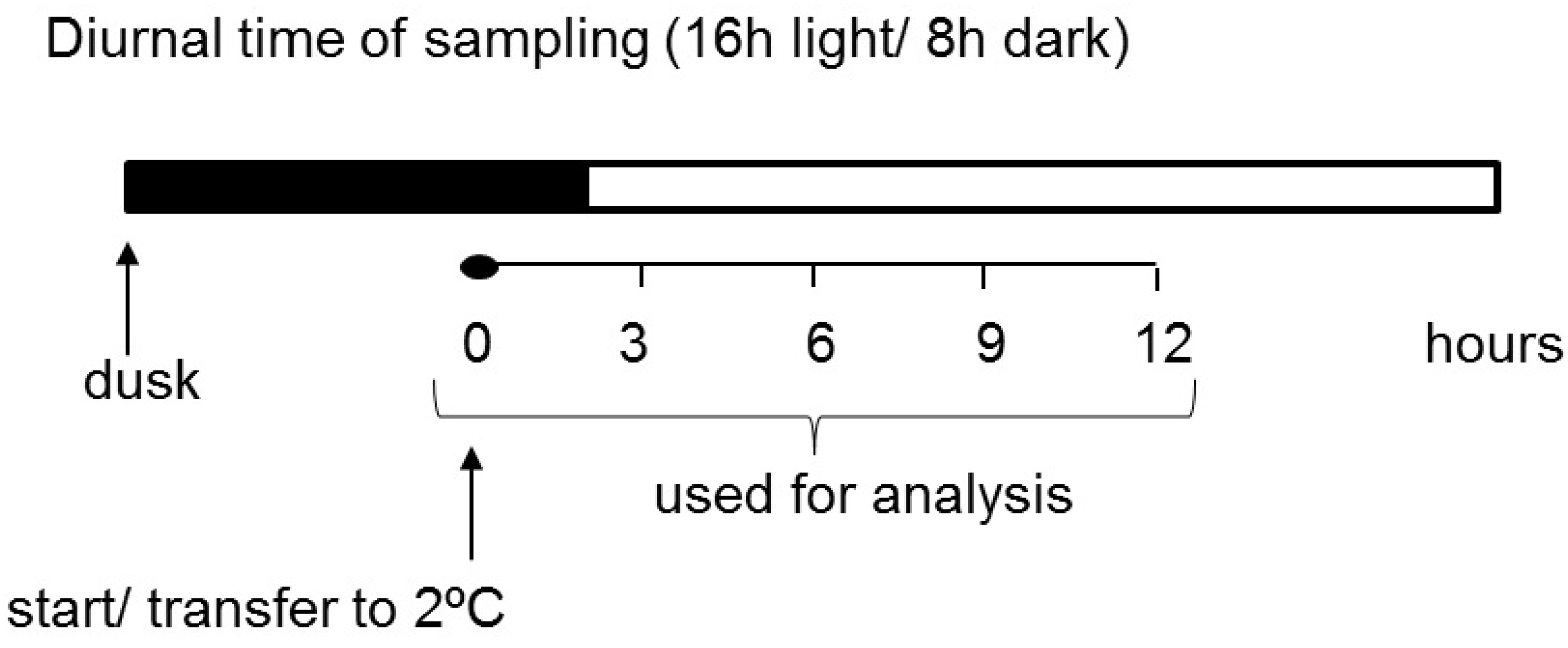
4.1. Plant Material and Cold Treatment
4.2. Relative Water Content (RWC)
4.3. RNA Extraction
4.4. Two-Step Real-Time PCR
4.5. Carbohydrate Analysis
4.6. Chloroplast Isolation
4.7. Leaf Crude Extract Preparation
4.8. Determination of Chlorophyll, Malondialdehyde, and Protein Content
4.9. Amylase, Glucan Phosphorylase, and Disproportioning Enzyme 2 Zymography
4.10. Measurement of Acid Invertase Activity
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAM | Beta-amylase |
| DPE | Disproportionating enzyme |
| GWD | Glucan, water dikinase |
| PHO | Glucan phosphorylase |
| NDA1 | Homolog of NADH dehydrogenase |
| MDA | Malondialdehyde |
| PWD | Phosphoglucan, water dikinase |
References
- Huner, N.P.A.; Öquist, G.; Sarhan, F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998, 3, 224–230. [Google Scholar] [CrossRef]
- Stone, J.M.; Palta, J.P.; Bamberg, J.B.; Weiss, L.S.; Harbage, J.F. Inheritance of freezing resistance in tuber-bearing Solanum species: Evidence for independent genetic control of nonacclimated freezing tolerance and cold acclimation capacity. Proc. Natl. Acad. Sci. USA 1993, 90, 7869–7873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotke, J.; Kopka, J.; Gatzke, N.; Heyer, A.G. Impact of soluble sugar concentrations on the acquisition of freezing tolerance in accessions of Arabidopsis thaliana with contrasting cold adaptation—Evidence for a role of raffinose in cold acclimation. Plant Cell Environ. 2004, 27, 1395–1404. [Google Scholar] [CrossRef]
- Slugina, M.A.; Meleshin, A.A.; Kochieva, E.Z.; Shchennikova, A.V. The opposite effect of low temperature on the Pho1a starch phosphorylase gene expression in Solanum tuberosum L. Tubers and Petota Species Leaves. Am. J. Potato Res. 2020, 97, 78–87. [Google Scholar] [CrossRef]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.; Dai, D.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857. [Google Scholar] [CrossRef] [PubMed]
- Hoermiller, I.I.; Naegele, T.; Augustin, H.; Stutz, S.; Weckwerth, W.; Heyer, A.G. Subcellular reprogramming of metabolism during cold acclimation in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 602–610. [Google Scholar] [CrossRef]
- Sitnicka, D.; Orzechowski, S. Cold-induced starch degradation in potato leaves—Intercultivar differences in the gene expression profile and activity of key enzymes. Biol. Plant. 2014, 58, 659–666. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005, 44, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, F.; Guy, C. β–amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [Green Version]
- Sicher, R. Carbon partitioning and the impact of starch deficiency on the initial response of Arabidopsis to chilling temperatures. Plant Sci. 2011, 181, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Minami, A.; Arakawa, K.; Fujikawa, S.; Takezawa, D. Rapid degradation of starch in chloroplasts and concomitant accumulation of soluble sugars associated with ABA-induced freezing tolerance in the moss Physcomitrella patens. J. Plant Physiol. 2005, 162, 169–180. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars–metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahlow, S.; Orzechowski, S.; Fettke, J. Starch phosphorylation: Insights and perspectives. Cell Mol. Life Sci. 2016, 73, 2753–2764. [Google Scholar] [CrossRef]
- Orzechowski, S.; Grabowska, A.; Sitnicka, D.; Simińska, J.; Feluś, M.; Dudkiewicz, M.; Fudali, S.; Sobczak, M. Analysis of the expression, subcellular and tissue localisation of phosphoglucan, water dikinase (PWD/GWD3) in Solanum tuberosum L.: A bioinformatics approach for the comparative analysis of two α-glucan, water dikinases (GWDs) from Solanum tuberosum L. Acta Physiol. Plant. 2013, 35, 483–500. [Google Scholar] [CrossRef] [Green Version]
- Knaupp, M.; Mishra, K.B.; Nedbal, L.; Heyer, A.G. Evidence for a role of raffinose in stabilizing photosystem II during freeze-thaw cycles. Planta 2011, 234, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.; Van Den Ende, W. Sugars as hydroxyl radical scavengers. Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef]
- Parvanova, D.; Ivanov, S.; Konstantinova, T.; Karanov, E.; Atanassov, A.; Tsvetkov, T.; Alexieva, V.; Djilianov, D. Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol. Biochem. 2004, 42, 57–63. [Google Scholar] [CrossRef]
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeekens, S.; Hellmann, H.A. Sugar sensing and signaling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of plant metabolism during cold acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234–235, 80–93. [Google Scholar] [CrossRef]
- Evers, D.; Bonnechère, S.; Hoffmann, L.; Hausman, J.F. Physiological aspects of abiotic stress response in potato. Belg. J. Bot. 2007, 140, 141–150. [Google Scholar]
- Evers, D.; Legay, S.; Lamoureux, D.; Hausman, J.F.; Hoffmann, L.; Renaut, J. Towards a synthetic view of potato cold and salt stress response by transcriptomic and proteomic analyses. Plant Mol. Biol. 2012, 78, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Oufir, M.; Legay, S.; Nicot, N.; Van Moer, K.; Hoffmann, L.; Renaut, J.; Hausman, J.-F.; Evers, D. Gene expression in potato during cold exposure: Changes in carbohydrate and polyamine metabolisms. Plant Sci. 2008, 175, 839–852. [Google Scholar] [CrossRef]
- Deryabin, A.N.; Burakhanova, E.A.; Trunova, T.I. Apoplastic sugars and cell-wall invertase are involved in formation of the tolerance of cold resistant potato plants to hypothermia. Dokl. Biochem. Biophys. 2015, 465, 366–369. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Klíma, M.; Roy, A.; Prášil, I.T. Biological networks underlying abiotic stress tolerance in temperate crops—A proteomic perspective. Int. J. Mol. Sci. 2015, 16, 20913–20942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, J. Responses of Plants to Environmental Stresses Volume I, Chilling, Freezing, and High Temperature Stresses; Academic Press: New York, NY, USA, 1980; pp. 137–141. [Google Scholar]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [Green Version]
- Ruelland, E.; Vaultier, M.-N.; Zachowski, A.; Hurry, V. Chapter 2 Cold Signalling and cold acclimation in plants. Adv. Bot. Res. 2009, 49, 35–150. [Google Scholar]
- Zeeman, S.C.; Thorneycroft, D.; Schupp, N.; Chapple, A.; Weck, M.; Dunstan, H.; Haldimann, P.; Bechtold, N.; Smith, A.M.; Smith, S.M. Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol. 2004, 135, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medini, W.; Farhat, N.; Al-Rawi, S.; Mahto, H.; Qasim, H.; Ben-Halima, E.; Bessrour, M.; Chibani, F.; Abdelly, C.; Fettke, J.; et al. Do carbohydrate metabolism and partitioning contribute to the higher salt tolerance of Hordeum marinum compared to Hordeum vulgare? Acta Physiol. Plant. 2019, 41, 190. [Google Scholar] [CrossRef]
- İşeri, Ö.D.; Körpe, D.A.; Sahin, I.F.; Haberal, M. Hydrogen peroxide pretreatment of roots enhanced oxidative stress response of tomato under cold stress. Acta Physiol. Plant. 2013, 35, 1905–1913. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Chia, T.; Thorneycroft, D.; Chapple, A.; Messerli, G.; Chen, J.; Zeeman, S.C.; Smith, S.M.; Smith, A.M. A cytosolic glucosyltransferase is required for conversion of starch to sucrose in Arabidopsis leaves at night. Plant J. 2004, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.R.; Blennow, A.; Burhenne, K.; Kossmann, J. Repression of a novel isoform of disproportionating enzyme (stDPE2) in potato leads to inhibition of starch degradation in leaves but not tubers stored at low temperature. Plant Physiol. 2004, 134, 1347–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fettke, J.; Chia, T.; Eckermann, N.; Smith, A.; Steup, M. A transglucosidase necessary for starch degradation and maltose metabolism in leaves at night acts on cytosolic heteroglycans (SHG). Plant J. 2006, 46, 668–684. [Google Scholar] [CrossRef]
- Fettke, J.; Fernie, A.R. Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism. Trends Plant Sci. 2015, 20, 490–497. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Moghaddam, M.R.; Van den Ende, W. Sugars, the clock and transition to flowering. Front. Plant Sci. 2013, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciereszko, I. Regulatory roles of sugars in plant growth and development. Acta Soc. Bot. Pol. 2018, 87, 3583. [Google Scholar] [CrossRef] [Green Version]
- Yadeghari, L.Z.; Heidari, R.; Carapetian, J. Cold Pretreatment-Induced Changes in Antioxidant Enzyme Activities and Relative Water Content and Soluble Sugars in Shoots and Roots of Soybean Seedlings. J. Biol. Sci. 2008, 7, 1525–1530. [Google Scholar] [CrossRef] [Green Version]
- Nevyl, F.S.; Battaglia, M.E. Developmental plasticity in Arabidopsis thaliana under combined cold and water deficit stresses during flowering stage. Planta 2021, 253, 50. [Google Scholar] [CrossRef] [PubMed]
- Svensson, Å.S.; Johansson, F.I.; Møller, I.M.; Rasmusson, A.G. Cold stress decreases the capacity for respiratory NADH oxi-dation on potato leaves. FEBS Lett. 2002, 517, 79–82. [Google Scholar] [CrossRef] [Green Version]
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive potato genotypes. Front. Plant Sci. 2020, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Yano, R.; Nakamura, M.; Yoneyama, T.; Nishida, I. Starch-related-glucan/water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol. 2005, 138, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejazi, M.; Mahlow, S.; Fettke, J. The glucan phosphorylation mediated by α-glucan, water dikinase (GWD) is also essential in the light phase for a functional transitory starch turn-over. Plant Signal. Behav. 2014, 9, e28892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejazi, M.; Fettke, J.; Haebel, S.; Edner, C.; Paris, O.; Frohberg, C.; Steup, M.; Ritte, G. Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J. 2008, 55, 323–334. [Google Scholar] [CrossRef]
- Calzadilla, P.I.; Vilas, J.M.; Escaray, F.J.; Unrein, F.; Carrasco, P.; Ruiz, O.A. The increase of photosynthetic carbon assimilation as a mechanism of adaptation to low temperature in Lotus japonicus. Sci. Rep. 2019, 9, 863. [Google Scholar] [CrossRef]
- Fettke, J.; Malinova, I.; Albrecht, T.; Hejazi, M.; Steup, M. Glucose-1-phosphate transport into protoplasts and chloroplasts from leaves of Arabidopsis. Plant Physiol. 2011, 155, 1723–1734. [Google Scholar] [CrossRef] [Green Version]
- Huber, S.C. Biochemical mechanism for regulation of sucrose accumulation in leaves during photosynthesis. Plant Physiol. 1989, 91, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Orawetz, T.; Malinova, I.; Orzechowski, S.; Fettke, J. Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol. Biochem. 2016, 100, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fettke, J.; Albrecht, T.; Hejazi, M.; Mahlow, S.; Nakamura, Y.; Steup, M. Glucose 1-phosphate is efficiently taken up by potato (Solanum tuberosum) tuber parenchyma cells and converted to reserve starch granules. New Phytol. 2010, 185, 663–675. [Google Scholar] [CrossRef]
- Fettke, J.; Poeste, S.; Eckermann, N.; Tiessen, A.; Pauly, M.; Geigenberger, P.; Steup, M. Analysis of cytosolic heteroglycans from leaves of transgenic potato (Solanum tuberosum L.) plants that under- or overexpress the Pho 2 phosphorylase isozyme. Plant Cell Physiol. 2005, 46, 1987–2004. [Google Scholar] [CrossRef]
- Nägele, T.; Kandel, B.A.; Frana, S.; Meissner, M.; Heyer, A.G. A systems biology approach for the analysis of carbohydrate dynamics during acclimation to low temperature in Arabidopsis thaliana. FEBS J. 2011, 278, 506–518. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Bergmeyer, H. Methoden der Enzymatischen Analyse, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1974. [Google Scholar]
- Jones, M.G.K.; Outlow, W.H.; Lowry, O.M. Enzymic assay of 10−7–10−14 moles of sucrose in plant tissues. Plant Physiol. 1977, 60, 379–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 159, 19–23. [Google Scholar] [CrossRef]
- Rödiger, A.; Baudisch, B.; Klösgen, R.B. Simultaneous isolation of intact mitochondria and chloroplasts from a single pulping of plant tissue. J. Plant Physiol. 2010, 167, 620–624. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A Method for extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein—Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Wessa, P. Free Statistics Software, Office for Research Development and Education, Version 1.1.23-r7. 2014. Available online: http://www.wessa.net/ (accessed on 3 July 2021).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orzechowski, S.; Sitnicka, D.; Grabowska, A.; Compart, J.; Fettke, J.; Zdunek-Zastocka, E. Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves. Int. J. Mol. Sci. 2021, 22, 7203. https://doi.org/10.3390/ijms22137203
Orzechowski S, Sitnicka D, Grabowska A, Compart J, Fettke J, Zdunek-Zastocka E. Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves. International Journal of Molecular Sciences. 2021; 22(13):7203. https://doi.org/10.3390/ijms22137203
Chicago/Turabian StyleOrzechowski, Sławomir, Dorota Sitnicka, Agnieszka Grabowska, Julia Compart, Joerg Fettke, and Edyta Zdunek-Zastocka. 2021. "Effect of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves" International Journal of Molecular Sciences 22, no. 13: 7203. https://doi.org/10.3390/ijms22137203





