Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels
Abstract
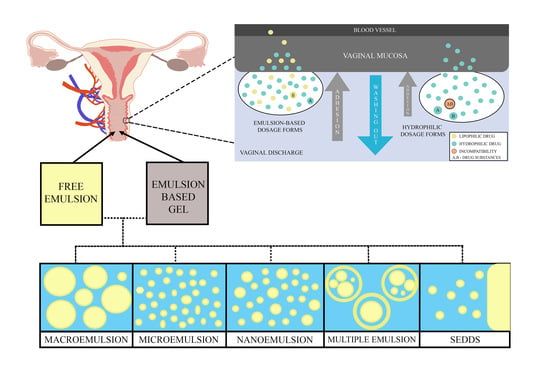
:1. Introduction
2. The Anatomical and Physiological Aspects Intravaginal Drug Application
2.1. Vaginal Anatomy
2.2. Vascularization of the Vagina
2.3. Vagina Surface Area
2.4. Vaginal Mucosa and Vaginal Discharge
2.5. The Influence of Vaginal Microbiota
3. Technological Aspects of Vaginal Formulations Development
3.1. Emulsions–Based Vaginal Dosage Forms (EVDF)
3.2. The Technological Aspects of Emulsion-Based Vaginal Dosage Forms
3.2.1. The Vaginal Drug Dosage Form Compositions
Oil Phase
Surfactants
Cosurfactants
Other Excipients
3.2.2. EVDF Preparation Methods
3.2.3. Vaginal Emulsion-Based Drug Delivery Systems Characterization Methods
pH and Osmolarity
Internal Droplets Measurements, Polydispersity Index and Zeta Potential
Viscosity and Adhesion
Spreadability
In Vitro Drug Release and Permeability Studies
In Vivo Studies
4. Emulsion-Based Vaginal Dosage Forms with Drugs from Different Therapeutic Groups—Biological Evaluation and Examples of In Vivo Applications
4.1. Antifungal Activity
4.1.1. Antimycotic Azoles
Fluconazole, Clotrimazole
Itraconazole
Sertaconazole
4.1.2. Nystatin
4.1.3. Antifungal Phytoconstituents
4.2. Antibacterial Activity
4.2.1. Antibiotics and Chemotherapeutics
4.2.2. Antiseptics
4.2.3. Emulsions with Phytoconstituents
4.3. Contraceptive and Sexually Transmitted Diseases Prevention
4.4. Other Diseases and Conditions
4.4.1. Pre-Term Birth Prevention and Hormonal Therapy
4.4.2. Tumors and Autoimmunological Diseases
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brannon-Peppas, L. Novel vaginal drug release applications. Adv. Drug Deliv. Rev. 1993, 11, 169–177. [Google Scholar] [CrossRef]
- Mallipeddi, R.; Rohan, L.C. Nanoparticle-based vaginal drug delivery systems for HIV prevention. Expert Opin. Drug Deliv. 2010, 7, 37–48. [Google Scholar] [CrossRef]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Uckun, F.M. Vaginal microbicides and their delivery platforms. Expert Opin. Drug Deliv. 2014, 11, 723–740. [Google Scholar] [CrossRef]
- das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef]
- Bassi, P.; Kaur, G. Innovations in bioadhesive vaginal drug delivery system. Expert Opin. Ther. Pat. 2012, 22, 1019–1032. [Google Scholar] [CrossRef]
- Major, I.; McConville, C. Vaginal drug delivery for the localised treatment of cervical cancer. Drug Deliv. Transl. Res. 2017, 7, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal Films for Drug Delivery. J. Pharm. Sci. 2013, 102, 2069–2081. [Google Scholar] [CrossRef]
- Rohan, L.C.; Sassi, A.B. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009, 11, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araújo Pereira, R.R.; Bruschi, M.L. Vaginal mucoadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2012, 38, 643–652. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control. Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2013, 24, 537–543. [Google Scholar] [CrossRef]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef]
- Das Neves, J.; Palmeira-De-Oliveira, R.; Palmeira-De-Oliveira, A.; Rodrigues, F.; Sarmento, B. Vaginal Mucosa and Drug Delivery. In Mucoadhesive Materials and Drug Delivery Systems; Khutoryanskiy, V.V., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; Volume 9781119941, pp. 99–132. ISBN 9781118794203. [Google Scholar]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Bruska, M. Anatomia czynnościowa i topograficzna narządów płciowych. In Ginekologia. Podręcznik dla Położnych, Pielęgniarek i Fizjoterapeutów; Opala, T., Ed.; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2003; pp. 30–31. ISBN 83-200-3353-5. [Google Scholar]
- Funt, M.I.; Thompson, J.D.; Birch, H. Normal Vaginal Axis. South. Med. J. 1978, 71, 1534–1535. [Google Scholar] [CrossRef]
- Anderson, J.R.; Genadry, R. Anatomy and Embryology. In Berek & Novak’s Gynecology; Berek, J.S., Ed.; Lippincott Williams & Wilkins, a Wolters Kluwer Business: Philadelphia, PA, USA, 2007; pp. 75–128. ISBN 978-0-78176-805-4. [Google Scholar]
- Valea, F.A. Reproductive Anatomy Gross and Microscopic, Clinical Correlations. In Comprehensive Gynecology; Lobo, R.A., Gershenson, D.M., Lentz, G.M., Valea, F.A., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 48–77. ISBN 978-0-323-32287-4. [Google Scholar]
- Bulletti, C.; De Ziegler, D.; Flamigni, C.; Giacomucci, E.; Polli, V.; Bolelli, G.; Franceschetti, F. Targeted drug delivery in gynaecology: The first uterine pass effect. Hum. Reprod. 1997, 12, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef]
- Wing, D.A.; Rahall, A.; Jones, M.M.; Goodwin, T.M.; Paul, R.H. Misoprostol: An effective agent for cervical ripening and labor induction. Am. J. Obstet. Gynecol. 1995, 172, 1811–1816. [Google Scholar] [CrossRef]
- Katz, D.F.; Yuan, A.; Gao, Y. Vaginal drug distribution modeling. Adv. Drug Deliv. Rev. 2015, 92, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor, Z.; Chmel, R. Differential diagnostics of female “sexual” fluids: A narrative review. Int. Urogynecol. J. 2018, 29, 621–629. [Google Scholar] [CrossRef]
- Miller, E.A.; Beasley, D.A.E.; Dunn, R.R.; Archie, E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 1936. [Google Scholar] [CrossRef]
- Gorodeski, G.I.; Hopfer, U.; Liu, C.C.; Margles, E. Estrogen acidifies vaginal pH by up-regulation of proton secretion via the apical membrane of vaginal-ectocervical epithelial cells. Endocrinology 2005, 146, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Mania-Pramanik, J.; Kerkar, S.C.; Mehta, P.B.; Potdar, S.; Salvi, V.S. Use of vaginal pH in diagnosis of infections and its association with reproductive manifestations. J. Clin. Lab. Anal. 2008, 22, 375–379. [Google Scholar] [CrossRef]
- Tucker, K.M.; Godha, K.; Mirkin, S.; Archer, D.F. Vaginal pH: A simple assessment highly correlated with vaginal morphology and symptoms in postmenopausal women. Menopause 2018, 25, 762–766. [Google Scholar] [CrossRef]
- Hainer, B.L.; Gibson, M.V. Vaginitis: Diagnosis and Treatment. Am. Fam. Physician 2011, 83, 807–815. [Google Scholar]
- Godha, K.; Tucker, K.M.; Biehl, C.; Archer, D.F.; Mirkin, S. Human vaginal pH and microbiota: An update. Gynecol. Endocrinol. 2018, 34, 451–455. [Google Scholar] [CrossRef]
- Kitaya, K.; Nagai, Y.; Arai, W.; Sakuraba, Y.; Ishikawa, T. Characterization of microbiota in endometrial fluid and vaginal secretions in infertile women with repeated implantation failure. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Sado, T.; Naruse, K.; Kobayashi, H. Vaginal fluid pH and buffer capacity for predicting false preterm labor in Japanese women. Int. J. Gynecol. Obstet. 2016, 134, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2019, 25, 298–325. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Fredlund, H.; Jensen, J.S.; Engstrand, L.; Unemo, M. Composition of the Vaginal Microbiota in Women of Reproductive Age—Sensitive and Specific Molecular Diagnosis of Bacterial Vaginosis Is Possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, R.; Sobel, J.; Akins, R.; Hassan, S.; Chaiworapongsa, T.; Kusanovic, J.; Romero, R. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 533–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379. [Google Scholar] [CrossRef]
- Donders, G.G.; Zodzika, J.; Rezeberga, D. Treatment of bacterial vaginosis: What we have and what we miss. Expert Opin. Pharmacother. 2014, 15, 645–657. [Google Scholar] [CrossRef]
- Kamińska, D.; Gajecka, M. Is the role of human female reproductive tract microbiota underestimated? Benef. Microbes 2017, 8, 327–343. [Google Scholar] [CrossRef]
- Haque, M.M.; Merchant, M.; Kumar, P.N.; Dutta, A.; Mande, S.S. First-trimester vaginal microbiome diversity: A potential indicator of preterm delivery risk. Sci. Rep. 2017, 7, 16145. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. Correction to: The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, A.; Yamagishi, Y.; Miyamoto, K.; Oka, K.; Takahashi, M.; Mikamo, H. Characterization of the vaginal microbiota of Japanese women. Anaerobe 2018, 54, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, C.; Silveira, P.; Rodrigues Sereia, A.F.; Christoff, A.P.; Mendes, H.; de Oliveira, L.F.V.; Podgaec, S. Microbiome profile of deep endometriosis patients: Comparison of vaginal fluid, endometrium and lesion. Diagnostics 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Bruning, E.; Chen, Y.; McCue, K.A.; Rubino, J.R.; Wilkinson, J.E.; Brown, A.D.G. A 28 day clinical assessment of a lactic acid-containing antimicrobial intimate gel wash formulation on skin tolerance and impact on the vulvar microbiome. Antibiotics 2020, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- WHO; UNFPA; FHI. Advisory Note Use and Procurement of Additional Lubricants for Male and Female Condoms: WHO/UNFPA/FHI360 Advisory Note; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Vaginal semisolid products: Technological performance considering physiologic parameters. Eur. J. Pharm. Sci. 2017, 109, 556–568. [Google Scholar] [CrossRef]
- Ensign, L.M.; Hoen, T.E.; Maisel, K.; Cone, R.A.; Hanes, J.S. Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials 2013, 34, 6922–6929. [Google Scholar] [CrossRef] [Green Version]
- Lacey, C.J.; Woodhall, S.; Qi, Z.; Sawant, S.; Cowen, M.; McCormack, S.; Jiang, S. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int. J. STD AIDS 2010, 21, 714–717. [Google Scholar] [CrossRef]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.-Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [Green Version]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J.Y. Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.A.; Giannasca, K.T.; Giannasca, P.; Hubert, R.; Lencer, W.I.; Neutra, M.R. From the Department of Pediatrics, Harvard Medical School and GI Cell Biology Laboratory, Children’s Hospital, Boston, Massachusetts 02115. J. Exp. Med. 1996, 184, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Kammona, O.; Waldner, C.; Kiparissides, C.; Bernkop-Schnürch, A. Nano-carrier systems: Strategies to overcome the mucus gel barrier. Eur. J. Pharm. Biopharm. 2015, 96, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chemie Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Wang, S.H.; Ciotti, S.; Makidon, P.E.; Smith, D.M.; Fan, Y.; Schuler, C.F.; Baker, J.R. Formulation and Characterization of Nanoemulsion Intranasal Adjuvants: Effects of Surfactant Composition on Mucoadhesion and Immunogenicity. Mol. Pharm. 2014, 11, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Emulsion. In IUPAC Compendium of Chemical Terminology; Nič, M.; Jirát, J.; Košata, B.; Jenkins, A.; McNaught, A. (Eds.) IUPAC: Research Triagle Park, NC, USA, 2009; ISBN 0-9678550-9-8. [Google Scholar]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Sanfeld, A.; Steinchen, A. Emulsions stability, from dilute to dense emulsions—Role of drops deformation. Adv. Colloid Interface Sci. 2008, 140, 1–65. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Friberg, S.E. Comments on “the definition of microemulsion”. Colloids Surf. 1982, 4, 201. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nano-emulsions and micro-emulsions: Clarifications of the critical differences. Pharm. Res. 2011, 28, 978–985. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Lu, Y.; Qi, J.; Wu, W. Absorption, Disposition and Pharmacokinetics of Nanoemulsions. Curr. Drug Metab. 2012, 13, 396–417. [Google Scholar] [CrossRef]
- Muschiolik, G.; Scherze, I.; Preissler, P.; Weiß, J.; Knoth, A.; Fechner, A. Multiple Emulsions—Preparation and Stability. In Proceedings of the 13th World Congress of Food Science & Technology, Nantes, France, 17–21 September 2006; EDP Sciences: Les Ulis, France, 2006. [Google Scholar]
- Raynal, S.; Grossiord, J.L.; Seiller, M.; Clausse, D. A topical W/O/W multiple emulsion containing several active substances: Formulation, characterization and study of release. J. Control. Release 1993, 26, 129–140. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Talegaonkar, S.; Azeem, A.; Ahmad, F.; Khar, R.; Pathan, S.; Khan, Z. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008, 2, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 1997, 25, 47–58. [Google Scholar] [CrossRef]
- Laffleur, F.; Bernkop-Schnürch, A. Strategies for improving mucosal drug delivery. Nanomedicine 2013, 8, 2061–2075. [Google Scholar] [CrossRef]
- Song, R.; Yan, F.; Cheng, M.; Dong, F.; Lin, Y.; Wang, Y.; Song, B. Ultrasound-assisted preparation of exopolysaccharide/nystatin nanoemulsion for treatment of vulvovaginal candidiasis. Int. J. Nanomed. 2020, 15, 2027–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos Ramos, M.A.; Da Silva, P.B.; De Toledo, L.G.; Oda, F.B.; Da Silva, I.C.; dos Santos, L.C.; dos Santos, A.G.; De Almeida, M.T.G.; Pavan, F.R.; Chorilli, M.; et al. Intravaginal delivery of syngonanthus nitens (Bong.) Ruhland fraction based on a nanoemulsion system applied to vulvovaginal candidiasis treatment. J. Biomed. Nanotechnol. 2019, 15, 1072–1089. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Uckun, F.M. Vaginal contraceptive activity of a chelated vanadocene. Contraception 2005, 72, 146–156. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Uckun, F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef]
- Frank, L.A.; Gazzi, R.P.; Mello, P.A.; Chaves, P.; Peña, F.; Beck, R.C.R.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Anti-HPV Nanoemulsified-Imiquimod: A New and Potent Formulation to Treat Cervical Cancer. AAPS PharmSciTech 2020, 21, 54. [Google Scholar] [CrossRef]
- Atinderpal, K.; Kapoor, N.; Gupta, S.; Tyag, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development and Characterization of Green Tea Catechins and Ciprofloxacin-loaded Nanoemulsion for Intravaginal Delivery to Treat Urinary Tract Infection. Indian J. Pharm. Sci. 2018, 80, 442–452. [Google Scholar] [CrossRef]
- Campaña-Seoane, M.; Pérez-Gago, A.; Vázquez, G.; Conde, N.; González, P.; Martinez, A.; Martínez, X.; García Varela, L.; Herranz, M.; Aguiar, P.; et al. Vaginal residence and pharmacokinetic preclinical study of topical vaginal mucoadhesive W/S emulsions containing ciprofloxacin. Int. J. Pharm. 2019, 554, 276–283. [Google Scholar] [CrossRef]
- Campaña-Seoane, M.; Peleteiro, A.; Laguna, R.; Otero-Espinar, F.J. Bioadhesive emulsions for control release of progesterone resistant to vaginal fluids clearance. Int. J. Pharm. 2014, 477, 495–505. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion-based vaginal gel of clotrimazole: Formulation, in vitro evaluation, and stability studies. AAPS PharmSciTech 2009, 10, 476–481. [Google Scholar] [CrossRef] [Green Version]
- Abu-Azzam, O.; Nasr, M. In vitro anti-inflammatory potential of phloretin microemulsion as a new formulation for prospective treatment of vaginitis. Pharm. Dev. Technol. 2020, 25, 930–935. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm. 2009, 365, 175–179. [Google Scholar] [CrossRef]
- Mirani, A.; Kundaikar, H.; Velhal, S.; Patel, V.; Bandivdekar, A.; Degani, M.; Patravale, V. Tetrahydrocurcumin-loaded vaginal nanomicrobicide for prophylaxis of HIV/AIDS: In silico study, formulation development, and in vitro evaluation. Drug Deliv. Transl. Res. 2019, 9, 828–847. [Google Scholar] [CrossRef]
- Patel, A.; Patel, J. Mucoadhesive Microemulsion Based Prolonged Release Vaginal Gel for Anti-Fungal Drug. Am. J. Pharma Tech. Res. 2012, 2, 650–661. [Google Scholar]
- Patki, M.; Giusto, K.; Gorasiya, S.; Reznik, S.E.; Patel, K. 17-α Hydroxyprogesterone Nanoemulsifying Preconcentrate-Loaded Vaginal Tablet: A Novel Non-Invasive Approach for the Prevention of Preterm Birth. Pharmaceutics 2019, 11, 335. [Google Scholar] [CrossRef] [Green Version]
- Giusto, K.; Patki, M.; Koya, J.; Ashby, C.R.; Munnangi, S.; Patel, K.; Reznik, S.E. A vaginal nanoformulation of a SphK inhibitor attenuates lipopolysaccharide-induced preterm birth in mice. Nanomedicine 2019, 14, 2835–2851. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Suñer-Carbó, J.; Calpena-Campmany, A.C.; Bozal-de Febrer, N.; Halbaut-Bellowa, L.; Boix-Montañés, A.; Souto, E.B.; Clares-Naveros, B. Clotrimazole multiple W/O/W emulsion as anticandidal agent: Characterization and evaluation on skin and mucosae. Colloids Surf. B Biointerfaces 2019, 175, 166–174. [Google Scholar] [CrossRef]
- Khattab, A.; Ismail, S. Formulation and evaluation of oxiconazole nitrate mucoadhesive nanoemulsion based gel for treatment of fungal vaginal infection. Int. J. Pharm. Pharm. Sci. 2016, 8, 33–40. [Google Scholar]
- Soriano-Ruiz, J.L.; Calpena-Capmany, A.C.; Cañadas-Enrich, C.; Febrer, N.B.-D.; Suñer-Carbó, J.; Souto, E.B.; Clares-Naveros, B. Biopharmaceutical profile of a clotrimazole nanoemulsion: Evaluation on skin and mucosae as anticandidal agent. Int. J. Pharm. 2019, 554, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gupta, S.; Tyagi, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development of nanoemulsion based gel loaded with phytoconstituents for the treatment of urinary tract infection and in vivo biodistribution studies. Adv. Pharm. Bull. 2017, 7, 611–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Santos, M.K.; Kreutz, T.; Danielli, L.J.; De Marchi, J.G.B.; Pippi, B.; Koester, L.S.; Fuentefria, A.M.; Limberger, R.P. A chitosan hydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 56, 101527. [Google Scholar] [CrossRef]
- Mirza, M.A.; Ahmad, S.; Mallick, M.N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef]
- Srivastava, N.; Patel, D.K.; Rai, V.K.; Pal, A.; Yadav, N.P. Development of emulgel formulation for vaginal candidiasis: Pharmaceutical characterization, in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2018, 48, 490–498. [Google Scholar] [CrossRef]
- Gündel, S.D.S.; de Godoi, S.N.; Santos, R.C.V.; da Silva, J.T.; Leite, L.B.D.M.; Amaral, A.C.; Ourique, A.F. In vivo antifungal activity of nanoemulsions containing eucalyptus or lemongrass essential oils in murine model of vulvovaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 57, 101762. [Google Scholar] [CrossRef]
- Pandit, A.; Kedar, A.; Koyate, K. Hollow pessary loaded with lawsone via self-microemulsifying drug delivery system for vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 60, 101955. [Google Scholar] [CrossRef]
- Kaur, A.; Saxena, Y.; Bansal, R.; Gupta, S.; Tyagi, A.; Sharma, R.K.; Ali, J.; Panda, A.K.; Gabrani, R.; Dang, S. Intravaginal Delivery of Polyphenon 60 and Curcumin Nanoemulsion Gel. AAPS PharmSciTech 2017, 18, 2188–2202. [Google Scholar] [CrossRef]
- McConville, C.; Friend, D. Development and characterisation of a self-microemulsifying drug delivery systems (SMEDDSs) for the vaginal administration of the antiretroviral UC-781. Eur. J. Pharm. Biopharm. 2013, 83, 322–329. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Yiv, S.H.; Waurzyniak, B.; Uckun, F.M. Contraceptive efficacy and safety studies of a novel microemulsion-based lipophilic vaginal spermicide. Fertil. Steril. 2001, 75, 115–124. [Google Scholar] [CrossRef]
- Tedajo, G.M.; Bouttier, S.; Grossiord, J.L.; Marty, J.P.; Seiller, M.; Fourniat, J. In vitro microbicidal activity of W/O/W multiple emulsion for vaginal administration. Int. J. Antimicrob. Agents 2002, 20, 50–56. [Google Scholar] [CrossRef]
- Özer, Ö.; Özyazici, M.; Tedajo, M.; Taner, M.S.; Köseoglu, K. W/O/W multiple emulsions containing nitroimidazole derivates for vaginal delivery. Drug Deliv. 2007, 14, 139–145. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, Z.; Wang, F.; He, J.; Yang, X.; Xie, W.; Liu, Y.; Zhang, Y. A thermosensitive gel based on w1/o/w2 multiple microemulsions for the vaginal delivery of small nucleic acid. Drug Deliv. 2019, 26, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Tedajo, G.M.; Bouttier, S.; Fourniat, J.; Grossiord, J.L.; Marty, J.P.; Seiller, M. Release of antiseptics from the aqueous compartments of a w/o/w multiple emulsion. Int. J. Pharm. 2005, 288, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Köllner, S.; Nardin, I.; Markt, R.; Griesser, J.; Prüfert, F.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: Design of a novel vaginal delivery system for curcumin. Eur. J. Pharm. Biopharm. 2017, 115, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Giovagnoli, S.; Pagano, C.; Rossi, C. Formulation studies of benzydamine mucoadhesive formulations for vaginal administration. Drug Dev. Ind. Pharm. 2009, 35, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C. Classification of Surface-Active Agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311. [Google Scholar]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef] [PubMed]
- Nazir, H.; Zhang, W.; Liu, Y.; Chen, X.; Wang, L.; Naseer, M.M.; Ma, G. Silicone oil emulsions: Strategies to improve their stability and applications in hair care products. Int. J. Cosmet. Sci. 2014, 36, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Vieira, O.V.; Hartmann, D.O.; Cardoso, C.M.P.; Oberdoerfer, D.; Baptista, M.; Santos, M.A.S.; Almeida, L.; Ramalho-Santos, J.; Vaz, W.L.C. Surfactants as microbicides and contraceptive agents: A systematic In Vitro study. PLoS ONE 2008, 3, e2913. [Google Scholar] [CrossRef] [PubMed]
- Inácio, Â.S.; Mesquita, K.A.; Baptista, M.; Ramalho-Santos, J.; Vaz, W.L.C.; Vieira, O.V. In vitro surfactant structure-toxicity relationships: Implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS ONE 2011, 6, e19850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niruthisard, S.; Roddy, R.E.; Chutivongse, S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 1991, 18, 176–179. [Google Scholar] [CrossRef]
- Jirwankar, P.; Gobbooru, S.; Shao, J. Self-Emulsified Nanoemulsion for Vaginal Administration: In Vitro Study of Effect on Lactobacillus acidophilus. J. Pharm. Sci. 2020, 109, 3145–3152. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hussain, A.; Hussain, M.S.; Mirza, M.A.; Iqbal, Z. Role of excipients in successful development of self-emulsifying/ microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev. Ind. Pharm. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Zeng, L.; Xin, X.; Zhang, Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. [Google Scholar] [CrossRef] [Green Version]
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, B.; Liu, W.; Li, S. Influence of hydroxypropyl methylcellulose, methylcellulose, gelatin, poloxamer 407 and poloxamer 188 on the formation and stability of soybean oil-in-water emulsions. Asian J. Pharm. Sci. 2017, 12, 521–531. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocoll. 2006, 20, 596–606. [Google Scholar] [CrossRef]
- Said dos Santos, R.; Rosseto, H.C.; Bassi da Silva, J.; Vecchi, C.F.; Caetano, W.; Bruschi, M.L. The effect of carbomer 934P and different vegetable oils on physical stability, mechanical and rheological properties of emulsion-based systems containing propolis. J. Mol. Liq. 2020, 307, 112969. [Google Scholar] [CrossRef]
- Mou, D.; Chen, H.; Du, D.; Mao, C.; Wan, J.; Xu, H.; Yang, X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int. J. Pharm. 2008, 353, 270–276. [Google Scholar] [CrossRef]
- Perez-Moral, N.; Watt, S.; Wilde, P. Comparative study of the stability of multiple emulsions containing a gelled or aqueous internal phase. Food Hydrocoll. 2014, 42, 215–222. [Google Scholar] [CrossRef]
- Sato, A.C.K.; Moraes, K.E.F.P.; Cunha, R.L. Development of gelled emulsions with improved oxidative and pH stability. Food Hydrocoll. 2014, 34, 184–192. [Google Scholar] [CrossRef]
- Ashara, K.C.; Paun, J.S.; Soniwala, M.M.; Chavda, J.R.; Mendapara, V.P.; Mori, N.M. Microemulgel: An overwhelming approach to improve therapeutic action of drug moiety. Saudi Pharm. J. 2016, 24, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Debeli, D.K.; Lin, C.; Mekbib, D.B.; Hu, L.; Deng, J.; Gan, L.; Shan, G. Controlling the Stability and Rheology of Copolyol Dispersions in Fatty Alcohol Ethoxylate (AEO9)-Stabilized Multiple Emulsions. Ind. Eng. Chem. Res. 2020, 59, 18307–18317. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Ph. Eur. 10.0, 1164 (01/2008). Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 11 June 2021).
- Nakamura, F.; Ohta, R.; Machida, Y.; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996, 134, 173–181. [Google Scholar] [CrossRef]
- Gué, E.; Since, M.; Ropars, S.; Herbinet, R.; Le Pluart, L.; Malzert-Fréon, A. Evaluation of the versatile character of a nanoemulsion formulation. Int. J. Pharm. 2016, 498, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; Van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H.D.; Cerqueira, M.A.; Vicente, A.A. Influence of surfactant and processing conditions in the stability of oil-in-water nanoemulsions. J. Food Eng. 2015, 167, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Edsman, K.; Hägerström, H. Pharmaceutical applications of mucoadhesion for the non-oral routes. J. Pharm. Pharmacol. 2005, 57, 3–22. [Google Scholar] [CrossRef]
- Tietz, K.; Klein, S. Simulated Genital Tract Fluids and Their Applicability in Drug Release/Dissolution Testing of Vaginal Dosage Forms. Dissolut. Technol. 2018, 25, 40–51. [Google Scholar] [CrossRef]
- Gurpret, K.; Singh, S.K. Singh Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Dissolution Test USP 28, 2nd Supplement, Official 1 August 2005. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q01_pf_ira_33_4_2007.pdf (accessed on 11 June 2021).
- Ph. Eur. 10.0, 20903 (01/2016). Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-10th-edition (accessed on 11 June 2021).
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Lírio, J.; Giraldo, P.C.; Amaral, R.L.; Sarmento, A.C.A.; Costa, A.P.F.; Goncalves, A.K. Antifungal (oral and vaginal) therapy for recurrent vulvovaginal candidiasis: A systematic review protocol. BMJ Open 2019, 9, e027489. [Google Scholar] [CrossRef] [Green Version]
- Kendirci, M.; Koç, A.N.; Kurtoglu, S.; Keskin, M.; Kuyucu, T. Vulvovaginal candidiasis in children and adolescents with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2004, 17, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Foxman, B.; Muraglia, R.; Dietz, J.P.; Sobel, J.D.; Wagner, J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013, 17, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Bhesania, A.H.; Narayankhedkar, A. Vulvovaginal Candidosis. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Konadu, D.G.; Owusu-Ofori, A.; Yidana, Z.; Boadu, F.; Iddrisu, L.F.; Adu-Gyasi, D.; Dosoo, D.; Awuley, R.L.; Owusu-Agyei, S.; Asante, K.P. Prevalence of vulvovaginal candidiasis, bacterial vaginosis and trichomoniasis in pregnant women attending antenatal clinic in the middle belt of Ghana. BMC Pregnancy Childbirth 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Kovács, K.; Stampf, G.; Klebovich, I.; Antal, I.; Ludányi, K. Aqueous solvent system for the solubilization of azole compounds. Eur. J. Pharm. Sci. 2009, 36, 352–358. [Google Scholar] [CrossRef]
- Bhesaniya, K.; Nandha, K.; Baluja, S. Thermodynamics of fluconazole solubility in various solvents at different temperatures. J. Chem. Eng. Data 2014, 59, 649–652. [Google Scholar] [CrossRef]
- Kracht, T.; Müller-Goymann, C.C. Antifungal efficacy of liquid poloxamer 407-based emulsions loaded with sertaconazole nitrate. Int. J. Pharm. 2020, 585, 119400. [Google Scholar] [CrossRef]
- Ferreira, P.G.; Noronha, L.; Teixeira, R.; Vieira, I.; Borba-Santos, L.P.; Viçosa, A.; de Moraes, M.; Calil-Elias, S.; de Freitas, Z.; da Silva, F.C.; et al. Investigation of a Microemulsion Containing Clotrimazole and Itraconazole for Transdermal Delivery for the Treatment of Sporotrichosis. J. Pharm. Sci. 2020, 109, 1026–1034. [Google Scholar] [CrossRef]
- Talaat, S.M.; Elnaggar, Y.S.R.; Abdalla, O.Y. Lecithin Microemulsion Lipogels Versus Conventional Gels for Skin Targeting of Terconazole: In Vitro, Ex Vivo, and In Vivo Investigation. AAPS PharmSciTech 2019, 20, 161. [Google Scholar] [CrossRef]
- Tiwari, N.; Sivakumar, A.; Mukherjee, A.; Chandrasekaran, N. Enhanced antifungal activity of Ketoconazole using rose oil based novel microemulsion formulation. J. Drug Deliv. Sci. Technol. 2018, 47, 434–444. [Google Scholar] [CrossRef]
- Suñer-Carbó, J.; Boix-Montañés, A.; Halbaut-Bellowa, L.; Velázquez-Carralero, N.; Zamarbide-Ledesma, J.; Bozal-de-Febrer, N.; Calpena-Campmany, A.C. Skin permeation of econazole nitrate formulated in an enhanced hydrophilic multiple emulsion. Mycoses 2017, 60, 166–177. [Google Scholar] [CrossRef]
- Suyal, J.; Bhatt, G.; Singh, N. Formulation and evaluation of nanoemulsion for enhanced bioavailability of itraconazole. Int. J. Pharm. Sci. Res. 2018, 9, 2927–2931. [Google Scholar] [CrossRef]
- Coneac, G.; Vlaia, V.; Olariu, I.; Muţ, A.M.; Anghel, D.F.; Ilie, C.; Popoiu, C.; Lupuleasa, D.; Vlaia, L. Development and Evaluation of New Microemulsion-Based Hydrogel Formulations for Topical Delivery of Fluconazole. AAPS PharmSciTech 2015, 16, 889–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Campos, V.E.B.; Cerqueira-Coutinho, C.S.; Capella, F.N.C.; Soares, B.G.; Holandino, C.; Mansur, C.R.E. Development and in vitro assessment of nanoemulsion for delivery of ketoconazole against Candida albicans. J. Nanosci. Nanotechnol. 2017, 17, 4623–4630. [Google Scholar] [CrossRef]
- Ebenazer, A.; Franklyne, J.S.; Tiwari, N.; Ch, P.A.R.; Mukherjee, A.; Chandrasekaran, N. In Vivo Testing and Extended Drug Release of Chitosan-Coated Itraconazole Loaded Microemulsion Using Volatile Oil Thymus vulgaris. Rev. Bras. Farmacogn. 2020, 30, 279–289. [Google Scholar] [CrossRef]
- Kaewbanjong, J.; Heng, P.W.S.; Boonme, P. Clotrimazole microemulsion and microemulsion-based gel: Evaluation of buccal drug delivery and irritancy using chick chorioallantoic membrane as the model. J. Pharm. Pharmacol. 2017, 69, 1716–1723. [Google Scholar] [CrossRef]
- Banerjee, K.; Curtis, E.; De San Lazaro, C.; Graham, J.C. Low prevalence of genital candidiasis in children. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 696–698. [Google Scholar] [CrossRef]
- Shapiro, R.A.; Schubert, C.J.; Siegel, R.M. Neisseria gonorrhea infections in girls younger than 12 years of age evaluated for vaginitis. Pediatrics 1999, 104, e72. [Google Scholar] [CrossRef] [Green Version]
- Giugno, S.; Risso, P.; Ocampo, D.; Rahman, G.; Rubinstein, D.A. Vulvovaginitis in a pediatric population: Relationship among etiologic agents, age and Tanner staging of breast development. Arch. Argent. Pediatr. 2014, 112, 65–70. [Google Scholar] [CrossRef]
- Randelović, G.; Mladenović, V.; Ristić, L.; Otašević, S.; Branković, S.; Mladenović-Antić, S.; Bogdanović, M.; Bogdanović, D. Microbiological aspects of vulvovaginitis in prepubertal girls. Eur. J. Pediatr. 2012, 171, 1203–1208. [Google Scholar] [CrossRef]
- Jones, R. Childhood vulvovaginitis and vaginal discharge in general practice. Fam. Pract. 1996, 13, 369–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, R.A. Haemophilus influenzae: An underrated cause of vulvovaginitis in young girls. J. Clin. Pathol. 1997, 50, 765–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumbuliene, Ž.; Venclavičiute, K.; Ramašauskaite, D.; Arlauskiene, A.; Bumbul, E.; Drasutiene, G. Microbiological findings of vulvovaginitis in prepubertal girls. Postgrad. Med. J. 2014, 90, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.E.; Celik, N.; Soylu, G.; Donmez, A.; Yuksel, C. Comparison of clinical and microbiological features of vulvovaginitis in prepubertal and pubertal girls. J. Formos. Med. Assoc. 2012, 111, 392–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Africa, C.W. Efficacy of methods used for the diagnosis of bacterial vaginosis. Expert Opin. Med. Diagn. 2013, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Silva Cunha, A.; Grossiord, J.L.; Seiller, M. Pharmaceutical applications. In Multiple Emulsions: Structure, Properties and Applications; Grossiord, J.L., Seiller, M., Eds.; Editions de Sante: Paris, France, 1996; pp. 279–312. ISBN 9782864111191. [Google Scholar]
- Morimoto, Y.; Sugibayashi, K.; Yamaguchi, Y.; Kato, Y. Detoxication capacity of a multiple (w/o/w) emulsion for the treatment of drug overdose: Drug extraction into the emulsion in the gastro-intestinal tract of rabbits. Chem. Pharm. Bull. 1979, 27, 3188–3192. [Google Scholar] [CrossRef] [Green Version]
- Trussell, J.; Kost, K. Contraceptive Failure in the United States: A Critical Review of the Literature. Stud. Fam. Plann. 1987, 18, 237. [Google Scholar] [CrossRef]
- Weir, S.S.; Roddy, R.E.; Zekeng, L.; Feldblum, P.J. Nonoxynol-9 use, genital ulcers, and HIV infection in a cohort of sex workers. Genitourin. Med. 1995, 71, 78–81. [Google Scholar] [CrossRef] [Green Version]
- D’Cruz, O.J.; Samuel, P.; Uckun, F.M. Conceival, a novel noncontraceptive vaginal vehicle for lipophilic microbicides. AAPS PharmSciTech 2005, 6, E56–E64. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Properties | |
|---|---|
| API |
|
| Formulation |
|
| Type of Formulation | Macroemulsion | Microemulsion | Nanoemulsion | Multiple Emulsion | SEDDS a |
|---|---|---|---|---|---|
| Appearance | Milky | Transparent | Translucent or transparent | Milky | Depending on the vehicle b |
| Droplet size | >500 nm | Typically < 1000 nm | <500 nm | >1000 nm | <100 nm |
| Droplet shape | Spherical | Spherical and non-spherical | Spherical | Multiple droplets | Spherical and non-spherical a |
| Polydispersity | Often high | Often low (single, narrow distribution peak) | Low or moderate (single or multiple distribution peaks) | Often high | Often low a |
| Stability | Thermodynamically and kinetically unstable | Thermodynamically stable | Kinetically stable | Often thermodynamically and kinetically unstable | - |
| Manufacturing methods | High and low-energy methods | Spontaneous formation | High and low-energy methods | Two-steps low-energy process | Spontaneous formation a |
| Excipients | Group/Function | Applied Excipient |
|---|---|---|
| Oil phase | Mineral oils | Paraffin oil/White Vaseline |
| Vegetable oils and essential oils | Copaiba oil, Eucalyptus essential oil, Geranium oil, Lemongrass essential oil Mentha essential oil, Soybean oil, Tea Tree oil | |
| Sterols | Cholesterol | |
| Phospholipids | Phospholipon 90G (soybean lecithin at 90% of phosphatidylcholine) | |
| Fatty acids | Oleic acid | |
| Fatty acid monoesters | Capryol 90 (propylene glycol monocaprylate), Cetyl palmitate, Glycerol monolaurate, Isopropyl myristate, Monoglycerides of caprylic acid | |
| Fatty acid diesters/triesters | Captex 300 (medium-chain triglyceride of caprylic and capric acid), Labrafac lipophile (medium-chain triglycerides of caprylic and capric acid), Labrasol (PEG-8 caprylic/capric glycerides), other undefined medium chain triglycerides | |
| Alkene derivates | Parleam (Hydrogenated polyisobutene) | |
| Organosilicon compounds | Cyclomethicon tetramer, Cyclomethicon pentamer | |
| Surfactants | Non-ionic surfactants | Polysorbates: Tween 20 (polysorbate 20), Tween 80 (polysorbate 80) Sorbitan esters: Span 60, Span 80 PEG derivatives: Gelucire 44/13 (mono/dri/triglycerides and PEG-32 mono- and diesters of lauric acid), Labrasol (PEG-8 caprylic/capric glycerides), Kolliphor EL/Cremophor EL (PEG-35 castor oil), Kolliphor HS (Macrogol (15)-hydroxystearate), Kolliphor RH 40/Cremophor RH 40 (PEG-40 castor oil) Polyoxyethylene derivatives: Brij 20 (polyoxyethylene (20) cetyl ether) Polyoxypropylene derivatives: Pluronic F68 (Poloxamer 188), Pluronic F127 (Poloxamer 407) |
| Amphoteric surfactants | Amino acid derivatives: Tego Betain F (Cocamidopropyl Betaine) | |
| Cationic surfactants | Amins: Cetylpyridinium chloride | |
| Other surfactants | Organosilicon compounds: Abil WE 09 (polyglyceryl-4 isostearate; Cetyl PEG/PPG-10/1 dimethicone; hexyl laurate), Abil EM 90 (Cetyl PEG/PPG-10/1 Dimethicone) Bacterial saccharides: Exopolysaccharide from B. vallismortis WF4 strain (mannose/glucose/xylose/arabinose) | |
| Cosurfactants | Alcohols | Ethanol, glycerol, propylene glycol, transcutol P (2-(2-ethoxyethoxy)ethanol) |
| Phospholipids | Soy phosphatidylcholine | |
| Fatty acids and their monoesters | Caprylic acid, Capryol 90 (propylene glycol monocaprylate) | |
| PEGs and PEGs derivatives | Labrasol (PEG-8 caprylic/capric glycerides), PEG 200, PEG 300, PEG 400 | |
| Polysorbates | Tween 20, Tween 80 | |
| Other | Gelling agents | Carbomers: CP 934 (Carbopol 934), CP 940 (Carbopol 940), CP ETD 2020 (Carbopol ETD 2020), CP U 10 NF (Carbopol Ultrez 10 NF), Tego Carbomer 341 Polyoxypropylene derivatives: Pluronic F127 (Poloxamer 407) Polysaccharides: Chitosan, HPMC (hydroxypropyl methylcellulose), NaCMC (sodium carboxymethyl cellulose), Xantural (XG, xanthan gum) |
| Preservatives | Benzyl alcohol, Chlorocresol, Methylparaben, Sodium benzoate | |
| pH regulators | Lactic acid, Phosphate buffer, Triethanolamine | |
| Electrolytes | Magnesium sulphate, Sodium chloride | |
| Humectants | Propylene glycol, PEG 200 |
| API (Indication) | Formulation | Oil Phase/Surfactant/Cosurfactant/Others | Particle Size (nm) | PDI | Zeta Potential (mV) | Manufacturing Method | Ref. |
|---|---|---|---|---|---|---|---|
| Vaginal macroemulsions | |||||||
| Benzydamine (Antibacterial/Anti-inflammatory) | Emulgel | white Vaseline, paraffin/n.a./n.a./Water phase: NaCMC, glycerol, citrate buffer | n.a. | n.a. | n.a. | Mixing | [106] |
| Progesterone (n.a.) | W/S emulsion | cyclomethicone pentamer/Abil WE 09/glycerol/Sodium chloride | 1000–3000 | n.a. | n.a. | Mixing | [81] |
| Ciprofloxacin (Antibacterial) | W/S emulsion | cyclomethicone pentamer ortetramer/Abil WE 09/glycerol/ Sodium chloride | 2230–2540 | n.a. | n.a. | Mixing | [80] |
| Vaginal microemulsion | |||||||
| - (Contraceptive) | Microemulgel | Captex 300/Cremophor EL, Phospholipon 90 G, Propylene Glycol/PEG 200/Seaspan carrageenan, Viscarin carrageenan, Sodium benzoate | 30–80 | n.a. | n.a. | n.a. | [100] |
| - (Contraceptive) | Microemulgel | Captex 300/Cremophor EL, Pluronic F68, Phospholipon 90G, Propylene glycol/Xanthan gum, Sodium benzoate | 30–80 | n.a. | n.a. | n.a. | [77] |
| Vanadocene (Contraceptive) | Microemulgel | Captex 300, Phospholipon 90G/Cremophor EL, Pluronic F68/Xanthan gum | 30–80 | n.a. | n.a. | n.a. | [76] |
| Fluconazole (Antifungal) | Microemulgel | Capryol 90/Cremophor EL/Benzyl alcohol, chlorocresol, CP ETD 2020 | 24 | 0.98 | n.a | Mixing | [84] |
| Clotrimazole (Antifungal) | Microemulgel | Capryol 90/Cremophor EL/Benzyl alcohol, chlorocresol, CP ETD 2020 | 48 | 0.75 | n.a | Mixing | [82] |
| Sertaconazole (Antifungal) | Microemulgel | Oleic Acid/Tween 80/Propylene glycol/CP 940 | 26 | 0.55 | 0.26 | Mixing, dissolving API under ultrasonication | [86] |
| Tetrahydro-curcumin (Vaginal microbicide, HIV protection) | Microemulgel | Gycerol monolaurate/Tween 20/Transcutol P/CP U 10 NF, triethanolamine | 130 | 0.18 | n.a. | Low-energy (mixing and heating) | [85] |
| Phloretin (Anti-inflammatory) | Microemulsion | Oleic acid/Tween 20/Ethanol | 11 | n.a | n.a | Mixing | [83] |
| Vaginal nanoemulsion | |||||||
| Itraconazole (Antifungal) | Nanoemulgel | Tea tree oil/Tween 20/Labrasol/CP 934, Poloxamer 407 | 42 | 0.12 | −44 | Low-energy method (mixing) | [94] |
| Oxiconazole (Antifungal) | Nanoemulgel | Isopropyl myristate/Cremophor EL/Ethanol/HPMC or XG or CP 934 | 26 | 0.55 | −34 | Low-energy method (mixing) | [90] |
| Clotrimazole (Antifungal) | Nanoemulsion | Labrafac lipophile/Labrasol/Capryol 90/Propylene glycol (aqueous phase) | 153–186 | 0.37–0.85 | −15–−1 | Low-energy method (mixing, heating), High-energy method (sonication) | [91] |
| Polyphenon 60, Curcumin (Antibacterial) | Nanoemulgel | Soybean oil/Tween 20/Propylene glycol/Chitosan | 211 | 0.34 | −33 | Low-energy method (mixing), High-energy method (high-speed homogenization and ultrasonication) | [98] |
| Polyphenon 60, cranberry (Antibacterial) | Nanoemulgel | Oleic acid/Tween 20/Glycerol/Chitosan, lactic acid | 58 | 0.20 | −16 | Low-energy method (mixing), High-energy method (high-speed homogenization and ultrasonication) | [92] |
| Mentha essential oil (Antifungal) | Nanoemulgel | Mentha essential oil/Tween 80/PEG 400/CP 940, methylparaben, triethanolamine | 178 | 0.18 | −32 | High-energy method (high-speed homogenization) | [95] |
| Nystatine (Antifungal) | Nanoemulsion | Paraffin oil/Exopolysaccharide/PEG 400 | 131 | 0.08 | −40 | Low-energy method (mixing), High-energy method (ultrasonication) | [74] |
| Ciprofloxacin, Polyphenon 60 (Antibacterial) | Nanoemulsion | Labrasol/Cetylperidinum chloride/Glycerol | 151 | 0.20 | 55 | Low-energy method (mixing), High-energy method (high-speed homogenization and ultrasonication) | [79] |
| Geranium oil (Antifungal) | Nanoemulgel | Geranium oil/Span 80/Tween 20/Chitosan | 281 | 0.32 | 53 | High-energy method (high-speed and high-pressure homogenization) | [93] |
| Syngonanthus nitens (Bong.) extract (Antifungal) | Nanoemulsion | Cholesterol/Brij 20/Soy phosphatidylcholine/Chitosan, phosphate buffer | 111 | 0.30 | 2 | Low-energy method (mixing), High-energy method (sonication) | [75] |
| Imiquimod (Cancer treatment) | Nanoemulsion | Copaiba oil/Span 60/Tween 80 | 190 | 0.11 | n.a. | Low-energy method (mixing and solvent evaporation) | [78] |
| Eucalyptus essential oil (Antifungal) | Nanoemulsion | Eucalyptus essential oil/Polysorbate 80/Sorbitan monooleate | 68 | 0.18 | −9 | High-energy (high-speed homogenization) | [96] |
| Lemongrass essential oil (Antifungal) | Nanoemulsion | Lemongrass essential oil/Polysorbate 80/Sorbitan monooleate | 90 | 0.21 | −8 | High-energy (high-speed homogenization) | [96] |
| API(s) (Indication) | Formulation | Oil Phase/Lipophilic Surfactant/Hydrophilic Surfactant/Other | Particle Size (nm) | PDI | Zeta Potential (mV) | Manufacturing Method | Ref. |
|---|---|---|---|---|---|---|---|
| W1: benzalkonium chloride O: octadecylamine W2: lactic acid (Antibacterial) | Multiple emulsion | Parleam/Abil EM 90/Poloxamer 407 | >5000 | n.a. | n.a. | Two-step process | [101] |
| W1: benzalkonium chloride W2: chlorhexidine (Antibacterial) | Multiple emulsion | Parleam/Abil EM 90/Poloxamer 407/Sodium chloride | >5000 | n.a. | n.a | Raynal method [71] | [104] |
| W1: metronidazole W2: Ornidazole (Antibacterial) | Multiple emulsion | Parleam/Abil EM 90/Poloxamer 407/Magnesium sulphate | >8000 | n.a. | n.a. | Raynal method [71] | [102] |
| O: Clotrimazole (Antifungal) | Multiple emulsion-based gel | Labrafac lipophile, Cetyl palmitate/Abil EM 90, Span 60/Cocamidopropyl Betaine/Tego Carbomer 341, Sodium chloride | >29,000 | n.a. | −55 | Modification of Raynal method [71] | [89] |
| API (Indication) | Formulation | Oil Phase/Surfactant/Cosurfactant/Others | Particle Size (nm) | PDI | Zeta Potential (mV) | Manufacturing Method | Ref. |
|---|---|---|---|---|---|---|---|
| UC 781 (HIV-protection) | SMEDDS | Mono- and diglycerides of caprylic acid/Cremophor RH40/PEG 300 | 13 | 0.25 | 32 | Mixing | [99] |
| Curcumin (HPV-protection) | SNEDDS | Medium chain triglycerides/Cremophor RH40/PEG 200, Caprylic acid, Tween 80 | 38 | 0.35 | −1 | Mixing | [105] |
| 17-α hydroxyprogesterone (Pre-term births prevention) | Solid-state SNEDDS Vaginal tablet | Captex 300/Kolliphor HS/Polyvinyl alcohol, calcium silicate, microcrystalline cellulose, Kollidon CL, Magnesium stearate | 50 | 0.09 | −7 | Mixing, tablet formation | [87] |
| The SphK inhibitor (Pre-term births prevention) | SNEDDS | Captex 300/Kolliphor HS/Dimethyl-acetamide | 37 | 0.05 | −5 | Mixing | [88] |
| W1: siRNA (Gene silencing) | Multiple microemulsion SEDDS gel | Medium chain triglycerides/Lipophilic: Cremophor RH40, Span 80; Hydrophilic: Cremophor RH 40/Lecithin (hydrophilic and lipophilic)/‘thermosensitive gel’ a | 167 | 0.18 | −7 | Two-step process | [103] |
| Lawsone (Antifungal) | SMEDDS hollow pessary | Capryol 90/Gelucire 44/14/Tween 80/Ovucire WL3460, beeswax | 12 | 0.27 | −11 | Mixing | [97] |
| API(s) (Formulation) | pH | Droplet Size | Viscosity c (Pa·s) | Spreadability | Bioadhesion | In Vitro Release/Permeability | In Vivo Studies | Ref. |
|---|---|---|---|---|---|---|---|---|
| Vaginal macroemulsions | ||||||||
| Benzydamine (Emulgel) | - | - | Plate-plate (100–700) | - | in vitro, T/DF, porcine VM | Franz cells | - | [106] |
| Progesterone (W/S * emulsion) | - | MS | Cone-plate (21.2–186.6) | - | in vitro, T/DF, bovine VM, GTL | USP II/Franz cells | rats | [81] |
| Ciprofloxacin (W/S * emulsion) | - | MS | Cone-plate (1.4–17.0) (1.5–14.0) | - | in vitro, T/DF, GTL | Franz cells and in vivo | rats | [80] |
| Vaginal microemulsion | ||||||||
| - (Microemulgel) | - | DLS | + a | - | - | - | rabbits | [100] |
| - (Microemulgel) | - | DLS | + a | - | - | - | rabbits | [77] |
| Vanadocene (Microemulgel) | - | DLS | Results n.a. | - | - | - | rabbits, pigs | [76] |
| Fluconazole (Microemulgel) | 4.5 b | DLS | Spindle (9800 at 5 rpm) | P-P | in vitro, NM, agar plate | - | rabbits, 11 female patients | [84] |
| Clotrimazole (Microemulgel) | 4.5 b | DLS | Spindle (9000 at 5 rpm) | P-P | in vitro, NM, agar plate | Modified Apparatus No. 1 USP 23 | - | [82] |
| Sertaconazole (Microemulgel) | 4.2 b | DLS | + (2.0) | P-P | in vitro, T/DF, goat VM | Franz cells | - | [86] |
| Tetrahydrocurcumin (Microemulgel) | 6.0 b | DLS | Spindle (11.5 at 5 rpm) | TA | - | Dialysis bag | - | [85] |
| Phloretin (Microemulsion) | - | DLS | - | - | - | - | - | [83] |
| Vaginal nanoemulsion | ||||||||
| Itraconazole (Nanoemulgel) | 5.5 b (nanoemulsion) | DLS | Spindle (0.91) | - | in vitro, T/DF, CM, in vivo (rats) | Franz Cells | rats | [94] |
| Oxiconazole (Nanoemulgel) | 6.9 b (gel with HPMC) | DLS | Cone-plate (8.43 at 50 rpm for gel with HPMC) | P-P | in vitro, NM, animal vagina | USP II | - | [90] |
| Clotrimazole (Nanoemulsion) | 5.7 b | DLS | Cone-plate (0.041–0.042 at 100/s) | P-P | - | Franz Cells | 10 women—skin tolerance | [91] |
| Polyphenon 60, Curcumin (Nanoemulgel) | - | DLS | + (0.66–141) | - | - | Dialysis bag | rats | [98] |
| Polyphenon 60, cranberry (Nanoemulgel) | 3.7 b | DLS | + (>141 at 0.01/s) | - | - | Dialysis cells | rats | [92] |
| Mentha essential oil (Nanoemulgel) | 5.2 b | DLS | Spindle (24.8) | TA | in vitro | - | mice | [95] |
| Nystatine (Nanoemulsion) | - | DLS | Spindle (0.12) | - | - | Dialysis bag | mice | [74] |
| Ciprofloxacin, Polyphenon 60 (Nanoemulsion) | - | DLS | - | - | - | USP II | rats | [79] |
| Geranium oil (Nanoemulgel) | 4.4 b | DLS | Spindle (0.4–0.5 at 50/s–0.01/s) | - | in vitro, T/DF, porcine VM | - | - | [93] |
| Syngonanthus nitens (Bong.) extract (Nanoemulsion) | - | DLS | Cone-plate | - | in vitro, T/DF, porcine VM | - | rats | [75] |
| Imiquimod (Nanoemulsion) | 6.0 b | DLS | - | - | - | Franz cells | - | [78] |
| Eucalyptus essential oil (Nanoemulsion) | 5.3 b | DLS | - | - | - | - | mice | [96] |
| Lemongrass essential oil (Nanoemulsion) | 4.6 b | DLS | - | - | - | - | mice | [96] |
| Vaginal multiple emulsions | ||||||||
| W1: benzalkonium chloride O: octadecylamine W2: lactic acid (Multiple emulsion) | 7.8 b | MS, GA | Cone-plate (3.2 at 100/s) | - | - | - | - | [101] |
| W1: benzalkonium chloride W2: chlorhexidine (Multiple emulsion) | - | MS | Cone-plate (Isosmotic condition: 0.003 at 100/s) | - | - | Conductometric (NaCl as a marker) | - | [104] |
| W1: metronidazole W2: ornidazole (Multiple emulsion) | W1: 5.7 b W2: 6.0 b | MS | - | - | - | Dialysis tube | rabbits | [102] |
| O: Clotrimazole (Multiple emulsion-based gel) | 6.5 b | LD | parallel plate-plate (0.29 at 100/s) | P-P | - | Franz cells | - | [89] |
| Vaginal Self-Emulsifying Drug Delivery Systems | ||||||||
| UC 781 (SMEDDS) | - | DLS | - | - | - | Dialysis bag (balloon) | - | [99] |
| Curcumin (SNEDDS) | - | DLS | Plate-plate (116.3) | OM | - | Transwell chambers | - | [105] |
| 17-α hydroxyprogesterone (Solid-state SNEDDS Vaginal tablet) | - | DLS | - | - | - | USP II | mice | [87] |
| The SphK inhibitor (SNEDDS) | - | DLS | Spindle (0.2-fold dilution: 0.53 0.4-fold dilution: 4.8 at 20 rpm) | - | - | USP II | mice | [88] |
| W1: siRNA (Multiple emulsion SEDDS gel) | - | DLS | - | - | - | Dialysis bag | mice | [103] |
| Lawsone (SMEDDS hollow pessary) | 4.2–4.8 b | DLS | Spindle (0.956–1.023) | - | - | USP I | - | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoleński, M.; Karolewicz, B.; Gołkowska, A.M.; Nartowski, K.P.; Małolepsza-Jarmołowska, K. Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels. Int. J. Mol. Sci. 2021, 22, 6455. https://doi.org/10.3390/ijms22126455
Smoleński M, Karolewicz B, Gołkowska AM, Nartowski KP, Małolepsza-Jarmołowska K. Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels. International Journal of Molecular Sciences. 2021; 22(12):6455. https://doi.org/10.3390/ijms22126455
Chicago/Turabian StyleSmoleński, Michał, Bożena Karolewicz, Anna M. Gołkowska, Karol P. Nartowski, and Katarzyna Małolepsza-Jarmołowska. 2021. "Emulsion-Based Multicompartment Vaginal Drug Carriers: From Nanoemulsions to Nanoemulgels" International Journal of Molecular Sciences 22, no. 12: 6455. https://doi.org/10.3390/ijms22126455