The Low-Molecular Weight Protein Arginine Phosphatase PtpB Affects Nuclease Production, Cell Wall Integrity, and Uptake Rates of Staphylococcus aureus by Polymorphonuclear Leukocytes
Abstract
:1. Introduction
2. Results and Discussion
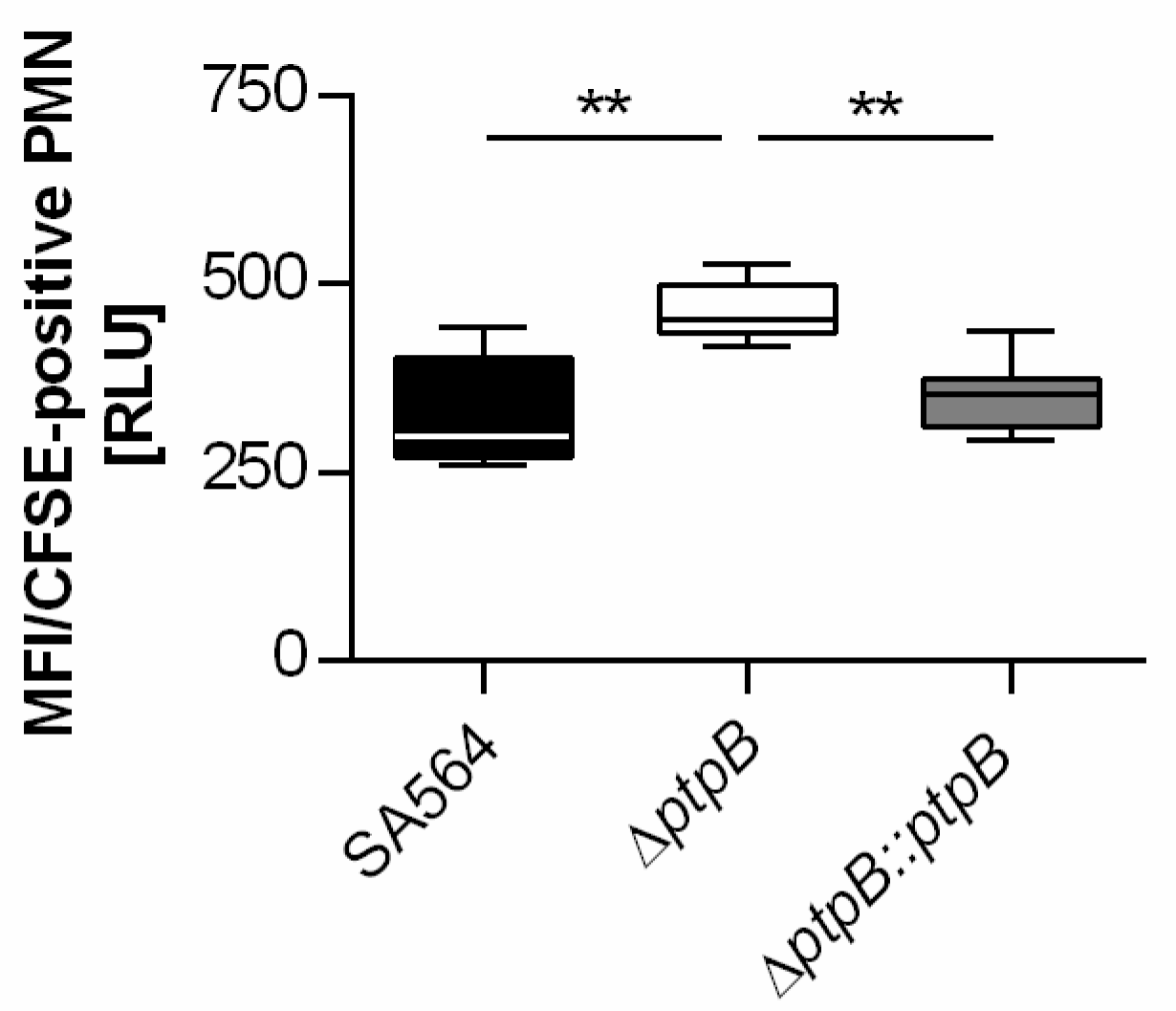
2.1. PtpB Affects the Ability of S. aureus to Evade Phagocytosis by PMNs
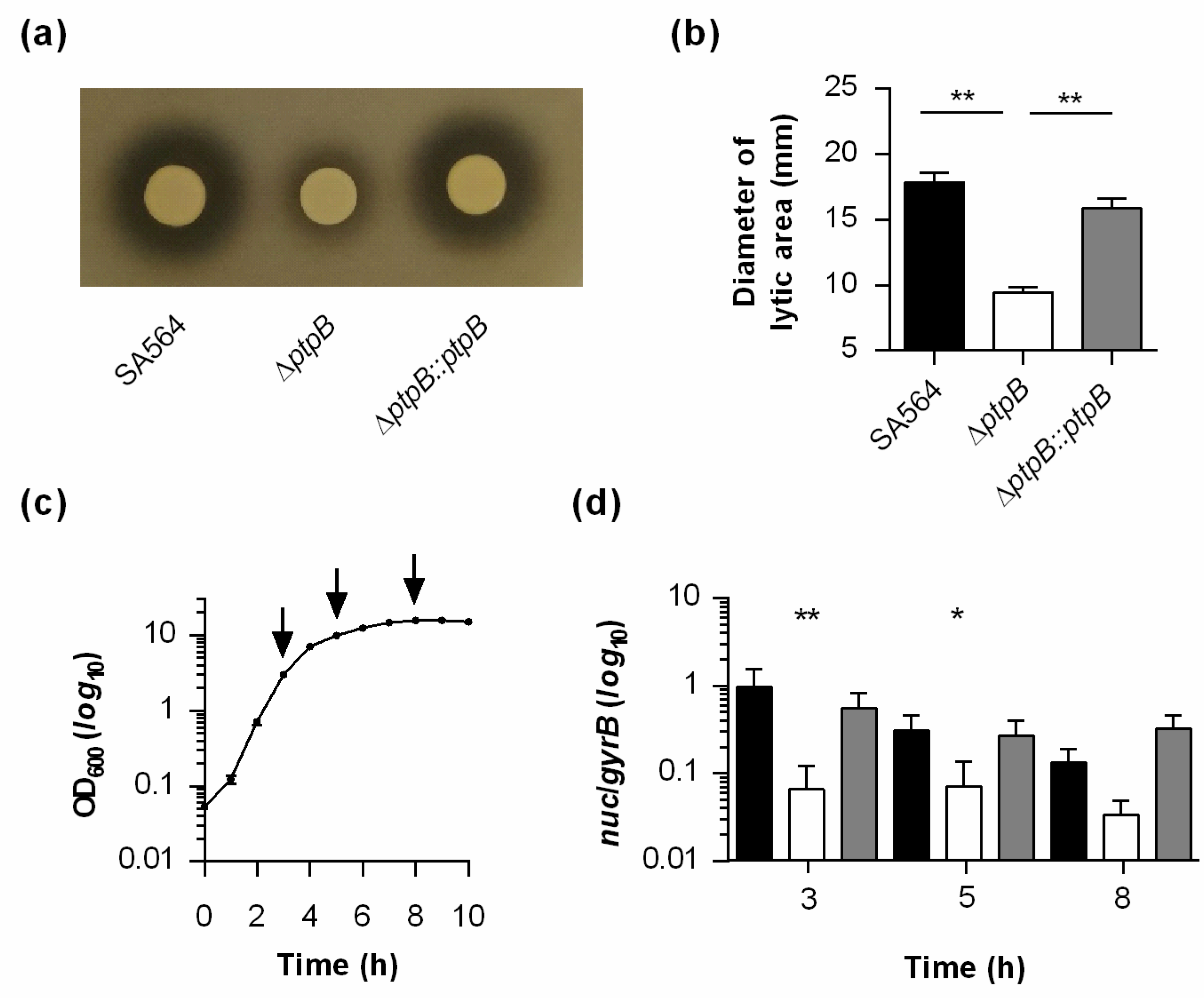
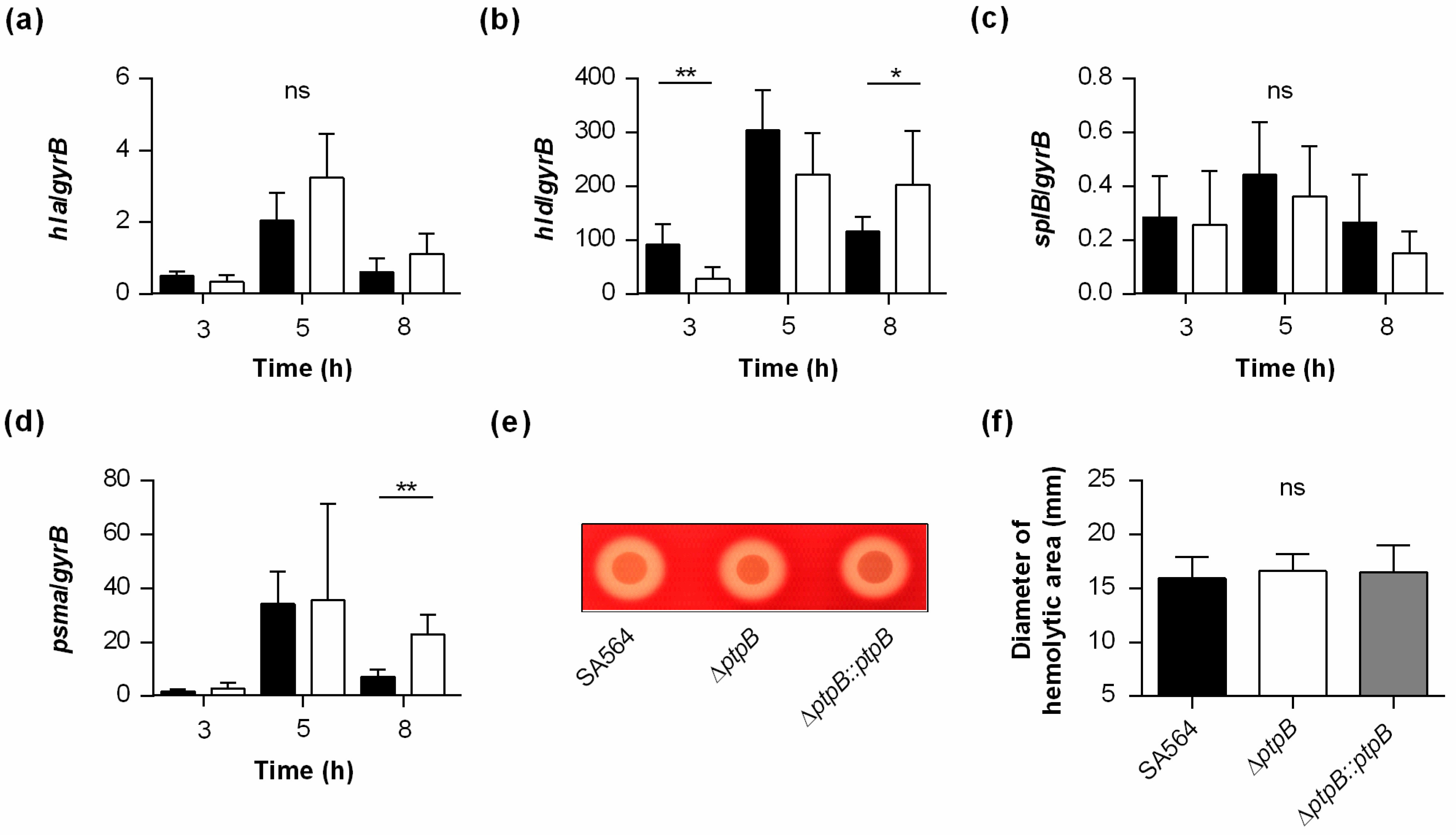
2.2. PtpB Promotes the Transcription and Secretion of Nuclease in S. aureus
2.3. PtpB Promotes the Transcription of the Aureolysin Encoding Gene aur in S. aureus
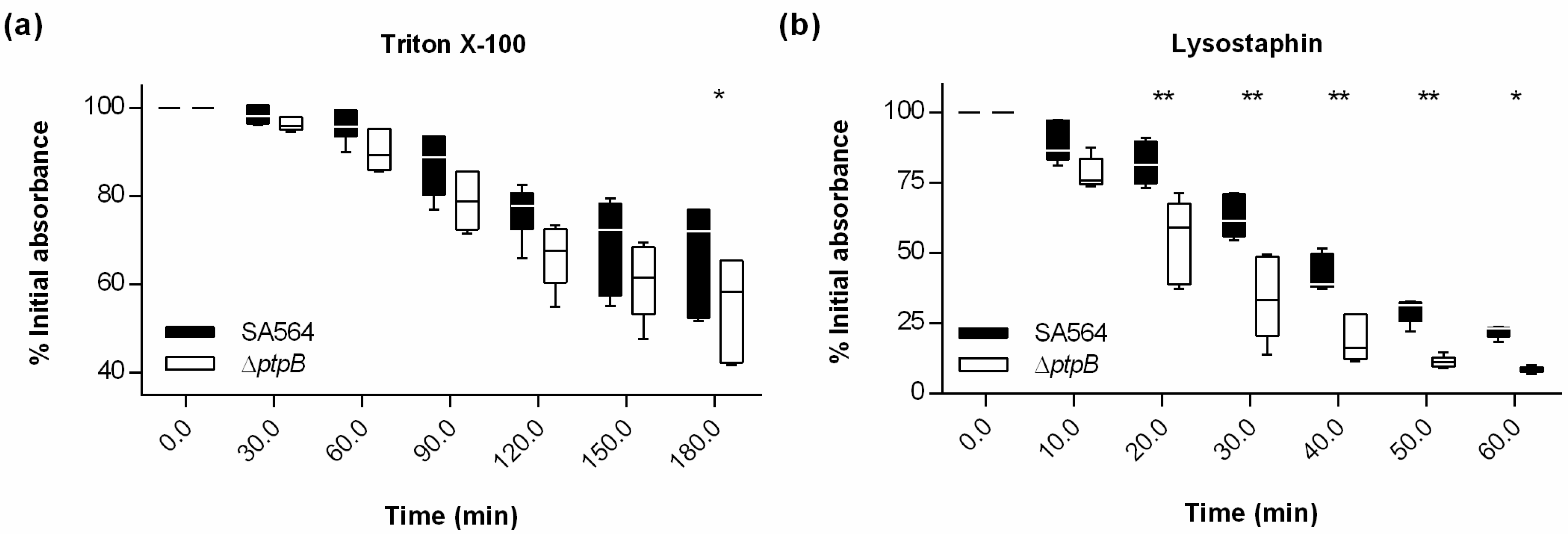
2.4. PtpB Reduces the Autolytic Activity of S. aureus
2.5. PtpB Does Not Alter MgrA Activity per Se
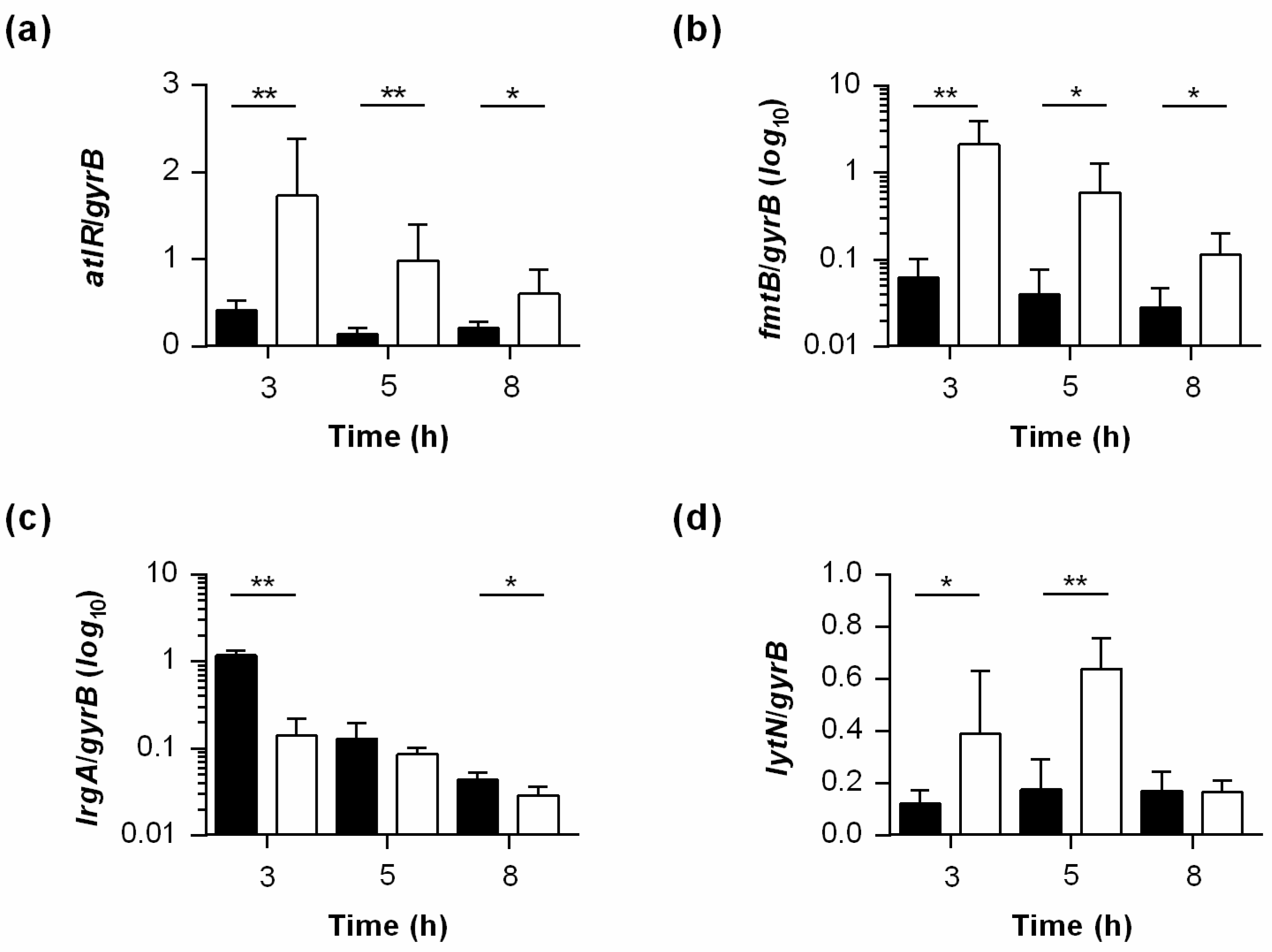
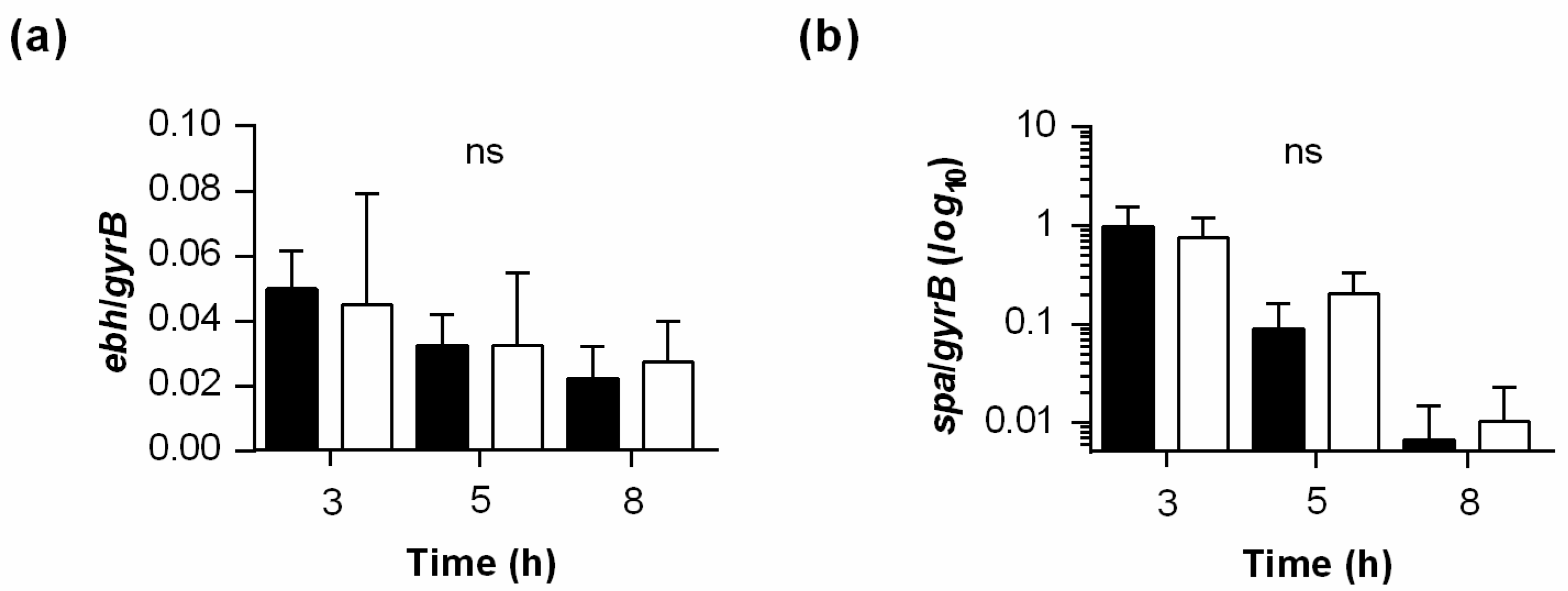
2.6. PtpB Alters the Transcription of Some but Not All SarA Regulated Genes
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strains, Media, and Growth Conditions
4.2. Human Whole Blood Phagocytosis Assay
4.3. Extracellular DNase-, Hemolytic- and Proteolytic Activity Assays
4.4. Triton X-100 Induced Autolysis Assay
4.5. Lysostaphin Induced Autolysis Assay
4.6. qRT-PCR Analyses
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haag, A.F.; Fitzgerald, J.R.; Penadés, J.R. Staphylococcus aureus in Animals. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Bischoff, M.; Rhomby, P. Genetic Regulation. In Staphylococcus: Genetics and Physiology; Somerville, G.A., Ed.; Caister Academic Press: Cambridge, MA, USA, 2016; pp. 301–334. [Google Scholar]
- Balasubramanian, D.; Harper, L.; Shopsin, B.; Torres, J.V. Staphylococcus aureus Pathogenesis in Diverse Host Environments. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Burnside, K.; Lembo, A.; Reyes, M.D.L.; Iliuk, A.; BinhTran, N.-T.; Connelly, J.E.; Lin, W.-J.; Schmidt, B.Z.; Richardson, A.R.; Fang, F.C.; et al. Regulation of Hemolysin Expression and Virulence of Staphylococcus aureus by a Serine/Threonine Kinase and Phosphatase. PLoS ONE 2010, 5, e11071. [Google Scholar] [CrossRef]
- Junker, S.; Maaß, S.; Otto, A.; Hecker, M.; Becher, D. Toward the Quantitative Characterization of Arginine Phosphorylations in Staphylococcus aureus. J. Proteom. Res. 2019, 18, 265–279. [Google Scholar]
- Junker, S.; Maaß, S.; Otto, A.; Michalik, S.; Morgenroth, F.; Gerth, U.; Hecker, M.; Becher, D. Spectral Library Based Analysis of Arginine Phosphorylations in Staphylococcus aureus. Mol. Cell. Proteom. 2018, 17, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Elhawy, M.I.; Huc-Brandt, S.; Pätzold, L.; Gannoun-Zaki, L.; Abdrabou, A.M.M.; Bischoff, M.; Molle, V. The Phos-Phoarginine Phosphatase PtpB from Staphylococcus aureus Is Involved in Bacterial Stress Adaptation During Infection. Cells 2021, 10, 645. [Google Scholar] [CrossRef]
- Wozniak, D.J.; Tiwari, K.B.; Soufan, R.; Jayaswal, R.K. The McsB Gene of the ClpC Operon Is Required for Stress Tolerance and Virulence in Staphylococcus aureus. Microbiology 2012, 158, 2568–2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trentini, D.B.; Suskiewicz, M.J.; Heuck, A.; Kurzbauer, R.; Deszcz, L.; Mechtler, K.; Clausen, T. Arginine Phosphorylation Marks Proteins for Degradation by a Clp Protease. Nature 2016, 539, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, J.; Schmidt, A.; Spiess, S.; Lehner, A.; Turgay, K.; Mechtler, K.; Charpentier, E.; Clausen, T. McsB Is a Protein Arginine Kinase That Phosphorylates and Inhibits the Heat-Shock Regulator CtsR. Science 2009, 324, 1323–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somerville, G.A.; Beres, S.B.; Fitzgerald, J.R.; DeLeo, F.R.; Cole, R.L.; Hoff, J.S.; Musser, J.M. In Vitro Serial Passage of Staphylococcus aureus: Changes in Physiology, Virulence Factor Production, and Agr Nucleotide Sequence. J. Bacteriol. 2002, 184, 1430–1437. [Google Scholar] [CrossRef] [Green Version]
- Giraud, C.; Hausmann, S.; Lemeille, S.; Prados, J.; Redder, P.; Linder, P. The C-Terminal Region of the RNA Helicase CshA Is Required for the Interaction with the Degradosome and Turnover of Bulk RNA in the Opportunistic Pathogen Staphylococcus aureus. RNA Biol. 2015, 12, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P.; Ross, H.F.; Projan, S.J.; Kornblum, J.; Kreiswirth, B.; Moghazeh, S. Synthesis of Staphylococcal Virulence Factors Is Controlled by a Regulatory RNA Molecule. EMBO J. 1993, 12, 3967–3975. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The Multifaceted Functions of Neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berends, E.T.; Horswill, A.R.; Haste, N.M.; Monestier, M.; Nizet, V.; Von Köckritz-Blickwede, M. Nuclease Expression by Staphylococcus aureus Facilitates Escape from Neutrophil Extracellular Traps. J. Innate Immun. 2010, 2, 576–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbeis, J.; Saffarzadeh, M.; Peisker, H.; Jung, P.; Thewes, N.; Preissner, K.T.; Herrmann, M.; Molle, V.; Geisbrecht, B.V.; Jacobs, K.; et al. The Staphylococcus aureus Extracellular Adherence Protein Eap Is a DNA Binding Protein Capable of Blocking Neutrophil Extracellular Trap Formation. Front. Cell. Infect. Microbiol. 2018, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Jusko, M.; Potempa, J.; Kantyka, T.; Bielecka, E.; Miller, H.K.; Kalinska, M.; Dubin, G.; Garred, P.; Shaw, L.N.; Blom, A.M. Staphylococcal Proteases Aid in Evasion of the Human Complement System. J. Innate Immun. 2014, 6, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Golonka, E.; Potempa, J.; Foster, S.J. The Role and Regulation of the Extracellular Proteases of Staphylococcus aureus. Microbiology 2004, 150, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahl, H.-G.; Pag, U.; Bonness, S.; Wagner, S.; Antcheva, N.; Tossi, A. Mammalian Defensins: Structures and Mechanism of Antibiotic Activity. J. Leukoc. Biol. 2004, 77, 466–475. [Google Scholar] [CrossRef]
- Schindler, C.A.; Schuhardt, V.T. Purification and Properties of lysostaphin—A Lytic Agent for Staphylococcus aureus. Biochim. Biophys. Acta (BBA) Gen. Subj. 1965, 97, 242–250. [Google Scholar] [CrossRef]
- Koehl, J.L.; Muthaiyan, A.; Jayaswal, R.K.; Ehlert, K.; Labischinski, H.; Wilkinson, B.J. Cell Wall Composition and Decreased Autolytic Activity and Lysostaphin Susceptibility of Glycopeptide-Intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 3749–3757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trotonda, M.P.; Xiong, Y.Q.; Memmi, G.; Bayer, A.S.; Cheung, A.L. Role of MgrA and SarA in Methicillin-Resistant Staphylococcus aureus Autolysis and Resistance to Cell Wall-Active Antibiotics. J. Infect. Dis. 2009, 199, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, A.C.; Ingavale, S.S.; Maloney, M.; Van Wamel, W.; Cheung, A.L. Identification of SarV (SA2062), a New Transcriptional Regulator, Is Repressed by SarA and MgrA (SA0641) and Involved in the Regulation of Autolysis in Staphylococcus aureus. J. Bacteriol. 2004, 186, 5267–5280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luong, T.T.; Dunman, P.M.; Murphy, E.; Projan, S.J.; Lee, C.Y. Transcription Profiling of the MgrA Regulon in Staphylococcus aureus. J. Bacteriol. 2006, 188, 1899–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosby, H.A.; Schlievert, P.M.; Merriman, J.A.; King, J.M.; Salgado-Pabón, W.; Horswill, A.R. The Staphylococcus aureus Global Regulator MgrA Modulates Clumping and Virulence by Controlling Surface Protein Expression. PLoS Pathog. 2016, 12, e1005604. [Google Scholar] [CrossRef] [PubMed]
- Groicher, K.H.; Firek, B.A.; Fujimoto, D.F.; Bayles, K.W. The Staphylococcus aureus LrgAB Operon Modulates Murein Hydrolase Activity and Penicillin Tolerance. J. Bacteriol. 2000, 182, 1794–1801. [Google Scholar] [CrossRef] [Green Version]
- Sugai, M.; Fujiwara, T.; Komatsuzawa, H.; Suginaka, H. Identification and Molecular Characterization of a Gene Homologous to Epr (endopeptidase Resistance Gene) in Staphylococcus aureus. Gene 1998, 224, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Komatsuzawa, H.; Ohta, K.; Sugai, M.; Fujiwara, T.; Glanzmann, P.; Berger-Bächi, B.; Suginaka, H. Tn551-Mediated Insertional Inactivation of the FmtB Gene Encoding a Cell Wall-Associated Protein Abolishes Methicillin Resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 45, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, D.F.; Brunskill, E.W.; Bayles, K.W. Analysis of Genetic Elements Controlling Staphylococcus aureus LrgAB Expression: Potential Role of DNA Topology in SarA Regulation. J. Bacteriol. 2000, 182, 4822–4828. [Google Scholar] [CrossRef] [Green Version]
- Ziebandt, A.K.; Weber, H.; Rudolph, J.; Schmid, R.; Höper, D.; Engelmann, S.; Hecker, M. Extracellular Proteins of Staphylococcus aureus and the Role of SarA and Sigma B. Proteomics 2001, 1, 480–493. [Google Scholar] [CrossRef]
- Dunman, P.M.; Murphy, E.; Haney, S.; Palacios, D.; Tucker-Kellogg, G.; Wu, S.; Brown, E.L.; Zagursky, R.J.; Shlaes, D.; Projan, S.J. Transcription Profiling-Based Identification of Staphylococcus aureus Genes Regulated by the Agr and/Or SarA Loci. J. Bacteriol. 2001, 183, 7341–7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.F.; Foster, S.J. Role of SarA in Virulence Determinant Production and Environmental Signal Trans-Duction in Staphylococcus aureus. J. Bacteriol. 1998, 180, 6232–6241. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Projan, S.J. Cloning and Sequencing of sarA of Staphylococcus aureus, a Gene Required for the Expression of agr. J. Bacteriol. 1994, 176, 4168–4172. [Google Scholar] [CrossRef] [Green Version]
- Novick, R.P. Autoinduction and Signal Transduction in the Regulation of Staphylococcal Virulence. Mol. Microbiol. 2003, 48, 1429–1449. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-Independent Target Gene Control by the Agr Quorum-Sensing System: Insight into the Evolution of Virulence Regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janczarek, M.; Vinardell, J.-M.; Lipa, P.; Karaś, M. Hanks-Type Serine/Threonine Protein Kinases and Phosphatases in Bacteria: Roles in Signaling and Adaptation to Various Environments. Int. J. Mol. Sci. 2018, 19, 2872. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Ding, Y.; Ji, Q.; Liang, Z.; Deng, X.; Wong, C.C.; Yi, C.; Zhang, L.; Xie, S.; Alvarez, S.; et al. Protein Cysteine Phosphorylation of SarA/MgrA Family Transcriptional Regulators Mediates Bacterial Virulence and Antibiotic Resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 15461–15466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiba, J.; Hartmann, T.; Cluzel, M.E.; Cohen-Gonsaud, M.; Delolme, F.; Bischoff, M.; Molle, V. A Novel Mode of Regu-Lation of the Staphylococcus aureus Catabolite Control Protein a (CcpA) Mediated by Stk1 Protein Phosphorylation. J. Biol. Chem. 2012, 287, 43607–43619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, M.; Wonnenberg, B.; Nippe, N.; Nyffenegger, N.; Voss, M.; Beisswenger, C.; Sunderkötter, C.; Molle, V.; Dinh, Q.T.; Lammert, F.; et al. CcpA Affects Infectivity of Staphylococcus aureus in a Hyperglycemic Environment. Front. Cell. Infect. Microbiol. 2017, 7, 172. [Google Scholar] [CrossRef]
- Maccari, R.; Ottanà, R. Low Molecular Weight Phosphotyrosine Protein Phosphatases as Emerging Targets for the Design of Novel Therapeutic Agents. J. Med. Chem. 2011, 55, 2–22. [Google Scholar] [CrossRef]
- Ruddraraju, K.V.; Aggarwal, D.; Zhang, Z.Y. Therapeutic Targeting of Protein Tyrosine Phosphatases from Mycobacterium tuberculosis. Microorganisms 2020, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Pardella, E.; Pranzini, E.; Leo, A.; Taddei, M.L.; Paoli, P.; Raugei, G. Oncogenic Tyrosine Phosphatases: Novel Therapeutic Targets for Melanoma Treatment. Cancers 2020, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y. Drugging the Undruggable: Therapeutic Potential of Targeting Protein Tyrosine Phosphatases. Accounts Chem. Res. 2017, 50, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.M.; Aleshin, A.E.; Zhang, V.; Ardecky, R.J.; Hedrick, M.P.; Zou, J.; Ganji, S.R.; Bliss, M.R.; Yamamoto, F.; Bobkov, A.A.; et al. Diabetes Reversal by Inhibition of the Low-Molecular-Weight Tyrosine Phosphatase. Nat. Chem. Biol. 2017, 13, 624–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballhausen, B.; Jung, P.; Kriegeskorte, A.; Makgotlho, P.E.; Ruffing, U.; Von Müller, L.; Köck, R.; Peters, G.; Herrmann, M.; Ziebuhr, W.; et al. LA-MRSA CC398 Differ from Classical Community Acquired-MRSA and Hospital Acquired-MRSA Lineages: Functional Analysis of Infection and Colonization Processes. Int. J. Med Microbiol. 2014, 304, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.; Tobin, P.; Jayaswal, R.K. Isolation and Characterization of Autolysis-Defective Mutants of Staphylococcus aureus Created by Tn917-LacZ Mutagenesis. J. Bacteriol. 1993, 175, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Peschel, A.; Vuong, C.; Otto, M.; Götz, F.; Cole, A.M.; Darouiche, R.O.; Legarda, D.; Connell, N.; Diamond, G. The D-Alanine Residues of Staphylococcus aureus Teichoic Acids Alter the Susceptibility to Vancomycin and the Activity of Autolytic Enzymes. Antimicrob. Agents Chemother. 2000, 44, 2039–2045. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, I.; Becker, P.; Grundmeier, M.; Bischoff, M.; Somerville, G.A.; Peters, G.; Sinha, B.; Harraghy, N.; Proctor, R.A.; Herrmann, M. Staphylococcus aureus ClpC Is Required for Stress Resistance, Aconitase Activity, Growth Recovery, and Death. J. Bacteriol. 2005, 187, 4488–4496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. Relative Quantification. In Real-Time PCR; Tevfik Dorak, M., Ed.; Taylor & Francis: Abingdon, UK, 2006; pp. 63–82. [Google Scholar]

| Strain | Description 1 | Reference |
|---|---|---|
| SA564 | S. aureus clinical isolate, wild type | [12] |
| SA564 ΔptpB | SA564 ΔptpB::lox66-erm(B)-lox71; ErmR | [8] |
| SA564 ΔptpB::ptpB | cis-complemented SA564 ΔptpB derivative | [8] |
| Gene Target | Primer | Sequence (5′-3′) |
|---|---|---|
| atlR | forward | AACTTATTACACTGACTAACAATG |
| reverse | TGTCCAAATCTTCTATTCACTAA | |
| aur | forward | AATAGTATTGACGGTGGATTT |
| reverse | AATGCTGATAATTTACCTTGATG | |
| ebh | forward | GTAATAATGAACAGACTGAGAATC |
| reverse | AGCGGATAATGATTGACTATT | |
| fmtB | forward | GATGCTTCAAGAATTACAACAA |
| reverse | ATCCTGAGAATAGACCTACAT | |
| gyrB | forward | GACTGATGCCGATGTGGA |
| reverse | AACGGTGGCTGTGCAATA | |
| hla | forward | AACCCGGTATATGGCAATCAACT |
| reverse | CTGCTGCTTTCATAGAGCCATTT | |
| hld | forward | AGGAGTGATTTCAATGGCACAAG |
| reverse | TGTGTCGATAATCCATTTTACTAAGTCA | |
| lrgA | forward | GCCGGTATCTCAGTTGTTAACTCTT |
| reverse | AAATGGTGCTTGGCTAATGACAC | |
| lytN | forward | CTATTGTCTTAAATGGTGATTATG |
| reverse | ATCTAAACTTTGGAACTTCATTA | |
| nuc | forward | TAGCTCAGCAAATGCATCACAA |
| reverse | GAACCACTTCTATTTACGCCATTATCT | |
| psma | forward | ATCAACAACTCATCACTATGTTAAATCAAC |
| reverse | GCCATCGTTTTGTCCTCCTGT | |
| ptpB | forward | AGCCCATTAGCGGAAAGTATTG |
| reverse | AAATTGATGATTTGGCATAACCTCT | |
| spa | forward | TACTTATATCTGGTGGCGTAA |
| reverse | GGTCGTCTTTAAGACTTTGA | |
| splB | forward | AAGGTAATGGTGGTATTTATTC |
| reverse | GAATGACTGATACATCTTCTTTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhawy, M.I.; Molle, V.; Becker, S.L.; Bischoff, M. The Low-Molecular Weight Protein Arginine Phosphatase PtpB Affects Nuclease Production, Cell Wall Integrity, and Uptake Rates of Staphylococcus aureus by Polymorphonuclear Leukocytes. Int. J. Mol. Sci. 2021, 22, 5342. https://doi.org/10.3390/ijms22105342
Elhawy MI, Molle V, Becker SL, Bischoff M. The Low-Molecular Weight Protein Arginine Phosphatase PtpB Affects Nuclease Production, Cell Wall Integrity, and Uptake Rates of Staphylococcus aureus by Polymorphonuclear Leukocytes. International Journal of Molecular Sciences. 2021; 22(10):5342. https://doi.org/10.3390/ijms22105342
Chicago/Turabian StyleElhawy, Mohamed Ibrahem, Virginie Molle, Sören L. Becker, and Markus Bischoff. 2021. "The Low-Molecular Weight Protein Arginine Phosphatase PtpB Affects Nuclease Production, Cell Wall Integrity, and Uptake Rates of Staphylococcus aureus by Polymorphonuclear Leukocytes" International Journal of Molecular Sciences 22, no. 10: 5342. https://doi.org/10.3390/ijms22105342






