BRAF Controls the Effects of Metformin on Neuroblast Cell Divisions in C. elegans
Abstract
:1. Introduction
2. Results
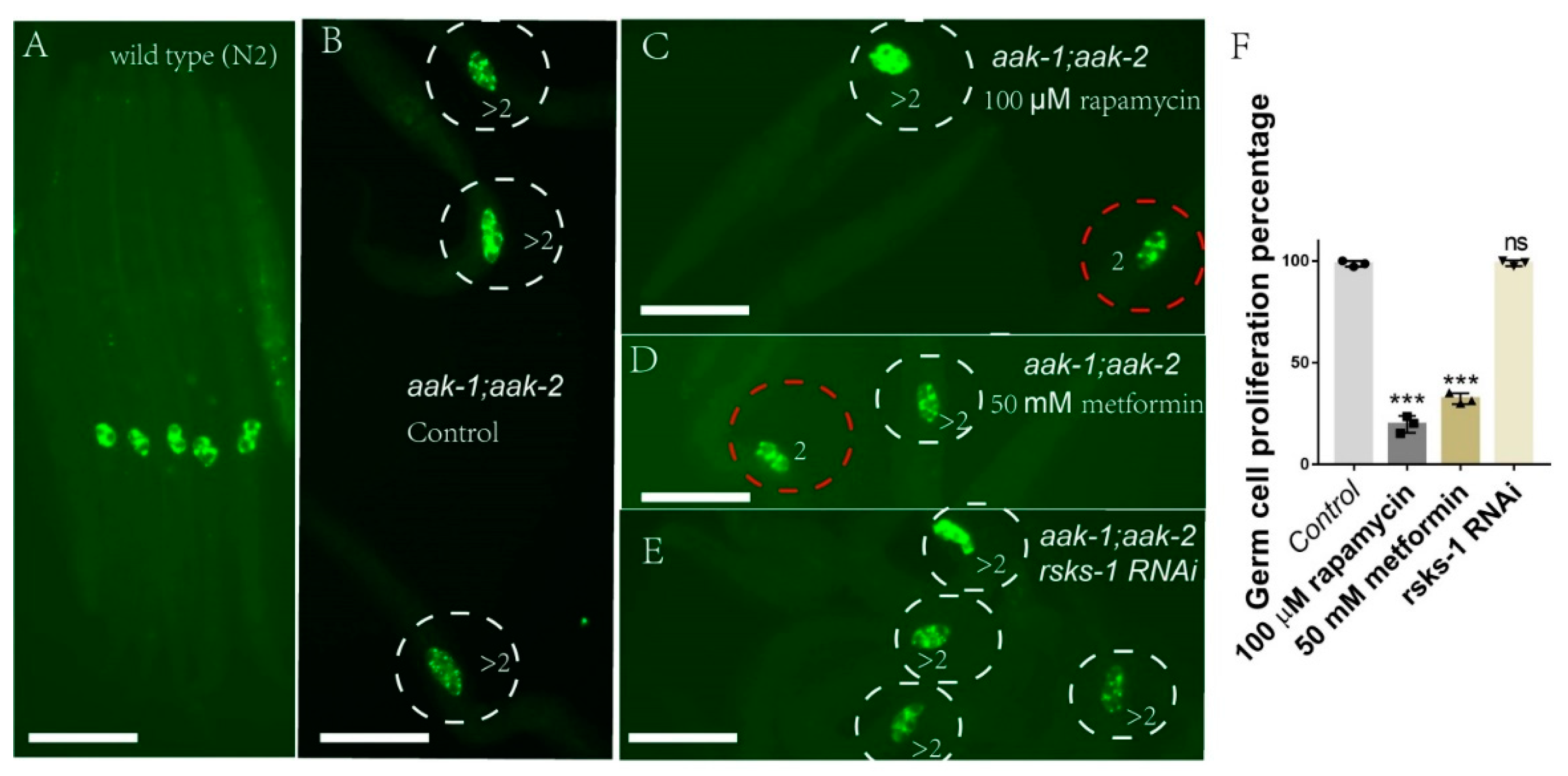
2.1. Metformin Suppresses the Q-Cell Divisions Occurring in AMPK-Deficient Worms during L1 Arrest
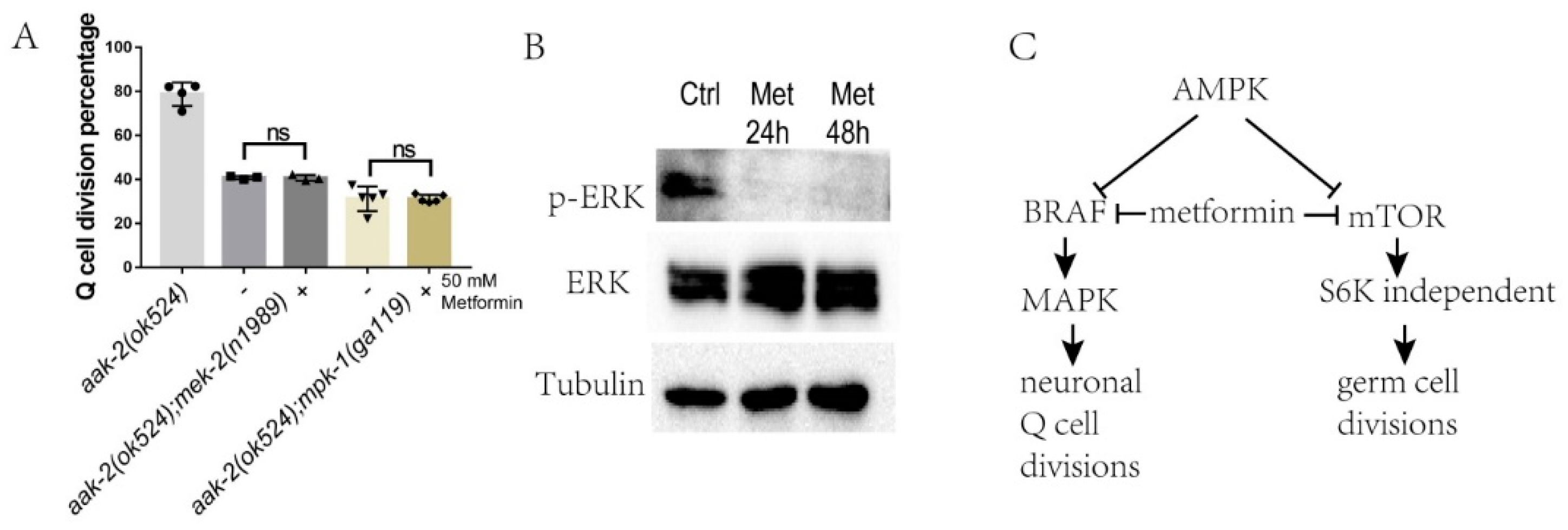
2.2. Metformin Suppresses Q-Cell Divisions Independent of mTOR
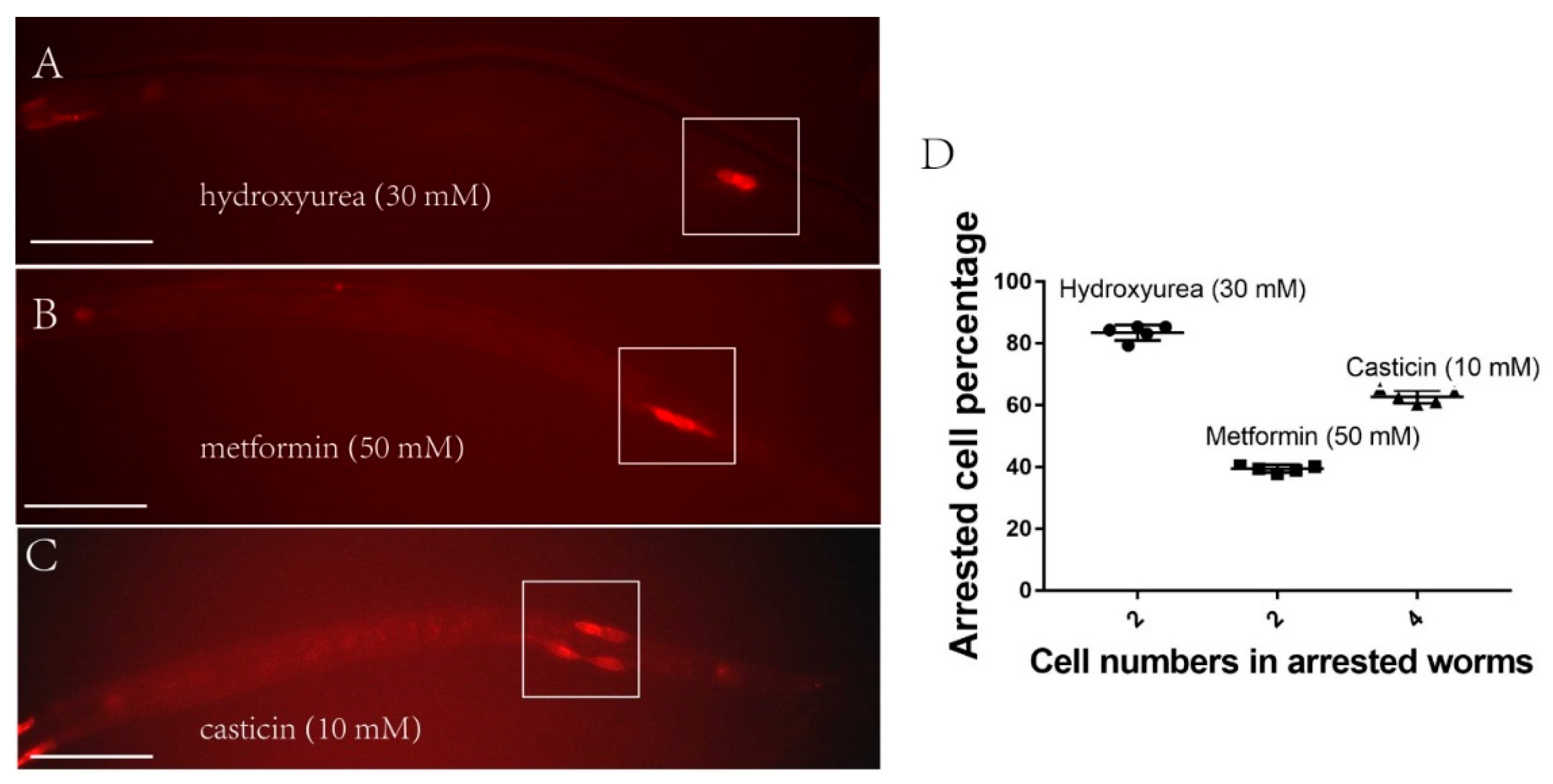
2.3. Q-Cells Treated with Metformin Arrest at the G1/S Stage In Vivo during L1 Arrest
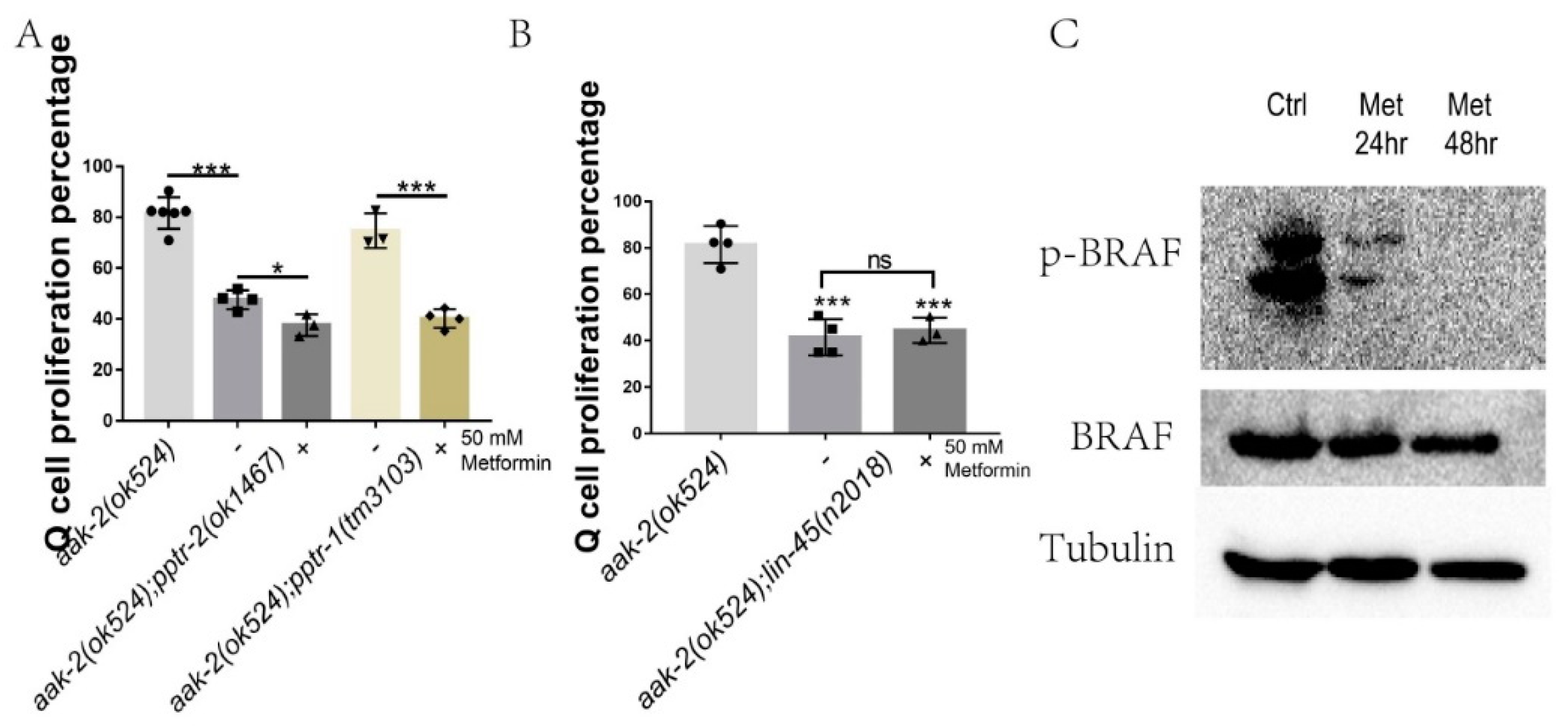
2.4. Metformin Regulates BRAF Function during L1 Arrest
2.5. Metformin Blocks BRAF-MAPK to Control Neuronal Q-Cell Divisions during L1 Arrest
3. Discussions
4. Materials and Methods
4.1. Strains
4.2. Reagents and Treatments
4.3. Analysis of Q-Cell Divisions
4.4. Antibody Staining
4.5. Cell Culture and Western Blot Analysis
4.6. RNAi
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasznicki, J.; Sliwinska, A.; Drzewoski, J. Metformin in cancer prevention and therapy. Ann. Transl. Med. 2014, 2, 57. [Google Scholar] [PubMed]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin–dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Zhou, B.; Oshiro-Rapley, N.; Li, M.; Paulo, J.A.; Webster, C.M.; Mou, F.; Kacergis, M.C.; Talkowski, M.E.; Carr, C.E.; et al. An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 2016, 167, 1705–1718.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Quan, J.I.; Blackwell, T.K. Metformin: Restraining nucleocytoplasmic shuttling to fight cancer and aging. Cell 2016, 167, 1670–1671. [Google Scholar] [CrossRef] [Green Version]
- Fryer, L.G.D.; Parbu-Patel, A.; Carling, D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 2002, 277, 25226–25232. [Google Scholar] [CrossRef] [Green Version]
- Hawley, S.A.; Gadalla, A.E.; Olsen, G.S.; Hardie, D.G. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002, 51, 2420–2425. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.J.; Kosmatka, M.; Bardeesy, N.; Hurley, R.L.; Witters, L.A.; Depinho, R.A.; Cantley, L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA 2004, 101, 3329–3335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Guo, H.; Zhang, C.-S.; Lin, S.-Y.; Yin, Z.; Peng, Y.; Luo, H.; Shi, Y.; Lian, G.; Zhang, C.; et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013, 18, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Bailey, C.J. Biguanides and NIDDM. Diabetes Care 1992, 15, 755–772. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [Green Version]
- Wolf, T.; Qi, W.; Schindler, V.; Runkel, E.D.; Baumeister, R. Doxycyclin ameliorates a starvation-induced germline tumor in C. elegans daf-18/PTEN mutant background. Exp. Gerontol. 2014, 56, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzai, M.; Jones, R.G.; Amaravadi, R.K.; Lum, J.J.; DeBerardinis, R.; Zhao, F.; Viollet, B.; Thompson, C.B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007, 67, 6745–6752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef] [Green Version]
- Fendt, S.-M.; Bell, E.L.; Keibler, M.A.; Davidson, S.M.; Wirth, G.J.; Fiske, B.P.; Mayers, J.R.; Schwab, M.; Bellinger, G.; Csibi, A.; et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 2013, 73, 4429–4438. [Google Scholar] [CrossRef] [Green Version]
- Deschemin, J.-C.; Foretz, M.; Viollet, B.; Vaulont, S. AMPK is not required for the effect of metformin on the inhibition of BMP6-induced hepcidin gene expression in hepatocytes. Sci. Rep. 2017, 7, 12679. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.-F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Ou, Y.H.; Li, Y.; Hu, S.M.; Shao, L.W.; Liu, Y. Metformin extends C. elegans lifespan through lyso-somal pathway. eLife 2017, 6, e31268. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Memmott, R.M.; Mercado, J.R.; Maier, C.R.; Kawabata, S.; Fox, S.D.; Dennis, P.A. Metformin prevents to-bacco carcinogen-induced lung tumorigenesis. Cancer Prev. Res. (Phila.) 2010, 3, 1066–1076. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowker, S.L.; Majumdar, S.R.; Veugelers, P.; Johnson, J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 2006, 29, 1989–1990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Kepp, O.; Heiden, M.G.V.; Kroemer, G. Metabolic targets for cancer therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Baugh, L.R. To grow or not to grow: Nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 2013, 194, 539–555. [Google Scholar] [CrossRef] [Green Version]
- Fukuyama, M.; Rougvie, A.E.; Rothman, J.H. C. elegans DAF-18/PTEN mediates nutrient-dependent arrest of cell cycle and growth in the germline. Curr. Biol. 2006, 16, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Qu, Z.; Zanetti, M.; Lam, B.; Chin-Sang, I.D. C. elegans PTEN and AMPK block neuroblast divisions by inhibiting a BMP-insulin-PP2A-MAPK pathway. Development 2018, 145, dev166876. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, T.; Geng, H.; Liu, Z.; Liang, Z.; Zhang, Z.; Min, J.; Yu, D.; Zhong, C. MAPK/AP-1 pathway regulates benzidine-induced cell proliferation through the control of cell cycle in human normal bladder epithelial cells. Oncol. Lett. 2018, 16, 4628–4634. [Google Scholar] [CrossRef] [Green Version]
- Narbonne, P.; Maddox, P.S.; Labbé, J.-C. DAF-18/PTEN signals through AAK-1/AMPK to inhibit MPK-1/MAPK in feedback control of germline stem cell proliferation. PLoS Genet. 2017, 13, e1006738. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.-H.; Yuan, P.; Perez-Lorenzo, R.; Zhang, Y.; Lee, S.X.; Ou, Y.; Asara, J.M.; Cantley, L.C.; Zheng, B. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Mol. Cell 2013, 52, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, M.V. Canonical RTK-Ras-ERK signaling and related alternative pathways. WormBook 2013, 1–38. [Google Scholar] [CrossRef]
- Sieburth, D.S.; Sundaram, M.; Howard, R.M.; Han, M. A PP2A regulatory subunit positively regulates Ras-mediated signaling during Caenorhabditis elegans vulval induction. Genes Dev. 1999, 13, 2562–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Thomas, E.L.; Kapahi, P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aladzsity, I.; Tóth, M.L.; Sigmond, T.; Szabó, E.; Bicsák, B.; Barna, J.; Regős, Á.; Orosz, L.; Kovács, A.L.; Vellai, T. Autophagy genes unc-51 and bec-1 are required for normal cell size in Caenorhabditis elegans. Genetics 2007, 177, 655–660. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.T.; Ashrafi, K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis. Model. Mech. 2009, 2, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.-I.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Robida-Stubbs, S.; Glover-Cutter, K.; Lamming, D.W.; Mizunuma, M.; Narasimhan, S.D.; Neumann-Haefelin, E.; Sabatini, D.M.; Blackwell, T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012, 15, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef]
- Vaid, S.; Ariz, M.; Chaturbedi, A.; Kumar, G.A.; Subramaniam, K. PUF-8 negatively regulates RAS/MAPK signalling to promote differentiation of C. elegans germ cells. Development 2013, 140, 1645–1654. [Google Scholar] [CrossRef] [Green Version]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2011, 122, 253–270. [Google Scholar] [CrossRef] [Green Version]
- Madiraju, A.K.; Qiu, Y.; Perry, R.J.; Rahimi, Y.; Zhang, X.M.; Zhang, D.; Camporez, J.G.; Cline, G.W.; Butrico, G.M.; Kemp, B.E.; et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018, 24, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Pellatt, L.J.; Bryan, S.J.; Whitehead, S.A.; Mason, H.D. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J. Clin. Endocrinol. Metab. 2011, 96, E427–E435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogg, S.; Ruvkun, G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol. Cell 1998, 2, 887–893. [Google Scholar] [CrossRef]
- Fürstenberger, G.; Senn, H.-J. Insulin-like growth factors and cancer. Lancet Oncol. 2002, 3, 298–302. [Google Scholar] [CrossRef]
- Fukuyama, M.; Sakuma, K.; Park, R.; Kasuga, H.; Nagaya, R.; Atsumi, Y.; Shimomura, Y.; Takahashi, S.; Kajiho, H.; Rougvie, A.; et al. C. elegans AMPKs promote survival and arrest germline development during nutrient stress. Biol. Open 2012, 1, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-J.; Chern, Y. AMPK-mediated regulation of neuronal metabolism and function in brain diseases. J. Neurogenet. 2015, 29, 50–58. [Google Scholar] [CrossRef]
- Peixoto, C.A.; de Oliveira, W.H.; Araújo, S.M.D.R.; Nunes, A.K.S. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol. 2017, 298, 31–41. [Google Scholar] [CrossRef]
- Saito, M.; Saito, M.; Das, B.C. Involvement of AMP-activated protein kinase in neuroinflammation and neurodegeneration in the adult and developing brain. Int. J. Dev. Neurosci. 2019, 77, 48–59. [Google Scholar] [CrossRef]
- Chen, D.; Li, P.W.-L.; Goldstein, B.A.; Cai, W.; Thomas, E.L.; Chen, F.; Hubbard, A.E.; Melov, S.; Kapahi, P. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep. 2013, 5, 1600–1610. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Li, M.; Liu, H.; Luo, L.; Gu, W.; Wu, Q.; Wang, D. Function of RSKS-1-AAK-2-DAF-16 signaling cascade in enhancing toxicity of multi-walled carbon nanotubes can be suppressed by mir-259 activation in Caenorhabditis elegans. Sci. Rep. 2016, 6, 32409. [Google Scholar] [CrossRef] [Green Version]
- Baugh, L.R.; Sternberg, P.W. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 2006, 16, 780–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Inoshita, S.; Nakashima, O.; Kuwahara, M.; Sasaki, S.; Marumo, F. Regulation of cyclin D1 expression and cell cycle progression by mitogen-activated protein kinase cascade. Kidney Int. 1999, 56, 1258–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repetto, M.V.; Winters, M.J.; Bush, A.; Reiter, W.; Hollenstein, D.M.; Ammerer, G.; Pryciak, P.M.; Colman-Lerner, A. CDK and MAPK synergistically regulate signaling dynamics via a shared multi-site phosphorylation region on the scaffold protein Ste5. Mol. Cell 2018, 69, 938–952.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabris, L.; Berton, S.; Pellizzari, I.; Segatto, I.; D’Andrea, S.; Armenia, J.; Bomben, R.; Schiappacassi, M.; Gattei, V.; Philips, M.R.; et al. p27kip1 controls H-Ras/MAPK activation and cell cycle entry via modulation of MT stability. Proc. Natl. Acad. Sci. USA 2015, 112, 13916–13921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [PubMed]
- Chin-Sang, I.D.; George, S.E.; Ding, M.; Moseley, S.L.; Lynch, A.S.; Chisholm, A.D. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell 1999, 99, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; De-Censi, A.; Bonanni, B.; et al. Combination of hypoglycemia and metformin impairs tumor metabolic plasticity and growth by modulating the PP2A-GSK3β -MCL-1 axis. Cancer Cell 2019, 35, 798–815.e5. [Google Scholar] [CrossRef]
- Calixto, A.; Chelur, D.S.; Topalidou, I.; Chen, X.; Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods 2010, 7, 554–559. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Ji, S.; Zheng, S. BRAF Controls the Effects of Metformin on Neuroblast Cell Divisions in C. elegans. Int. J. Mol. Sci. 2021, 22, 178. https://doi.org/10.3390/ijms22010178
Qu Z, Ji S, Zheng S. BRAF Controls the Effects of Metformin on Neuroblast Cell Divisions in C. elegans. International Journal of Molecular Sciences. 2021; 22(1):178. https://doi.org/10.3390/ijms22010178
Chicago/Turabian StyleQu, Zhi, Shaoping Ji, and Shanqing Zheng. 2021. "BRAF Controls the Effects of Metformin on Neuroblast Cell Divisions in C. elegans" International Journal of Molecular Sciences 22, no. 1: 178. https://doi.org/10.3390/ijms22010178
APA StyleQu, Z., Ji, S., & Zheng, S. (2021). BRAF Controls the Effects of Metformin on Neuroblast Cell Divisions in C. elegans. International Journal of Molecular Sciences, 22(1), 178. https://doi.org/10.3390/ijms22010178




