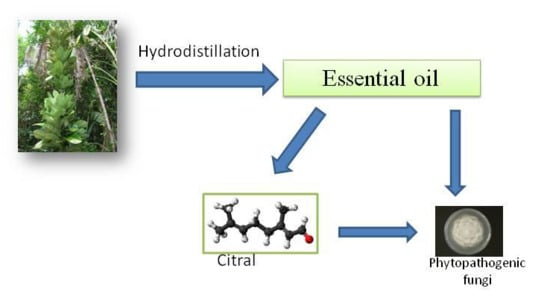
Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
2.2. Antifungal Activity
3. Materials and Methods
3.1. Plant Material and Essential Oil
3.2. Fungi Strains
3.3. Agar Dilution Method
3.4. Determination of Minimum Inhibitory Concentration (MIC)
3.5. Determination of Minimum Fungicidal Concentration (MFC)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VEO | V. macrophylla leaf essential oil |
| EOs | Essential oils |
| F. avenaceum | Fusarium avenaceum |
| F. poae | Fusarium poae |
| F. graminearum | Fusarium graminearum |
| F. semitectum | Fusarium semitectum |
| A. solani | Alternaria solani |
| P. cryptogea | Phytophthora cryptogea |
| MIC | Minimal Inhibitory Concentration |
| MFC | Minimum Fungicide Concentration |
References
- Ghosh, S.; Ozek, T.; Tabanca, N.; Ali, A.; Ur Rehman, J.; Khan, I.A.; Rangan, L. Chemical composition and bioactivity studies of Alpinia nigra essential oils. Ind. Crop. Prod. 2014, 53, 111–119. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ormancey, X.; Sialli, S.; Coutiere, P. Formulation of essential oils in functional perfumery. Parfum. Cosmet. Actual. 2001, 157, 30–40. [Google Scholar]
- Sawamura, M. Aroma and functional properties of Japanese yuzu (Citrus junos Tanaka) essential oil. Aroma Res. 2000, 1, 14–19. [Google Scholar]
- Giamperi, L.; Bucchini, A.; Fraternale, D.; Genovese, S.; Curini, M.; Ricci, D. Composition and antioxidant activity of Inula crithmoides essential oil grown in central Italy (Marche region). Nat. Prod. Commun. 2010, 5, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Devi, W.R.; Singh, S.B.; Singh, C.B. Antioxidant and anti-dermatophytic properties leaf and stem bark of Xylosma longifolium clos. BMC Complment. Altern. Med. 2013, 13, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguefack, J.; Leth, V.; Amvam Zollo, P.H.; Mathur, S.B. Evaluation of five essential oils from aromatic plants of Cameroon for controlling food spoilage and mycotoxin producing fungi. Int. J. Food Microbiol. 2004, 94, 329–334. [Google Scholar] [CrossRef]
- Rahamefy, S.; Beville, G. Plantes Aromatiques et Médicinales à Madagascar. In Proceedings of the Actes du Séminaire sur les Plantes Aromatiques et Médicinales à Madagascar, Tenu au CITE, Antananarivo, Madagascar, 17–22 June 1996. [Google Scholar]
- Novy, J.W. Medicinal Plants of eastern region of Madagascar. J. Ethnopharmacol. 1997, 55, 119–126. [Google Scholar] [CrossRef]
- Kremen, C.; Cameron, A.; Moilanen, A.; Phillips, S.J.; Thomas, C.D.; Beentje, H.; Dransfield, J.; Fisher, B.L.; Glaw, F.; Good, T.C.; et al. Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 2008, 320, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.; Charmillon, J.M.; Roux, E.; Sutour, S.; Rakotozafy, J.B.; Désiré, O.; Paoli, M.; Tomi, F.; Rabehaja, J.R. Chemical composition of leaf and bark essential oils of Vepris unifoliata from Madagascar. JEOR 2017, 3, 214–220. [Google Scholar] [CrossRef]
- Billet, D.; Favre-Bonvin, J. Constituants de l’huile essentielle de Vepris madagascarica Essential oil composition of Vepris madagascarica. Phytochemistry 1973, 2, 1194–1197. [Google Scholar] [CrossRef]
- Rabehaja, D.J.R.; Ihandriharison, H.; Ramanoelina, P.A.R.; Ratsimamanga-Urveg, S.; Bighelli, A.; Casanova, J.; Tomi, F. Leaf oil from Vepris madagascarica (Rutaceae), source of (E)- anethole. Nat. Prod. Commun. 2013, 8, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Poitou, F. Chemical composition of Vepris elliotii essential oil. J. Essent. Oil Res. 1995, 7, 447–449. [Google Scholar] [CrossRef]
- Rakotondraibe, L.H.R.; Rakotovao, M.; Ramanandraibe, V. Composition and antimicrobial activity of leaf oils of Vepris leandriana H. Perr. J. Essent. Oil Res. 2001, 13, 467–469. [Google Scholar] [CrossRef]
- Maggi, F.; Randriana, R.F.; Rasoanaivo, P.; Nicoletti, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; et al. Chemical composition and in vitro biological activities of the essential oil of Veprys macrophylla (Baker) I. Verd. Endemic to Madagascar. Chem. Biodivers. 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Grayson, D.H. Monoterpenoids. Nat. Prod. Rep. 1998, 15, 435–439. [Google Scholar] [CrossRef]
- Saddiq, A.A.; Khayyat, S.A. Chemical and antimicrobial studies of monoterpene: Citral. Pestic. Biochem. Phys. 2010, 98, 89–93. [Google Scholar] [CrossRef]
- Boiteau, M.; Boiteau, P.; Allorge–Boiteau, L. Dictionaire des noms Malgaches des Végétaux; Editoins Alzieu: Grenoble, France, 1999. [Google Scholar]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical composition and antibacterial activity of seven uncommon essential oils. JEOR 2018, 3, 233–243. [Google Scholar] [CrossRef]
- Nakahara, K.; Alzoreky, N.S.; Yoshihashi, T.; Nguyen, H.T.T.; Trakoontivakorn, G. Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (Citronella Grass). JARQ 2003, 37, 249–252. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Pereira, F.; Mendes, J.M.; Lima, I.O.; de Lira Mota, S.; de Oliveira, W.A.; de Oliveira Lima, E. Antifungal activity of geraniol and citronellol, two monoterpenes alcohols, against Tricophyton rubrum involves inhbition of ergosterol biosynthesy. Pharm. Biol. 2015, 53, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Necha, L.L.; Garduno-Pizana, C.; Garcia-Barrera, L.J. In vitro antifungal activity of essential oils and their compounds on mycelial growth of Fusarium oxysporum f. sp. gladioli (Massey) Snyder and Hansen. Plant Pathol. J. 2009, 8, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Bucchini, A.; Ricci, D.; Messina, F.; Marcotullio, M.C.; Curini, M.; Giamperi, L. Antioxidant and antifungal activity of different extracts obtained from aerial parts of Inula crithmoides L. Nat. Prod. Res. 2015, 29, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Giamperi, L.; Bucchini, A.E.A.; Ricci, D.; Maggi, F. Essential oil of Achillea ligustica All. (Asteraceae) as a good antifungal agent against phytopathogenic fungi. Nat. Prod. Commun. 2018, 13, 1171–1174. [Google Scholar]
- Carta, C.; Arras, G. Azione inibitrice in vitro di olii essenziali nei confronti di alcuni patogeni di piante ornamentali. La Difesa delle Piante 1987, 10, 195–202. [Google Scholar]
- Thompson, D.P. Fungitoxic activity of essential oil components of food storage fungi. Mycologia 1989, 81, 51–53. [Google Scholar] [CrossRef]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef]
| Positive Control | Concentration (µg/mL) | |||||
|---|---|---|---|---|---|---|
| Fungi | Days | 50 (µg/mL) | 100 | 200 | 400 | 800 |
| A. solani | 1° | 57.5 ± 6.99 aA | 20.1 ± 5.30 bD | 25.9 ± 6.48 bG | 24.3 ± 3.15 bI | 45.8 ± 4.56 cN |
| 3° | 78.0 ± 2.20 dB | 30.4 ± 3.95 eE | 30.6 ± 3.75 eG | 34.2 ± 4.50 eL | 53.8 ± 2.75 fN | |
| 6° | 100.0 ± 0.00 gC | 57.2 ± 6.98 hF | 60.8 ± 7.88 hH | 66.3 ± 4.90 hM | 73.3 ± 7.00 iO | |
| P. cryptogea | 1° | 57.5 ± 6.99 aA | 23.7 ± 3.80 bD | 55.3 ± 4.56 aF | 60.0 ± 2.41 aH | 100.0 ± 0.00 cM |
| 3° | 78.0 ± 2.20 dB | 31.5 ± 3.31 eD | 62.6 ± 3.01 fF | 77.0 ± 2.05 dI | 100.0 ± 0.00 gM | |
| 6° | 100.0 ± 0.00 hC | 73.8 ± 5.51 iE | 100 ± 0.00 hG | 100.0 ± 0.00 hL | 100.0 ± 0.00 hM | |
| F. avenaceum | 1° | 60.0± 6.99 aA | 23.5 ± 3.31 bD | 36.1 ± 3.97 cG | 60.0 ± 2.40 aL | 72.5 ± 1.88 dO |
| 3° | 88.0 ± 7.20 eB | 34.2 ± 5.92 fE | 62.8 ± 3.59 gH | 77.7 ± 2.47 hM | 82.3 ± 3.60 eO | |
| 6° | 100.0 ± 0.00 iC | 74.9 ± 7.14 lF | 100.0 ± 0.00 iI | 100.0 ± 0.00 iN | 100.0 ± 0.00 iP | |
| F. poae | 1° | 67.5 ± 6.99 aA | 35.9 ± 3.72 bC | 37.2 ± 4.13 bE | 39.4 ± 1.43 bG | 42.2 ± 4.5 bL |
| 3° | 75.0 ± 5.20 cA | 43.5 ± 4.12 dC | 47.0 ± 3.60 dE | 52.1 ± 4.45 dH | 60.1 ± 7.06 eM | |
| 6° | 100.0 ± 0.00 fB | 66.3 ± 4.95 gD | 72.8 ± 5.26 gF | 100.0 ± 0.00 fI | 100.0 ± 0.00 fN | |
| F. graminearum | 1° | 57.5 ± 6.99 aA | 45.1 ± 3.82 bD | 53.3 ± 2.08 aF | 62.8 ± 2.65 aI | 62.8 ± 2.65 aN |
| 3° | 77.0 ± 5.20 cB | 51.0 ± 1.40 dD | 70.0 ± 5.02 cG | 71.0 ± 3.81 cL | 71.0 ± 3.81 cN | |
| 6° | 100.0 ± 0.00 eC | 70.0 ± 7.17 fE | 88.0 ± 8.51 gH | 100.0 ± 0.00 eM | 100.0 ± 0.00 eO | |
| F. semitectum | 1° | 57.5 ± 6.99 aA | 39.4 ± 3.78 bD | 54.4 ± 5.41 aF | 61.4 ± 1.40 aH | 67.1 ± 3.65 aL |
| 3° | 79.0 ± 6.20 cB | 45.7 ± 2.23 dD | 59.4 ± 4.96 eF | 62.5 ± 3.59 eH | 67.4 ± 3.14 eL | |
| 6° | 100.0 ± 0.00 fC | 59.1 ± 5.70 gE | 70.1 ± 4.13 hG | 74.7 ± 4.16 hI | 78.7 ± 5.67 hM | |
| Fungi | Essential Oil | Citral | Citronellol | Nystatin | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MFC (µg/mL) | |
| P. cryptogea | 130.40a | 390.30d | 100e | 510f | 510g | 790h | 45i | 50m |
| F. avenaceum | 130.40a | 390.30d | 100e | 510f | 510g | 790h | 45i | 50m |
| F. poae | 300.20b | /* | 100e | 510f | 510 | >800 | 45i | 50m |
| F. graminearum | 215.00c | /* | 100e | 510f | 510g | >800 | 45i | 50m |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giamperi, L.; Bucchini, A.E.A.; Ricci, D.; Tirillini, B.; Nicoletti, M.; Rakotosaona, R.; Maggi, F. Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi. Int. J. Mol. Sci. 2020, 21, 2776. https://doi.org/10.3390/ijms21082776
Giamperi L, Bucchini AEA, Ricci D, Tirillini B, Nicoletti M, Rakotosaona R, Maggi F. Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi. International Journal of Molecular Sciences. 2020; 21(8):2776. https://doi.org/10.3390/ijms21082776
Chicago/Turabian StyleGiamperi, Laura, Anahi Elena Ada Bucchini, Donata Ricci, Bruno Tirillini, Marcello Nicoletti, Rianasoambolanoro Rakotosaona, and Filippo Maggi. 2020. "Vepris macrophylla (Baker) I. Verd Essential Oil: An Antifungal Agent against Phytopathogenic Fungi" International Journal of Molecular Sciences 21, no. 8: 2776. https://doi.org/10.3390/ijms21082776