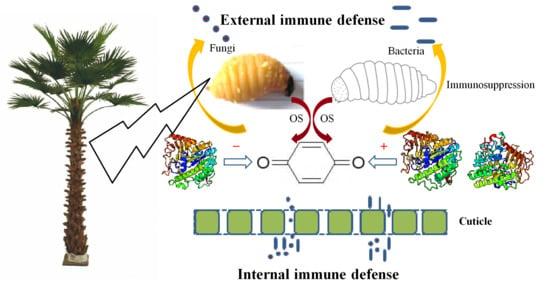
Identification of Novel ARSB Genes Necessary for p-Benzoquinone Biosynthesis in the Larval Oral Secretion Participating in External Immune Defense in the Red Palm Weevil
Abstract
:1. Introduction
2. Results
2.1. Sequence Characteristics and Phylogenetic Analysis of RfARSBs
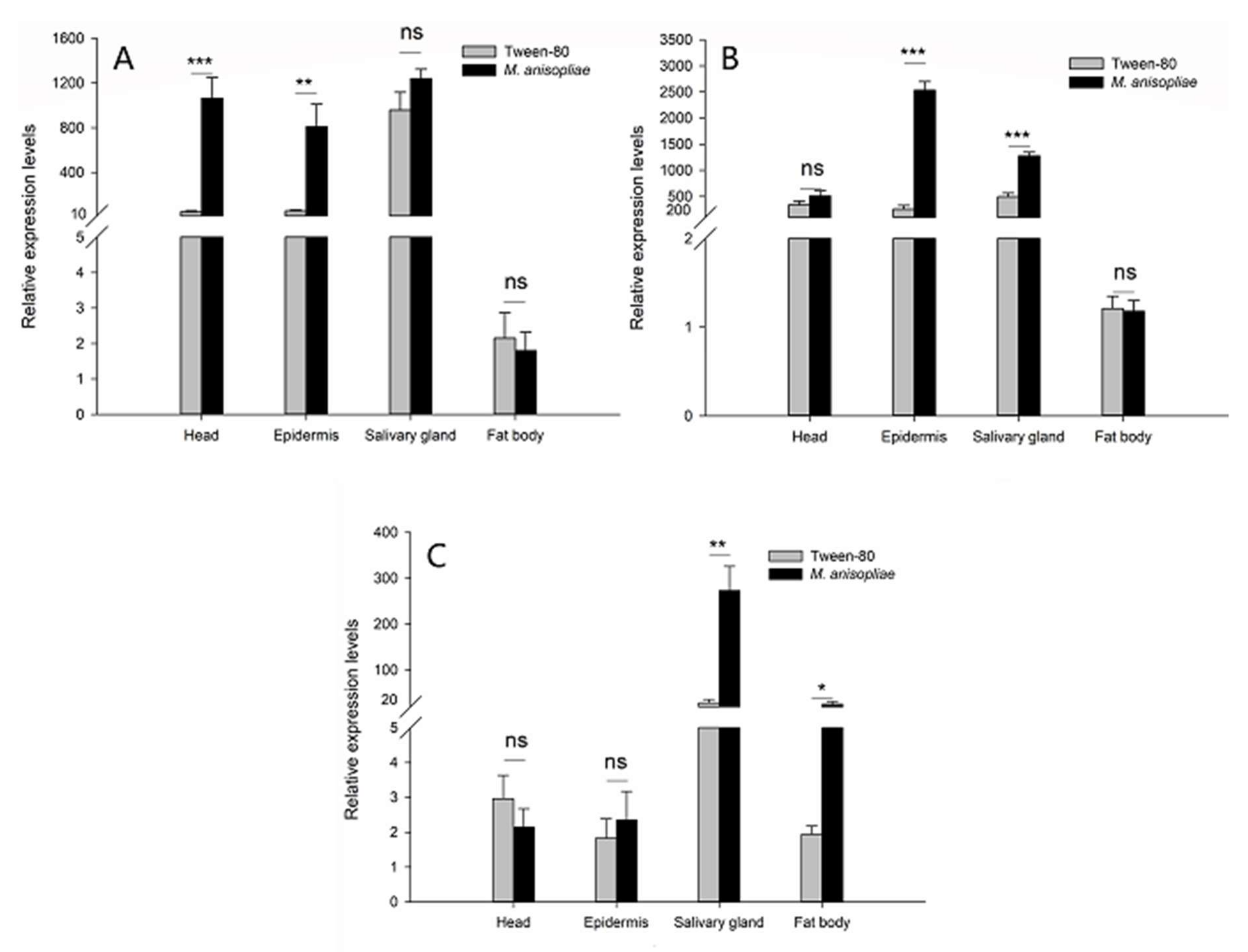
2.2. Tissue/Organ Expression Profiles of RfARSBs
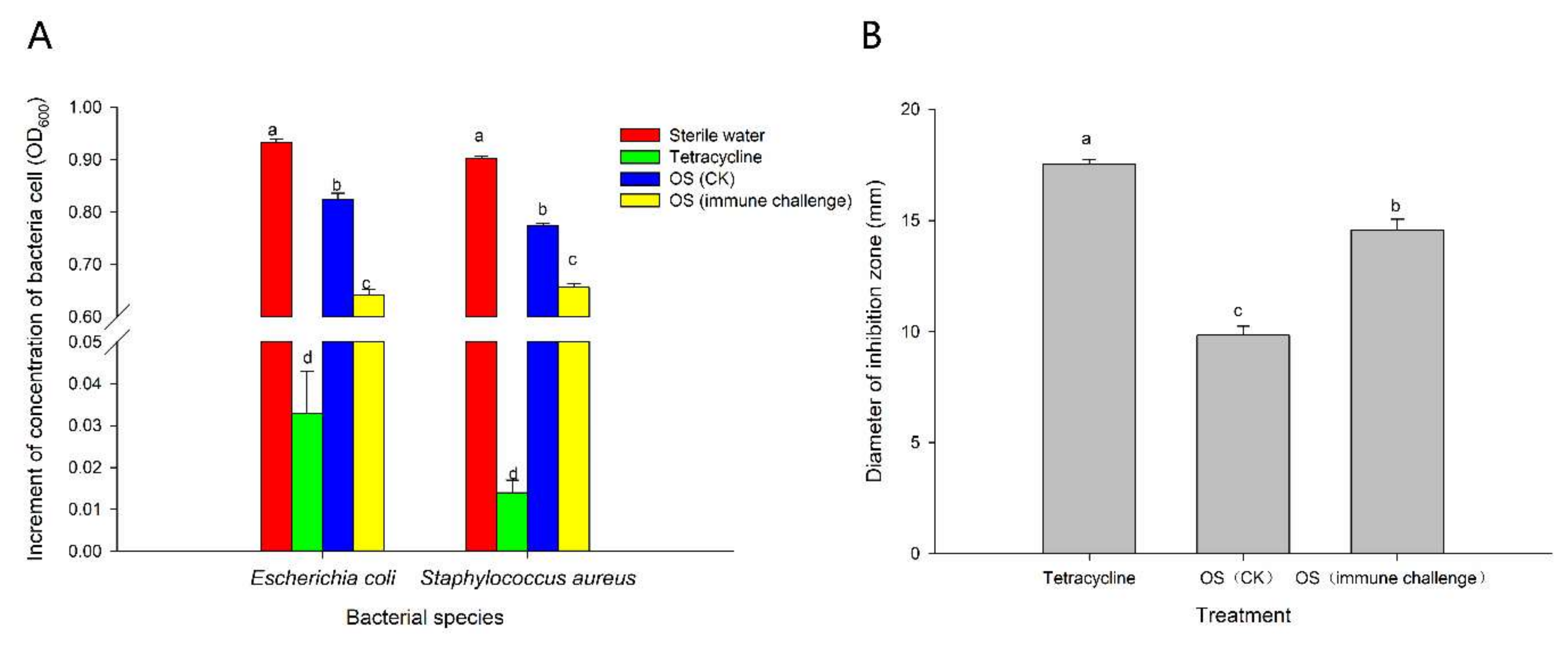
2.3. Antimicrobial Activity of Larval Oral Secretions Induced by M. anisopliae Infection
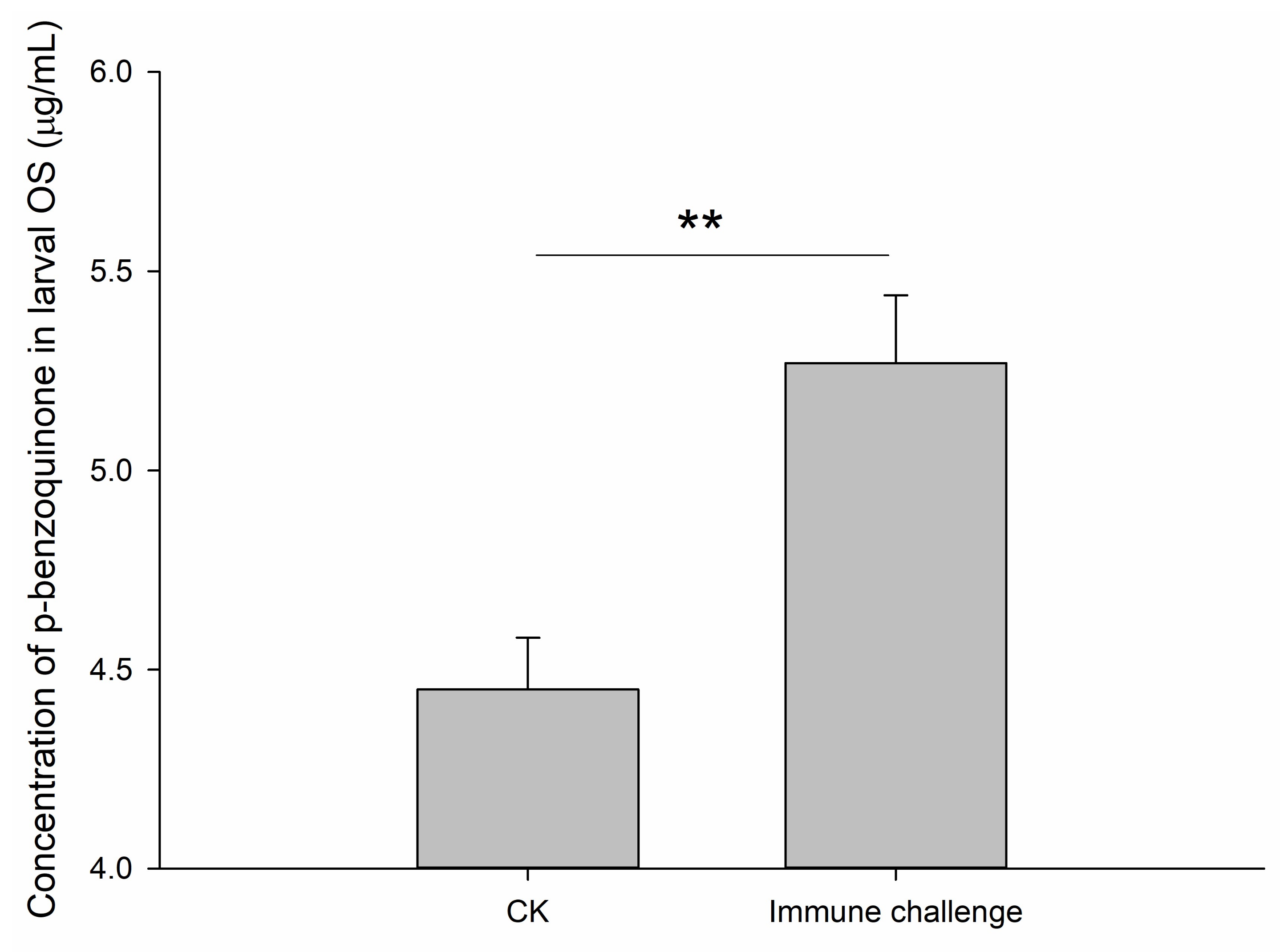
2.4. Contents of p-Benzoquinone in Oral Secretions Produced by Larvae Following Infection with M. anisopliae
2.5. Expression Levels of RfARSBs after Immune Challenge
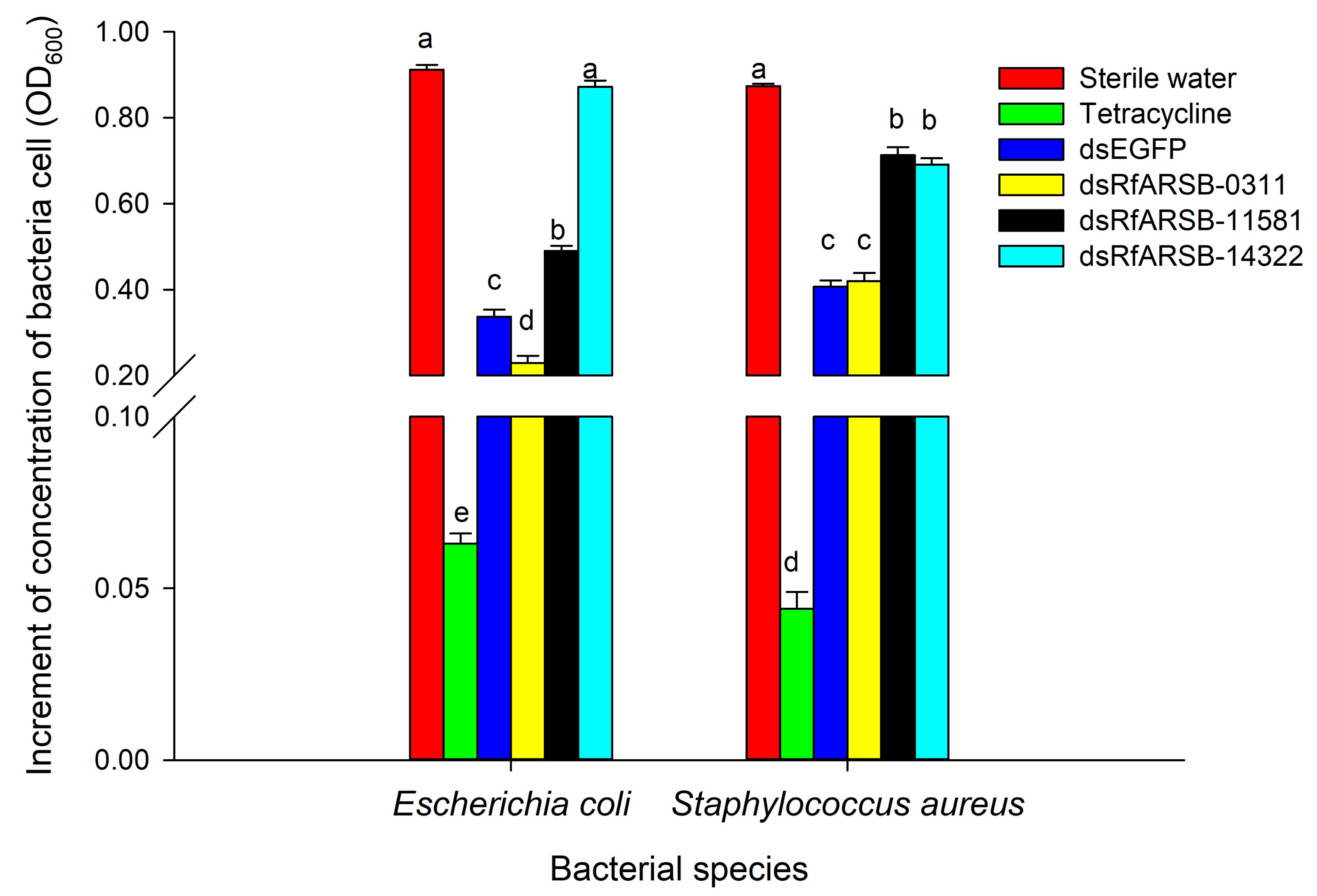
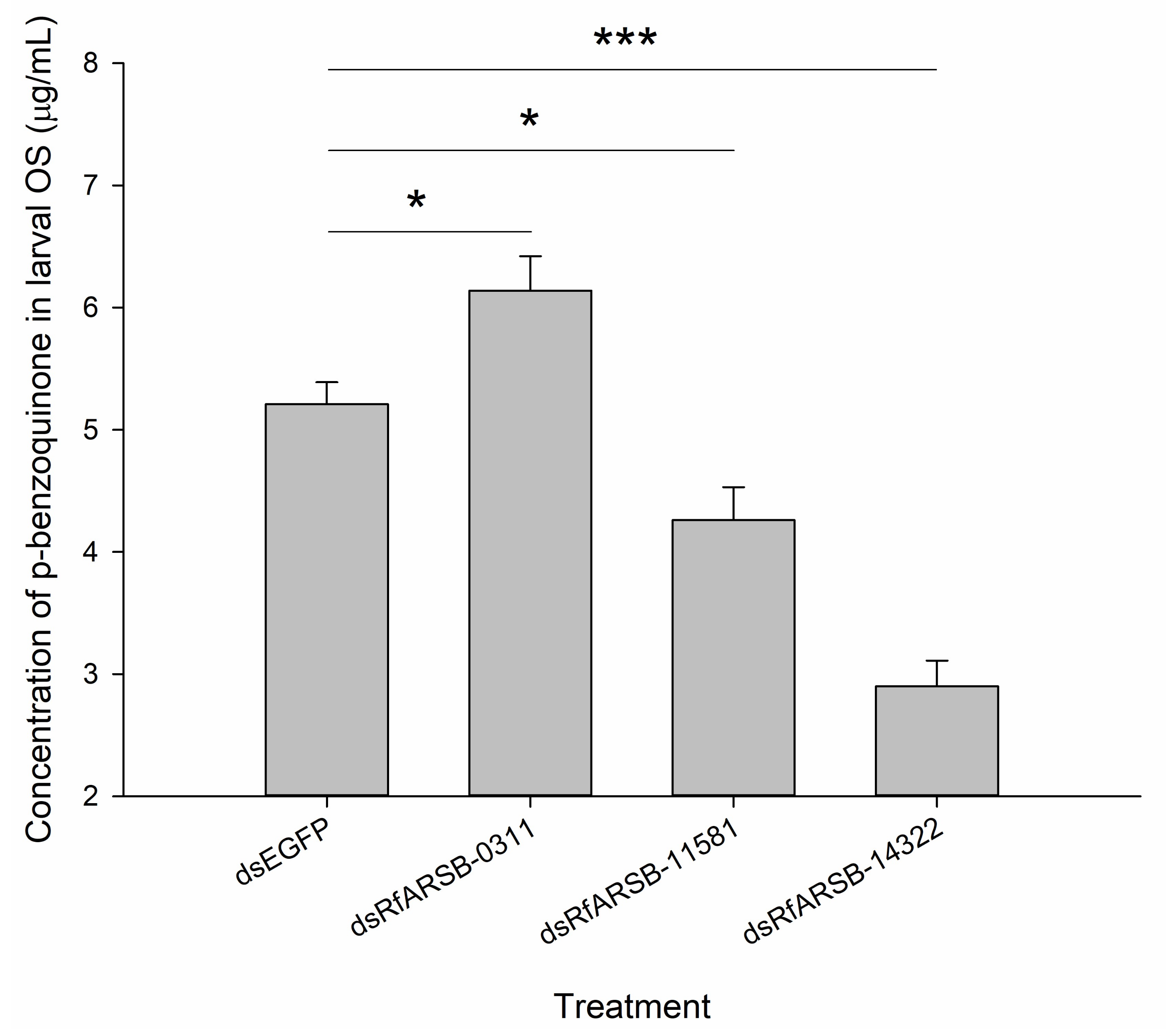
2.6. Roles of RfARSBs in External Immunity
3. Discussion
4. Materials and Methods
4.1. Collection and Rearing of Insects
4.2. Microbial Strains and Cultures
4.3. Immune Challenges in Vitro
4.4. Collection of Larval Oral Secretions
4.5. Amplification, Purification, and Analysis of Full-Length cDNA Sequences of RfARSB Genes
4.6. Evaluation of Gene Expression Profiles by Real-Time Quantitative PCR
4.7. RNA Interference
4.8. Microbial Inhibition Assays
4.9. Quantification of p-Benzoquinone in Larval Oral Secretions
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chapman, A.D. Numbers of Living Species in Australia and the World, 2nd ed.; Australian Biological Resources Study (ABRS): Canberra, Australia, 2009; p. 80. [Google Scholar]
- Eisner, T. Chemical defense against predation in arthropods. In Chemical Ecology; Sondheimer, E., Simeone, J.B., Eds.; Academic Press: New York, NY, USA, 1970; pp. 157–217. [Google Scholar]
- Joop, G.; Roth, O.; Schmid-Hempel, P.; Kurtz, J. Experimental evolution of external immune defences in the red flour beetle. J. Evol. Biol. 2014, 27, 1562–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Y.C.; Hou, Y.M.; Shi, Z.H.; Liang, X.Y. Defensive secretions and the trade-off between internal and external immunity in insects. Acta Entomol. Sinica 2017, 60, 962–974. [Google Scholar]
- Ulrich, K.R.; Kramer, M.; Feldlaufer, M.F. Ability of bed bug (Hemiptera: Cimicidae) defensive secretions (E)-2-hexenal and (E)-2-octenal to attract adults of the common bed bug Cimex lectularius. Physiol. Entomol. 2016, 41, 103–110. [Google Scholar] [CrossRef]
- Li, J.; Lehmann, S.; Weißbecker, B.; Naharros, I.O.; Schütz, S.; Joop, G.; Wimmer, E.A. Odoriferous defensive stink gland transcriptome to identify novel genes necessary for quinone synthesis in the red flour beetle, Tribolium castaneum. PLoS Genet. 2013, 9, e1003596. [Google Scholar] [CrossRef]
- Alexander, P.; Barton, D.H.R. The excretion of ethylquinone by the flour beetle. Biochem. J. 1943, 37, 463–465. [Google Scholar] [CrossRef] [Green Version]
- Qiang, C.K.; Yang, Z.F.; Zhang, S.Y. Analysis of chemical constituent in defensive secretions of Tenebrio molitor by GC/MS. Chin. Bull. Entomol. 2006, 43, 385–389. [Google Scholar]
- Meinwald, J.; Koch, K.F.; Rogers, J.E.; Eisner, T. Biosynthesis of arthropod secretions. III. synthesis of simple p-benzoquinones in a beetle (Eleodes longicollis). J. Am. Chem. Soc. 1966, 88, 341–345. [Google Scholar] [CrossRef]
- Happ, G.M. Quinone and hydrocarbon production in the defensive glands of Eleodes longicolis and Tribolium castaneum (Coleoptera, Tenebrionidae). J. Insect Physiol. 1968, 14, 1821–1837. [Google Scholar] [CrossRef]
- Blum, M.S. Chemical Defenses of Arthropods; Academic Press: London, UK, 1981; p. 562. [Google Scholar]
- Dierks, T.; Miech, C.; Hummerjohann, J.; Schmidt, B.; Kertesz, M.A.; von Figura, K. Posttranslational formation of formylglycine in prokaryotic sulfatases by modification of either cysteine or serine. J. Biol. Chem. 1998, 273, 25560–25564. [Google Scholar] [CrossRef] [Green Version]
- Sardiello, M. Sulfatases and sulfatase modifying factors: An exclusive and promiscuous relationship. Hum. Mol. Genet. 2005, 14, 3203–3217. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Tobacman, J.K. Arylsulfatase B regulates colonic epithelial cell migration by effects on MMP9 expression and Rho A activation. Clin. Exp. Metastas. 2009, 26, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.; Schmidt, B.; von Figura, K. Conversion of cysteine to formylglycine: A protein modification in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1997, 94, 11963–11968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Selmer, T.; Ingendoh, A.; von Figura, K. A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell 1995, 82, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Dierks, T. Sequence determinants directing conversion of cysteine to formylglycine in eukaryotic sulfatases. EMBO J. 1999, 18, 2084–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Tisa, L.S.; Rosen, B.P. Membrane topology of the ArsB protein, the membrane subunit of an anion-translocating ATPase. J. Biol. Chem. 1992, 267, 12570–12576. [Google Scholar]
- Prakash, S.; Cooper, G.; Singhi, S.; Saier, M.H. The ion transporter superfamily. BBA Biomembranes 2003, 1618, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.L.; Liu, Z.; Rosen, B.P. As (III) and Sb (III) uptake by GlpF and efflux by ArsB in Escherichia coli. J. Biol. Chem. 2004, 279, 18334–18341. [Google Scholar] [CrossRef] [Green Version]
- Mascio, C.; White, D.J.; Tisa, L.S. Construction and purification of His-tagged staphylococcal ArsB protein, an integral membrane protein that is involved in arsenical salt resistance. Indian J. Microbiol. 2009, 49, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeo, R.; Polishchuk, E.V.; Volpi, N.; Polishchuk, R.S.; Auricchio, A. Pharmacological read-through of nonsense ARSB mutations as a potential therapeutic approach for mucopolysaccharidosis VI. J. Inherit. Metab. Dis. 2013, 36, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Litjens, T.; Hopwood, J.J. Mucopolysaccharidosis type VI: Structural and clinical implications of mutations in N-acetylgalactosamine-4-sulfatase. Hum. Mutat. 2001, 18, 282–295. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Yezerski, A.; Gilmor, T.P.; Stevens, L. Genetic analysis of benzoquinone production in Tribolium confusum. J. Chem. Ecol. 2004, 30, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Song, T.X.; Tian, L.X.; Xie, W.; Cui, H.Y.; Xiang, W.S.; Zhang, Y.J. Molecular cloning and expression analysis of arylsulfatase B gene in Bemisia tabaci Q biotype. J. Plant Protect. 2019, 46, 40–48. [Google Scholar]
- Lefroy, H.M. The More Important Insects Injurious to Indian Agriculture; Government of India Press: Calcutta, India, 1906.
- Ge, X.Z.; He, S.Y.; Wang, T.; Yan, W.; Zong, S.X. Potential distribution predicted for Rhynchophorus ferrugineus in China under different climate warming scenarios. PLoS ONE 2016, 10, e0141111. [Google Scholar] [CrossRef]
- Dawadi, B.; Wang, X.H.; Xiao, R.; Muhammad, A.; Hou, Y.M.; Shi, Z.H. PGRP-LB homolog acts as a negative modulator of immunity in maintaining the gut-microbe symbiosis of red palm weevil, Rhynchophorus ferrugineus Olivier. Dev. Comp. Immunol. 2018, 86, 65–77. [Google Scholar] [CrossRef]
- Pu, Y.C.; Hou, Y.M. Isolation and identification of bacterial strains with insecticidal activities from Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae). J. Appl. Entomol. 2016, 140, 617–626. [Google Scholar] [CrossRef]
- Pu, Y.C.; Ma, T.L.; Hou, Y.M.; Sun, M. An entomopathogenic bacterium strain, Bacillus thuringiensis, as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Pest Manag. Sci. 2017, 73, 1494–1502. [Google Scholar] [CrossRef]
- Pu, Y.C.; Xiang, H.J.; Liang, X.Y.; Wang, Y.; Hou, Y.M.; Fu, L.; Wang, R. External immune inhibitory efficiency of external secretions and their metabolic profiling in red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Front. Physiol. 2020, 10, 1624. [Google Scholar] [CrossRef]
- He, S.; Johnston, P.R.; Kuropka, B.; Lokatis, S.; Weise, C.; Plarre, R.; Kunte, H.J.; McMahon, D.P. Termite soldiers contribute to social immunity by synthesizing potent oral secretions. Insect Mol. Biol. 2018, 27, 564–576. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, Y.Q.; Song, W.M.; Chen, D.Y.; Chen, F.Y.; Chen, F.Y.; Chen, X.Y.; Chen, Z.W.; Ge, S.X.; Wang, C.Z.; et al. An effector from cotton bollworm oral secretion impairs host plant defense signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 14331–14338. [Google Scholar] [CrossRef] [Green Version]
- Riberiro, J.M.C.; Martin-Martin, I.; Moreira, F.R.; Bernard, K.A.; Calvo, E. A deep insight into the male and female sialotranscriptome of adult Culex tarsalis mosquitoes. Insect Biochem. Mol. Biol. 2018, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.; Best, M.D.; Hanson, S.R.; Wong, C.H. Sulfotransferases: Structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 2004, 43, 3526–3548. [Google Scholar] [CrossRef] [PubMed]
- Cotter, S.C.; Littlefair, J.E.; Grantham, P.J.; Kilner, R.M. A direct physiological trade-off between personal and social immunity. J. Anim. Ecol. 2013, 82, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otti, O.; Tragust, S.; Feldhaar, H. Unifying external and internal immune defences. Trends Ecol. Evol. 2014, 29, 625–634. [Google Scholar] [CrossRef]
- Prendeville, H.R.; Stevens, L. Microbe inhibition by Tribolium flour beetles varies with beetle species, strain, sex, and microbe group. J. Chem. Ecol. 2002, 28, 1183–1190. [Google Scholar] [CrossRef]
- Yezerski, A.; Ciccone, C.; Rozitski, J.; Volingavage, B. The effects of a naturally produced benzoquinone on microbes common to flour. J. Chem. Ecol. 2007, 33, 1217–1225. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, Z.Q.; Liang, S.Z. Metarhizium anisopliae and its mechanism for killing insects. Chin. J. Pestic. 2004, 43, 342–345. [Google Scholar]
- Pu, Z.L.; Li, Z.Z. Insect Mycology; Anhui Science and Technology Press: Hefei, China, 1996; pp. 112–130. [Google Scholar]
- Zhang, J.; Qin, W.Q.; Yan, W.; Peng, Z.Q. Detection of pathogenicity of Meatarhiziums against Rhynchophorus ferrugineus in laboratory. Chin. J. Trop. Crop. 2012, 33, 899–905. [Google Scholar]
- Manickam, S.; Hanine, B. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar]
- Liang, C.C.; Peng, J.; Huang, Z.; Xie, Y.P.; Huang, J.S. Advances on fermentation technology for Metarhizium spp. Chin. J. Trop. Agr. 2008, 28, 87–91. [Google Scholar]
- Turlings, T.C.; Mccall, P.J.; Alborn, H.T.; Tumlinson, J.H. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. J. Chem. Ecol. 1993, 19, 411–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.H.; Hou, Y.M. The transcriptome data of the red palm weevil larvae. Unpublished.
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Lin, Y.T.; Hou, Y.M. Mother-derived trans-generational immune priming in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera, Dryophthoridae). Bull. Entomol. Res. 2014, 104, 742–750. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, Y.-C.; Liang, X.-Y.; Zhang, H.; Zhang, H.-J.; Xu, L.-N.; Ji, Y.-N.; Huang, S.-N.; Bai, J.; Hou, Y.-M. Identification of Novel ARSB Genes Necessary for p-Benzoquinone Biosynthesis in the Larval Oral Secretion Participating in External Immune Defense in the Red Palm Weevil. Int. J. Mol. Sci. 2020, 21, 1610. https://doi.org/10.3390/ijms21051610
Pu Y-C, Liang X-Y, Zhang H, Zhang H-J, Xu L-N, Ji Y-N, Huang S-N, Bai J, Hou Y-M. Identification of Novel ARSB Genes Necessary for p-Benzoquinone Biosynthesis in the Larval Oral Secretion Participating in External Immune Defense in the Red Palm Weevil. International Journal of Molecular Sciences. 2020; 21(5):1610. https://doi.org/10.3390/ijms21051610
Chicago/Turabian StylePu, Yu-Chen, Xin-Yu Liang, He Zhang, Hua-Jian Zhang, Li-Na Xu, Ya-Nan Ji, Shu-Ning Huang, Juan Bai, and You-Ming Hou. 2020. "Identification of Novel ARSB Genes Necessary for p-Benzoquinone Biosynthesis in the Larval Oral Secretion Participating in External Immune Defense in the Red Palm Weevil" International Journal of Molecular Sciences 21, no. 5: 1610. https://doi.org/10.3390/ijms21051610