Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function
Abstract
:1. Introduction
2. HDL Metabolism, Structure, and Composition
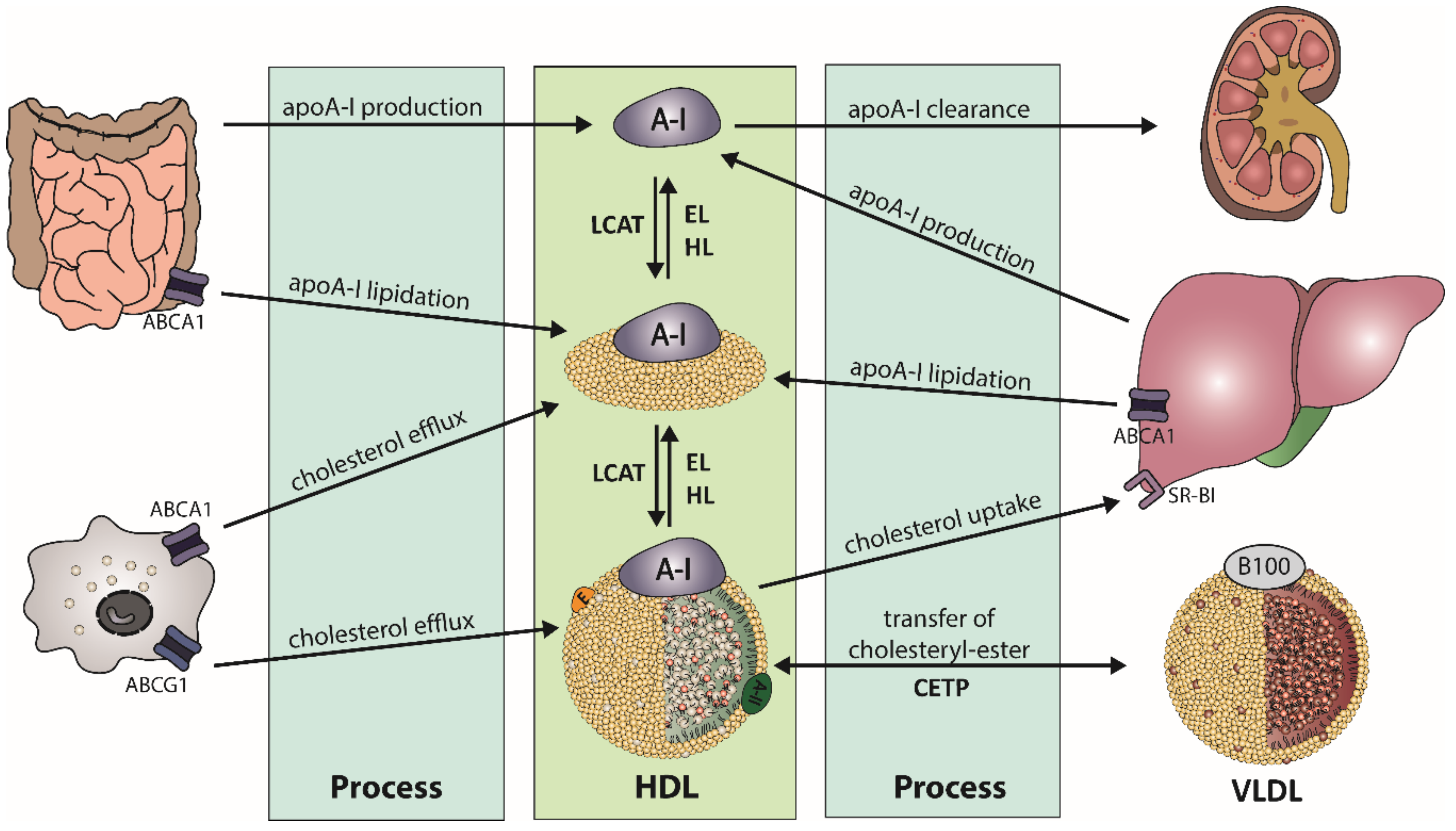
2.1. HDL Metabolism
2.2. HDL Structure and Composition
2.3. HDL Subclasses
2.4. Important Functions of HDL
3. HDL-C-Raising Therapies and Cardiovascular Outcome
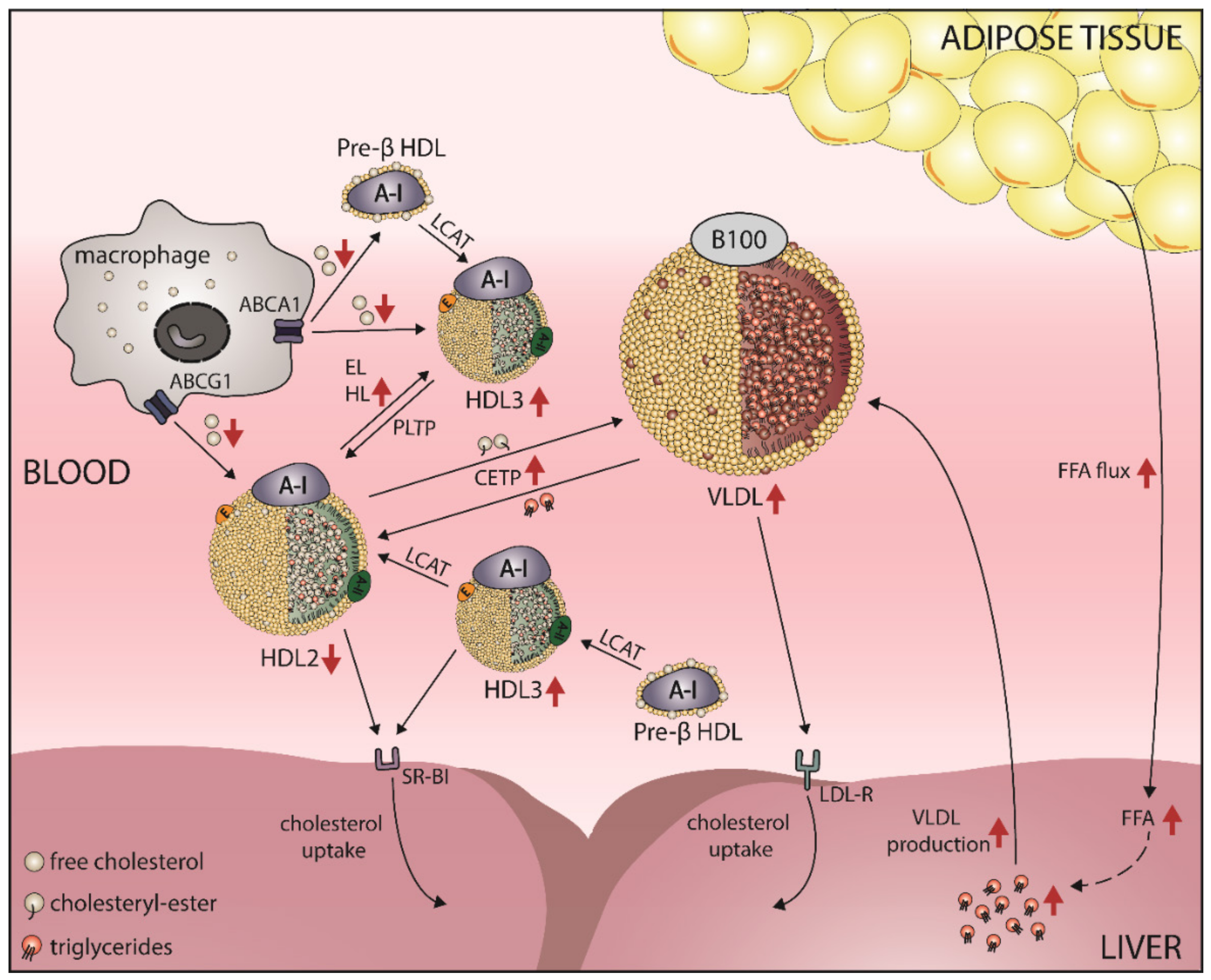
4. Obesity Alters HDL-C Levels
5. Obesity, HDL, and Cardiovascular Risk
5.1. Obesity Leads to a Shift in HDL Subclass Distribution
5.2. Obesity Affects HDL Function
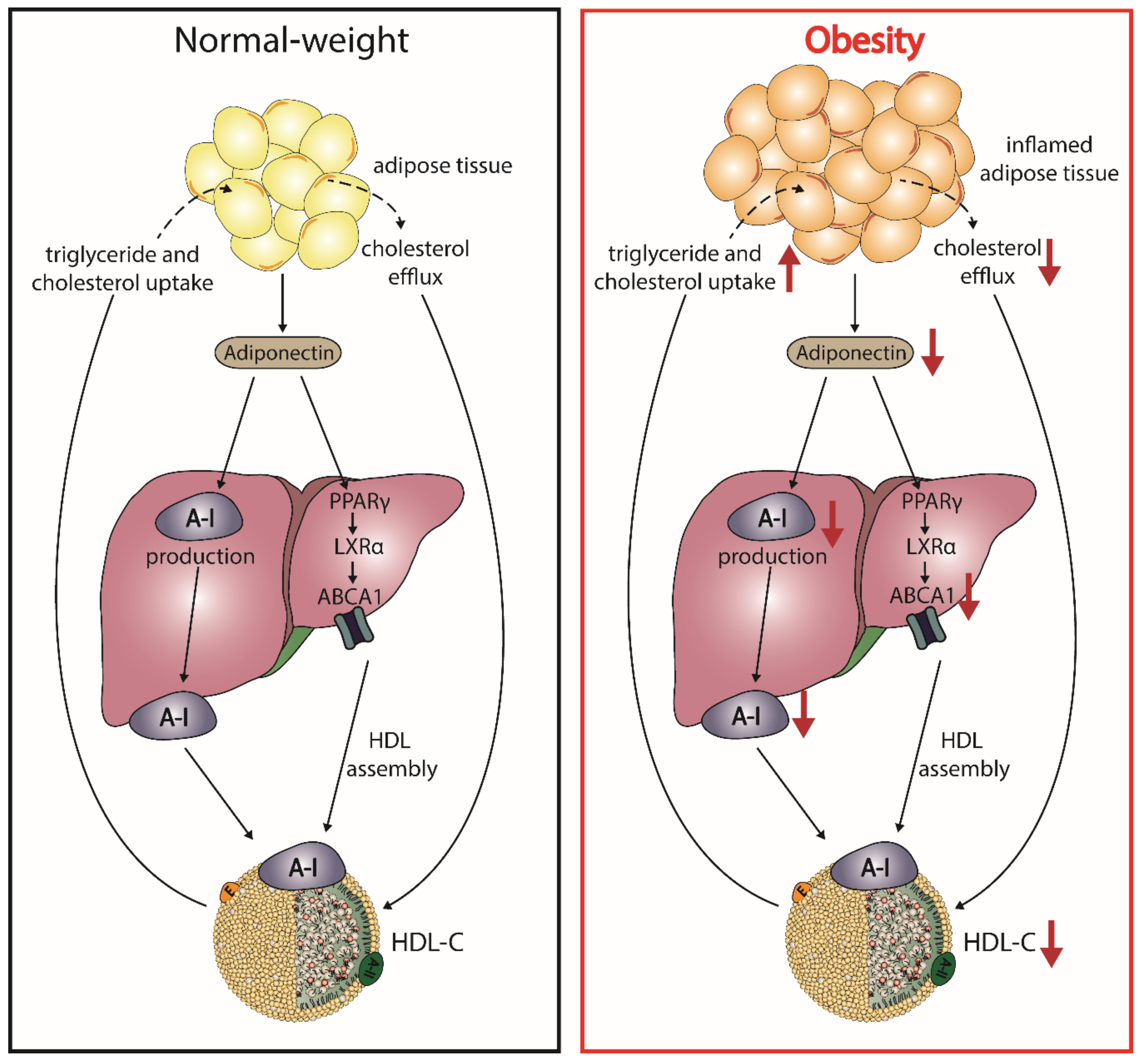
5.3. Adiponectin and HDL
5.4. Obesity and HDL-Associated Sphingosine-1-Phosphate (S1P)
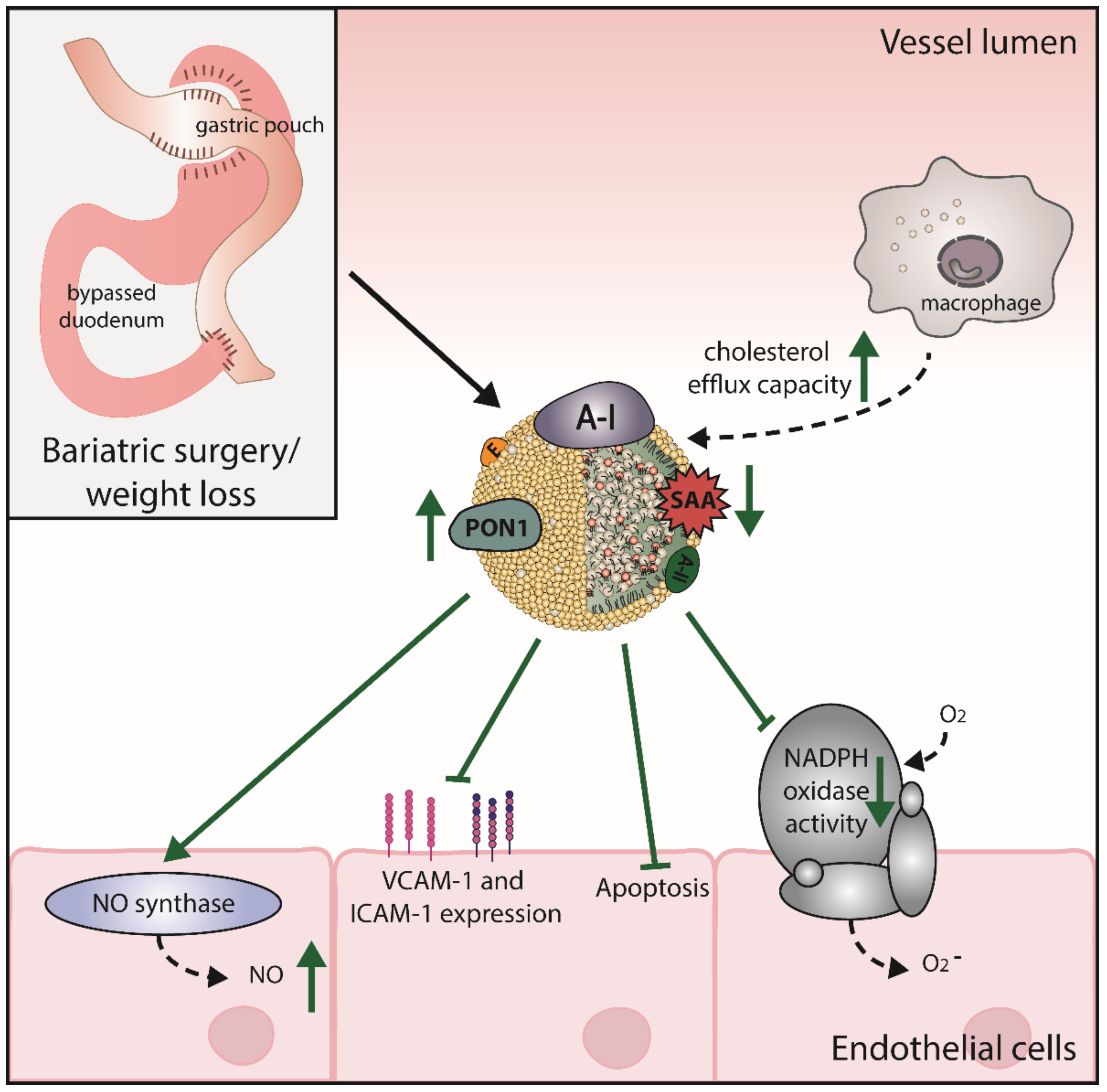
6. Bariatric Surgery Improves HDL Levels and Function
7. Effects of Pharmacological Anti-Obesity Interventions on HDL Levels and Function
8. Effects of Dietary Approaches on HDL Levels and Function
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Knight, J.A. Diseases and Disorders Associated with Excess Body Weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Steg, P.G.; Ravisy, J.; Lorgis, L.; Laurent, Y.; Sicard, P.; Janin-Manificat, L.; Beer, J.C.; Makki, H.; Lagrost, A.C.; et al. Relation Between Body Mass Index, Waist Circumference, and Death After Acute Myocardial Infarction. Circulation 2008, 118, 482–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Narkiewicz, K. Obesity and hypertension—The issue is more complex than we thought. Nephrol. Dial. Transplant. 2006, 21, 264–267. [Google Scholar] [CrossRef] [Green Version]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Genest, J. Effect of obesity on high-density lipoprotein metabolism. Obesity 2007, 15, 2875–2888. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Lecour, S.; Goedecke, J.H. HDL Subclass Distribution Shifts with Increasing Central Adiposity. Available online: https://www.hindawi.com/journals/jobe/2019/2107178/ (accessed on 10 August 2020).
- Wang, H.; Peng, D.-Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Rader, D.J.; Tall, A.R. Is it time to revise the HDL cholesterol hypothesis? Nat. Med. 2012, 18, 1344–1346. [Google Scholar] [CrossRef]
- Parks, J.S.; Chung, S.; Shelness, G.S. Hepatic ABC transporters and triglyceride metabolism. Curr. Opin. Lipidol. 2012, 23, 196–200. [Google Scholar] [CrossRef] [Green Version]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- Norum, K.R.; Remaley, A.T.; Miettinen, H.E.; Strøm, E.H.; Balbo, B.E.P.; Sampaio, C.A.T.L.; Wiig, I.; Kuivenhoven, J.A.; Calabresi, L.; Tesmer, J.J.; et al. Lecithin:cholesterol acyltransferase: Symposium on 50 years of biomedical research from its discovery to latest findings. J. Lipid Res. 2020, 61, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Sorci-Thomas, M.G.; Bhat, S.; Thomas, M.J. Activation of lecithin: Cholesterol acyltransferase by HDL ApoA-I central helices. Clin. Lipidol. 2009, 4, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Anthanont, P.; Asztalos, B.F. HDL metabolism, composition, function and deficiency. Curr. Opin. Lipidol. 2014, 25, 194–199. [Google Scholar] [CrossRef]
- Kozarsky, K.F.; Donahee, M.H.; Rigotti, A.; Iqbal, S.N.; Edelman, E.R.; Krieger, M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 1997, 387, 414–417. [Google Scholar] [CrossRef]
- Duong, M.; Psaltis, M.; Rader, D.J.; Marchadier, D.; Barter, P.J.; Rye, K.-A. Evidence that hepatic lipase and endothelial lipase have different substrate specificities for high-density lipoprotein phospholipids. Biochemistry 2003, 42, 13778–13785. [Google Scholar] [CrossRef]
- Albers, J.J.; Vuletic, S.; Cheung, M.C. Role of Plasma Phospholipid Transfer Protein in Lipid and Lipoprotein Metabolism. Biochim. Biophys. Acta 2012, 1821, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Toth, P.P.; Barter, P.J.; Rosenson, R.S.; Boden, W.E.; Chapman, M.J.; Cuchel, M.; D’Agostino, R.B.; Davidson, M.H.; Davidson, W.S.; Heinecke, J.W.; et al. High-density lipoproteins: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 484–525. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; Friedman, G.D.; Glennon, W.E.; Mcnamara, P.M. Risk Factors in coronary heart disease. An evaluation of several serum lipids as predictors of coronary heart disease; The Framingham Study. Ann. Intern. Med. 1964, 61, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birner-Gruenberger, R.; Schittmayer, M.; Holzer, M.; Marsche, G. Understanding high-density lipoprotein function in disease: Recent advances in proteomics unravel the complexity of its composition and biology. Prog. Lipid Res. 2014, 56, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle subclasses and molecular components. Handb. Exp. Pharmacol. 2015, 224, 3–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostner, G.; Alaupovic, P. Composition and structure of plasma lipoproteins. Separation and quantification of the lipoprotein families occurring in the high density lipoproteins of human plasma. Biochemistry 1972, 11, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Hernando, C. Antiatherogenic Properties of High-Density Lipoprotein–Enriched MicroRNAs. Arterioscler. Thromb. Vasc. Biol. 2014, 34, e13–e14. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Chapman, M.J. Antiatherogenic small, dense HDL--guardian angel of the arterial wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Duriez, P.; Fruchart, J.C. High-density lipoprotein subclasses and apolipoprotein A-I. Clin. Chim. Acta Int. J. Clin. Chem. 1999, 286, 97–114. [Google Scholar] [CrossRef]
- Litvinov, D.; Mahini, H.; Garelnabi, M. Antioxidant and Anti-Inflammatory Role of Paraoxonase 1: Implication in Arteriosclerosis Diseases. N. Am. J. Med. Sci. 2012, 4, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Serna, J.; García-Seisdedos, D.; Alcázar, A.; Lasunción, M.Á.; Busto, R.; Pastor, Ó. Quantitative lipidomic analysis of plasma and plasma lipoproteins using MALDI-TOF mass spectrometry. Chem. Phys. Lipids 2015, 189, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yetukuri, L.; Söderlund, S.; Koivuniemi, A.; Seppänen-Laakso, T.; Niemelä, P.S.; Hyvönen, M.; Taskinen, M.-R.; Vattulainen, I.; Jauhiainen, M.; Oresic, M. Composition and lipid spatial distribution of HDL particles in subjects with low and high HDL-cholesterol. J. Lipid Res. 2010, 51, 2341–2351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesner, P.; Leidl, K.; Boettcher, A.; Schmitz, G.; Liebisch, G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J. Lipid Res. 2009, 50, 574–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, A.; Kézdy, K.E.; Wald, J.H. Defined apolipoprotein A-I conformations in reconstituted high density lipoprotein discs. J. Biol. Chem. 1989, 264, 4818–4824. [Google Scholar]
- Woudberg, N.J.; Pedretti, S.; Lecour, S.; Schulz, R.; Vuilleumier, N.; James, R.W.; Frias, M.A. Pharmacological Intervention to Modulate HDL: What Do We Target? Front. Pharmacol. 2018, 8, 989. [Google Scholar] [CrossRef] [Green Version]
- De la Llera-Moya, M.; Drazul-Schrader, D.; Asztalos, B.F.; Cuchel, M.; Rader, D.J.; Rothblat, G.H. The Ability to Promote Efflux via ABCA1 Determines the Capacity of Serum Specimens with Similar HDL-C to Remove Cholesterol from Macrophages. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 796–801. [Google Scholar] [CrossRef]
- Davidson, W.S.; Silva, R.A.G.D.; Chantepie, S.; Lagor, W.R.; Chapman, M.J.; Kontush, A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: Relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 870–876. [Google Scholar] [CrossRef]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef] [Green Version]
- Nofer, J.R.; Van Der Giet, M.; Tölle, M.; Wolinska, I.; von Wnuck Lipinski, K.; Baba, H.A.; Tietge, U.J.; Gödecke, A.; Ishii, I.; Kleuser, B.; et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Investig. 2004, 113, 569–581. [Google Scholar] [CrossRef]
- Argraves, K.M.; Gazzolo, P.J.; Groh, E.M.; Wilkerson, B.A.; Matsuura, B.S.; Twal, W.O.; Hammad, S.M.; Argraves, W.S. High Density Lipoprotein-associated Sphingosine 1-Phosphate Promotes Endothelial Barrier Function. J. Biol. Chem. 2008, 283, 25074–25081. [Google Scholar] [CrossRef] [Green Version]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Attie, A.D.; Kastelein, J.P.; Hayden, M.R. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001, 42, 1717–1726. [Google Scholar] [PubMed]
- Tang, C.; Oram, J.F. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2009, 1791, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, Á.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Rothblat, G.H.; Phillips, M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010, 21, 229. [Google Scholar] [CrossRef]
- Phillips, M.C.; Johnson, W.J.; Rothblat, G.H. Mechanisms and Consequences of Cellular Cholesterol Exchange and Transfer. Available online: https://pubmed.ncbi.nlm.nih.gov/3297153/ (accessed on 14 October 2020).
- Marsche, G.; Heine, G.H.; Stadler, J.T.; Holzer, M. Current Understanding of the Relationship of HDL Composition, Structure and Function to Their Cardioprotective Properties in Chronic Kidney Disease. Biomolecules 2020, 10, 1348. [Google Scholar] [CrossRef]
- Assmann, G.; Gotto, A.M. HDL Cholesterol and Protective Factors in Atherosclerosis. Circulation 2004, 109, III-8–III-14. [Google Scholar] [CrossRef] [Green Version]
- Nofer, J.-R.; Assmann, G. Atheroprotective effects of high-density lipoprotein-associated lysosphingolipids. Trends Cardiovasc. Med. 2005, 15, 265–271. [Google Scholar] [CrossRef]
- Nofer, J.-R.; Kehrel, B.; Fobker, M.; Levkau, B.; Assmann, G.; von Eckardstein, A. HDL and arteriosclerosis: Beyond reverse cholesterol transport. Atherosclerosis 2002, 161, 1–16. [Google Scholar] [CrossRef]
- Navab, M.; Ananthramaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fonarow, G.C.; Vahabzadeh, K.; Hama, S.; Hough, G.; Kamranpour, N.; et al. The oxidation hypothesis of atherogenesis: The role of oxidized phospholipids and HDL. J. Lipid Res. 2004, 45, 993–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norata, G.D.; Callegari, E.; Inoue, H.; Catapano, A.L. HDL3 Induces Cyclooxygenase-2 Expression and Prostacyclin Release in Human Endothelial Cells Via a p38 MAPK/CRE-Dependent Pathway: Effects on COX-2/PGI-Synthase Coupling. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beitz, J.; Förster, W. Influence of human low density and high density lipoprotein cholesterol on the in vitro prostaglandin I2 synthetase activity. Biochim. Biophys. Acta BBA-Lipids Lipid Metab. 1980, 620, 352–355. [Google Scholar] [CrossRef]
- Drew, B.G.; Fidge, N.H.; Gallon-Beaumier, G.; Kemp, B.E.; Kingwell, B.A. High-density lipoprotein and apolipoprotein AI increase endothelial NO synthase activity by protein association and multisite phosphorylation. Proc. Natl. Acad. Sci. USA 2004, 101, 6999–7004. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-A.; Titlow, W.B.; Jackson, B.A.; Giltiay, N.; Nikolova-Karakashian, M.; Uittenbogaard, A.; Smart, E.J. High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner. J. Biol. Chem. 2002, 277, 11058–11063. [Google Scholar] [CrossRef] [Green Version]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001, 7, 853–857. [Google Scholar] [CrossRef]
- Van Linthout, S.; Spillmann, F.; Lorenz, M.; Meloni, M.; Jacobs, F.; Egorova, M.; Stangl, V.; De Geest, B.; Schultheiss, H.P.; Tschope, C. Vascular-Protective Effects of High-Density Lipoprotein Include the Downregulation of the Angiotensin II Type 1 Receptor. Hypertension 2009, 53, 682–687. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, H.; Rizzo, A.N.; Zhang, W.; Mai, Y.; Ye, M. Endothelial nitric oxide synthase dimerization is regulated by heat shock protein 90 rather than by phosphorylation. PLoS ONE 2014, 9, e105479. [Google Scholar] [CrossRef] [Green Version]
- Panzenböck, U.; Stocker, R. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim. Biophys. Acta BBA-Proteins Proteom. 2005, 1703, 171–181. [Google Scholar] [CrossRef]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.A.; Stocker, R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aviram, M.; Billecke, S.; Sorenson, R.; Bisgaier, C.; Newton, R.; Rosenblat, M.; Erogul, J.; Hsu, C.; Dunlop, C.; La Du, B. Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: Selective action of human paraoxonase allozymes Q and R. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, M.; Smith, J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat. Genet. 1996, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A.; Chapman, M.J. Functionally defective high-density lipoprotein: A new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006, 58, 342–374. [Google Scholar] [CrossRef]
- Goulinet, S.; Chapman, M.J. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids. Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef]
- Navab, M.; Imes, S.S.; Hama, S.Y.; Hough, G.P.; Ross, L.A.; Bork, R.W.; Valente, A.J.; Berliner, J.A.; Drinkwater, D.C.; Laks, H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Investig. 1991, 88, 2039–2046. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. High-density lipoproteins inhibit cytokine-induced expression of endothelial cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1987–1994. [Google Scholar] [CrossRef]
- Calabresi, L.; Franceschini, G.; Sirtori, C.R.; De Palma, A.; Saresella, M.; Ferrante, P.; Taramelli, D. Inhibition of VCAM-1 expression in endothelial cells by reconstituted high density lipoproteins. Biochem. Biophys. Res. Commun. 1997, 238, 61–65. [Google Scholar] [CrossRef]
- Bursill, C.A.; Castro, M.L.; Beattie, D.T.; Nakhla, S.; van der Vorst, E.; Heather, A.K.; Barter, P.J.; Rye, K.-A. High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1773–1778. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W.; Abbott, R.D.; Castelli, W.P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1988, 8, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tall, A.R.; Yvan-Charvet, L.; Wang, N. The failure of torcetrapib: Was it the molecule or the mechanism? Arterioscler. Thromb. Vasc. Biol. 2007, 27, 257–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, S.E.; Tardif, J.-C.; Nicholls, S.J.; Revkin, J.H.; Shear, C.L.; Duggan, W.T.; Ruzyllo, W.; Bachinsky, W.B.; Lasala, G.P.; Lasala, G.P.; et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 2007, 356, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; van Leuven, S.I.; Burgess, L.; Evans, G.W.; Kuivenhoven, J.A.; Barter, P.J.; Revkin, J.H.; Grobbee, D.E.; Riley, W.A.; Shear, C.L.; et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N. Engl. J. Med. 2007, 356, 1620–1630. [Google Scholar] [CrossRef]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and reduces VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Rudel, L.L.; Conner, A.; Akeefe, H.; Kostner, G.; Baki, T.; Rothblat, G.; de la Llera-Moya, M.; Asztalos, B.; Perlman, T.; et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J. Lipid Res. 2009, 50, 894–907. [Google Scholar] [CrossRef] [Green Version]
- Hovingh, G.K.; Smits, L.P.; Stefanutti, C.; Soran, H.; Kwok, S.; de Graaf, J.; Gaudet, D.; Keyserling, C.H.; Klepp, H.; Frick, J.; et al. The effect of an apolipoprotein A-I–containing high-density lipoprotein–mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am. Heart J. 2015, 169, 736–742.e1. [Google Scholar] [CrossRef]
- Parolini, C.; Marchesi, M.; Lorenzon, P.; Castano, M.; Balconi, E.; Miragoli, L.; Chaabane, L.; Morisetti, A.; Lorusso, V.; Martin, B.J.; et al. Dose-Related Effects of Repeated ETC-216 (Recombinant Apolipoprotein A-IMilano/1-Palmitoyl-2-Oleoyl Phosphatidylcholine Complexes) Administrations on Rabbit Lipid-Rich Soft Plaques: In Vivo Assessment by Intravascular Ultrasound and Magnetic Resonance Imaging. J. Am. Coll. Cardiol. 2008, 51, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Tardif, J.-C.; Grégoire, J.; L’Allier, P.L.; Ibrahim, R.; Lespérance, J.; Heinonen, T.M.; Kouz, S.; Berry, C.; Basser, R.; Lavoie, M.-A.; et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: A randomized controlled trial. JAMA 2007, 297, 1675–1682. [Google Scholar] [CrossRef] [Green Version]
- Waksman, R.; Torguson, R.; Kent, K.M.; Pichard, A.D.; Suddath, W.O.; Satler, L.F.; Martin, B.D.; Perlman, T.J.; Maltais, J.-A.B.; Weissman, N.J.; et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2010, 55, 2727–2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Christodoulidis, G.; Cheng, J.; Lerakis, S.; Vittorio, T.J. High-Density Lipoprotein Functionality in Coronary Artery Disease. Am. J. Med. Sci. 2014, 347, 504–508. [Google Scholar] [CrossRef]
- Ashen, M.D.; Blumenthal, R.S. Clinical practice. Low HDL cholesterol levels. N. Engl. J. Med. 2005, 353, 1252–1260. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Fernandez-Garcia, D.; Gomez-Huelgas, R.; Tinahones, F.J.; Cardona, F. Adipose Tissue Gene Expression of Factors Related to Lipid Processing in Obesity. PLoS ONE 2011, 6, e24783. [Google Scholar] [CrossRef] [Green Version]
- Walton, R.G.; Zhu, B.; Unal, R.; Spencer, M.; Sunkara, M.; Morris, A.J.; Charnigo, R.; Katz, W.S.; Daugherty, A.; Howatt, D.A.; et al. Increasing Adipocyte Lipoprotein Lipase Improves Glucose Metabolism in High Fat Diet-induced Obesity. J. Biol. Chem. 2015, 290, 11547–11556. [Google Scholar] [CrossRef] [Green Version]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Taskinen, M.-R.; Nikkilä, E.A. Lipoprotein lipase of adipose tissue and skeletal muscle in human obesity: Response to glucose and to semistarvation. Metabolism 1981, 30, 810–817. [Google Scholar] [CrossRef]
- Feng, R.; Luo, C.; Li, C.; Du, S.; Okekunle, A.P.; Li, Y.; Chen, Y.; Zi, T.; Niu, Y. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: A case—Control study. Lipids Health Dis. 2017, 16, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, S.; Uffelman, K.D.; Lewis, G.F. The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J. Diabetes Complicat. 2002, 16, 24–28. [Google Scholar] [CrossRef]
- Carr, M.C.; Hokanson, J.E.; Zambon, A.; Deeb, S.S.; Barrett, P.H.; Purnell, J.Q.; Brunzell, J.D. The contribution of intraabdominal fat to gender differences in hepatic lipase activity and low/high density lipoprotein heterogeneity. J. Clin. Endocrinol. Metab. 2001, 86, 2831–2837. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sparks, D.L. Hepatic Lipase, High Density Lipoproteins, and Hypertriglyceridemia. Am. J. Pathol. 2011, 178, 1429–1433. [Google Scholar] [CrossRef]
- Blades, B.; Vega, G.L.; Grundy, S.M. Activities of lipoprotein lipase and hepatic triglyceride lipase in postheparin plasma of patients with low concentrations of HDL cholesterol. Arterioscler. Thromb. J. Vasc. Biol. 1993, 13, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.; Barrett, P.H.R.; Uffelman, K.D.; Watanabe, T.; Adeli, K.; Lewis, G.F. Lipolytically Modified Triglyceride-Enriched HDLs Are Rapidly Cleared from the Circulation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Lönnqvist, F.; Nordfors, L.; Jansson, M.; Thörne, A.; Schalling, M.; Arner, P. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J. Clin. Investig. 1997, 99, 2398–2404. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Peterson, T.E.; Sert-Kuniyoshi, F.H.; Glenn, J.A.; Davison, D.E.; Romero-Corral, A.; Pusalavidyasagar, S.; Jensen, M.D.; Somers, V.K. Leptin signaling in adipose tissue: Role in lipid accumulation and weight gain. Circ. Res. 2012, 111, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.M.; Shen, M.H.; Chu, N.F. Relationship between plasma leptin levels and lipid profiles among school children in Taiwan--the Taipei Children Heart Study. Eur. J. Epidemiol. 2001, 17, 911–916. [Google Scholar] [CrossRef]
- Rainwater, D.L.; Comuzzie, A.G.; VandeBerg, J.L.; Mahaney, M.C.; Blangero, J. Serum leptin levels are independently correlated with two measures of HDL. Atherosclerosis 1997, 132, 237–243. [Google Scholar] [CrossRef]
- Lundåsen, T.; Liao, W.; Angelin, B.; Rudling, M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J. Biol. Chem. 2003, 278, 43224–43228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dullaart, R.P.F.; de Vries, R.; Dallinga-Thie, G.M.; van Tol, A.; Sluiter, W.J. Plasma cholesteryl ester transfer protein mass and phospholipid transfer protein activity are associated with leptin in type 2 diabetes mellitus. Biochim. Biophys. Acta 2007, 1771, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Radeau, T.; Lau, P.; Robb, M.; McDonnell, M.; Ailhaud, G.; McPherson, R. Cholesteryl ester transfer protein (CETP) mRNA abundance in human adipose tissue: Relationship to cell size and membrane cholesterol content. J. Lipid Res. 1995, 36, 2552–2561. [Google Scholar] [PubMed]
- Bamba, V.; Rader, D.J. Obesity and Atherogenic Dyslipidemia. Gastroenterology 2007, 132, 2181–2190. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Hirose, H.; Saito, I.; Tomita, M.; Taniyama, M.; Matsubara, K.; Okazaki, Y.; Ishii, T.; Nishikai, K.; Saruta, T. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin. Sci. Lond. Engl. 2002, 103, 137–142. [Google Scholar] [CrossRef]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. Off. J. Jpn. Circ. Soc. 2004, 68, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N.; Wannamethee, G.; Sarwar, N.; Tchernova, J.; Cherry, L.; Wallace, A.; Danesh, J.; Whincup, P. Adiponectin and Coronary Heart Disease. Circulation 2006, 114, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.J.; Woo, J.G.; Daniels, S.R.; Goodman, E.; Dolan, L.M. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. J. Clin. Endocrinol. Metab. 2005, 90, 4255–4259. [Google Scholar] [CrossRef] [Green Version]
- Baratta, R.; Amato, S.; Degano, C.; Farina, M.G.; Patanè, G.; Vigneri, R.; Frittitta, L. Adiponectin relationship with lipid metabolism is independent of body fat mass: Evidence from both cross-sectional and intervention studies. J. Clin. Endocrinol. Metab. 2004, 89, 2665–2671. [Google Scholar] [CrossRef] [Green Version]
- Després, J.P.; Couillard, C.; Gagnon, J.; Bergeron, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1932–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, M.C.; Ayyobi, A.F.; Murdoch, S.J.; Deeb, S.S.; Brunzell, J.D. Contribution of hepatic lipase, lipoprotein lipase, and cholesteryl ester transfer protein to LDL and HDL heterogeneity in healthy women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 667–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.F.; Lamarche, B.; Uffelman, K.D.; Heatherington, A.C.; Honig, M.A.; Szeto, L.W.; Barrett, P.H. Clearance of postprandial and lipolytically modified human HDL in rabbits and rats. J. Lipid Res. 1997, 38, 1771–1778. [Google Scholar] [PubMed]
- Rashid, S.; Trinh, D.K.; Uffelman, K.D.; Cohn, J.S.; Rader, D.J.; Lewis, G.F. Expression of human hepatic lipase in the rabbit model preferentially enhances the clearance of triglyceride-enriched versus native high-density lipoprotein apolipoprotein A-I. Circulation 2003, 107, 3066–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.F.; Murdoch, S.; Uffelman, K.; Naples, M.; Szeto, L.; Albers, A.; Adeli, K.; Brunzell, J.D. Hepatic lipase mRNA, protein, and plasma enzyme activity is increased in the insulin-resistant, fructose-fed Syrian golden hamster and is partially normalized by the insulin sensitizer rosiglitazone. Diabetes 2004, 53, 2893–2900. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Choi, S.; Kundu, R.K.; Hirata, K.-I.; Rubin, E.M.; Cooper, A.D.; Quertermous, T. Endothelial lipase is a major determinant of HDL level. J. Clin. Investig. 2003, 111, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Millar, J.S.; Broedl, U.; Glick, J.M.; Rader, D.J. Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J. Clin. Investig. 2003, 111, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Cilingiroglu, M.; Otvos, J.D.; Ballantyne, C.M.; Marian, A.J.; Chan, L. Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism. Proc. Natl. Acad. Sci. USA 2003, 100, 2748–2753. [Google Scholar] [CrossRef] [Green Version]
- Maugeais, C.; Tietge, U.J.F.; Broedl, U.C.; Marchadier, D.; Cain, W.; McCoy, M.G.; Lund-Katz, S.; Glick, J.M.; Rader, D.J. Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation 2003, 108, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Jaye, M.; Lynch, K.J.; Krawiec, J.; Marchadier, D.; Maugeais, C.; Doan, K.; South, V.; Amin, D.; Perrone, M.; Rader, D.J. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 1999, 21, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Badellino, K.O.; Wolfe, M.L.; Reilly, M.P.; Rader, D.J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 2006, 3, e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lemos, A.S.; Wolfe, M.L.; Long, C.J.; Sivapackianathan, R.; Rader, D.J. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002, 106, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Méd. Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Chung, S.; Cuffe, H.; Marshall, S.M.; McDaniel, A.L.; Ha, J.H.; Kavanagh, K.; Hong, C.; Tontonoz, P.; Temel, R.E.; Parks, J.S. Dietary Cholesterol Promotes Adipocyte Hypertrophy and Adipose Tissue Inflammation in Visceral, but Not in Subcutaneous, Fat in Monkeys. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1880–1887. [Google Scholar] [CrossRef] [Green Version]
- Kovanen, P.T.; Nikkilä, E.A.; Miettinen, T.A. Regulation of cholesterol synthesis and storage in fat cells. J. Lipid Res. 1975, 16, 211–223. [Google Scholar]
- Farkas, J.; Angel, A.; Avigan, M.I. Studies on the compartmentation of lipid in adipose cells. II. Cholesterol accumulation and distribution in adipose tissue components. J. Lipid Res. 1973, 14, 344–356. [Google Scholar]
- Schreibman, P.H.; Dell, R.B. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. J. Clin. Investig. 1975, 55, 986–993. [Google Scholar] [CrossRef]
- Krause, B.R.; Hartman, A.D. Adipose tissue and cholesterol metabolism. J. Lipid Res. 1984, 25, 97–110. [Google Scholar]
- McGillicuddy, F.C.; Reilly, M.P. Adipose tissue modulation of HDL. Clin. Lipidol. 2010, 5, 601–606. [Google Scholar] [CrossRef]
- Zhang, Y.; McGillicuddy, F.C.; Hinkle, C.C.; O’Neill, S.; Glick, J.M.; Rothblat, G.H.; Reilly, M.P. Adipocyte Modulation of High-Density Lipoprotein Cholesterol. Circulation 2010, 121, 1347–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016, 15, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woudberg, N.J.; Mendham, A.E.; Katz, A.A.; Goedecke, J.H.; Lecour, S. Exercise intervention alters HDL subclass distribution and function in obese women. Lipids Health Dis. 2018, 17, 232. [Google Scholar] [CrossRef] [Green Version]
- Davidson, W.S.; Heink, A.; Sexmith, H.; Dolan, L.M.; Gordon, S.M.; Otvos, J.D.; Melchior, J.T.; Elder, D.A.; Khoury, J.; Geh, E.; et al. Obesity is associated with an altered HDL subspecies profile among adolescents with metabolic disease. J. Lipid Res. 2017, 58, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Holzer, M.; Schilcher, G.; Curcic, S.; Trieb, M.; Ljubojevic, S.; Stojakovic, T.; Scharnagl, H.; Kopecky, C.M.; Rosenkranz, A.R.; Heinemann, A.; et al. Dialysis Modalities and HDL Composition and Function. J. Am. Soc. Nephrol. 2015, 26, 2267–2276. [Google Scholar] [CrossRef] [Green Version]
- Holzer, M.; Birner-Gruenberger, R.; Stojakovic, T.; El-Gamal, D.; Binder, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Uremia Alters HDL Composition and Function. J. Am. Soc. Nephrol. 2011, 22, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Farbstein, D.; Levy, A.P. HDL dysfunction in diabetes: Causes and possible treatments. Expert Rev. Cardiovasc. Ther. 2012, 10, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Trieb, M.; Horvath, A.; Birner-Gruenberger, R.; Spindelboeck, W.; Stadlbauer, V.; Taschler, U.; Curcic, S.; Stauber, R.E.; Holzer, M.; Pasterk, L.; et al. Liver disease alters high-density lipoprotein composition, metabolism and function. Biochim. Biophys. Acta 2016, 1861, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Holzer, M.; Wolf, P.; Curcic, S.; Birner-Gruenberger, R.; Weger, W.; Inzinger, M.; El-Gamal, D.; Wadsack, C.; Heinemann, A.; Marsche, G. Psoriasis alters HDL composition and cholesterol efflux capacity. J. Lipid Res. 2012, 53, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Trieb, M.; Wolf, P.; Knuplez, E.; Weger, W.; Schuster, C.; Peinhaupt, M.; Holzer, M.; Trakaki, A.; Eichmann, T.; Lass, A.; et al. Abnormal composition and function of high-density lipoproteins in atopic dermatitis patients. Allergy 2019, 74, 398–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakaki, A.; Sturm, G.J.; Pregartner, G.; Scharnagl, H.; Eichmann, T.O.; Trieb, M.; Knuplez, E.; Holzer, M.; Stadler, J.T.; Heinemann, A.; et al. Allergic rhinitis is associated with complex alterations in high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.A.; Besler, C.; Rohrer, L.; Meyer, M.; Heinrich, K.; Bahlmann, F.H.; Mueller, M.; Horváth, T.; Doerries, C.; Heinemann, M.; et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010, 121, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Perségol, L.; Vergès, B.; Foissac, M.; Gambert, P.; Duvillard, L. Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 2006, 49, 1380–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasahara, T.; Nestel, P.; Fidge, N.; Sviridov, D. Cholesterol transport between cells and high density lipoprotein subfractions from obese and lean subjects. J. Lipid Res. 1998, 39, 544–554. [Google Scholar]
- Marsche, G.; Zelzer, S.; Meinitzer, A.; Kern, S.; Meissl, S.; Pregartner, G.; Weghuber, D.; Almer, G.; Mangge, H. Adiponectin Predicts High-Density Lipoprotein Cholesterol Efflux Capacity in Adults Irrespective of Body Mass Index and Fat Distribution. J. Clin. Endocrinol. Metab. 2017, 102, 4117–4123. [Google Scholar] [CrossRef]
- Talbot, C.P.J.; Plat, J.; Joris, P.J.; Konings, M.; Kusters, Y.H.A.M.; Schalkwijk, C.G.; Ritsch, A.; Mensink, R.P. HDL cholesterol efflux capacity and cholesteryl ester transfer are associated with body mass, but are not changed by diet-induced weight loss: A randomized trial in abdominally obese men. Atherosclerosis 2018, 274, 23–28. [Google Scholar] [CrossRef]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol Efflux Capacity, High-Density Lipoprotein Function, and Atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Nishida, M.; Matsuyama, A.; Okamoto, Y.; Ishigami, M.; Kuriyama, H.; Kishida, K.; Nishizawa, H.; et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 2001, 103, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, F.; Oku, H.; Koseki, M.; Sandoval, J.C.; Yuasa-Kawase, M.; Tsubakio-Yamamoto, K.; Masuda, D.; Maeda, N.; Tsujii, K.; Ishigami, M.; et al. Adiponectin accelerates reverse cholesterol transport by increasing high density lipoprotein assembly in the liver. Biochem. Biophys. Res. Commun. 2007, 358, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Oku, H.; Matsuura, F.; Koseki, M.; Sandoval, J.C.; Yuasa-Kawase, M.; Tsubakio-Yamamoto, K.; Masuda, D.; Maeda, N.; Ohama, T.; Ishigami, M.; et al. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 2007, 581, 5029–5033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsubakio-Yamamoto, K.; Matsuura, F.; Koseki, M.; Oku, H.; Sandoval, J.C.; Inagaki, M.; Nakatani, K.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 2008, 375, 390–394. [Google Scholar] [CrossRef]
- Liang, B.; Wang, X.; Guo, X.; Yang, Z.; Bai, R.; Liu, M.; Xiao, C.; Bian, Y. Adiponectin upregulates ABCA1 expression through liver X receptor alpha signaling pathway in RAW 264.7 macrophages. Int. J. Clin. Exp. Pathol. 2015, 8, 450–457. [Google Scholar]
- Chawla, A.; Boisvert, W.A.; Lee, C.-H.; Laffitte, B.A.; Barak, Y.; Joseph, S.B.; Liao, D.; Nagy, L.; Edwards, P.A.; Curtiss, L.K.; et al. A PPARγ-LXR-ABCA1 Pathway in Macrophages Is Involved in Cholesterol Efflux and Atherogenesis. Mol. Cell 2001, 7, 161–171. [Google Scholar] [CrossRef]
- Vergès, B.; Petit, J.M.; Duvillard, L.; Dautin, G.; Florentin, E.; Galland, F.; Gambert, P. Adiponectin is an important determinant of apoA-I catabolism. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1364–1369. [Google Scholar] [CrossRef] [Green Version]
- Hafiane, A.; Daskalopoulou, S.S. Adiponectin’s mechanisms in high-density lipoprotein biogenesis and cholesterol efflux. Metabolism 2020, 113, 154393. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; Posadas-Romero, C.; Mendoza-Pérez, E.; Caracas-Portilla, N.A.; Cardoso-Saldaña, G.; Medina-Urrutia, A.; Jorge-Galarza, E.; Juárez-Rojas, J.G. Cholesterol efflux and metabolic abnormalities associated with low high-density-lipoprotein-cholesterol and high triglycerides in statin-treated coronary men with low-density lipoprotein-cholesterol <70 mg/dl. Am. J. Cardiol. 2012, 109, 636–641. [Google Scholar] [CrossRef]
- Wang, M.; Wang, D.; Zhang, Y.; Wang, X.; Liu, Y.; Xia, M. Adiponectin increases macrophages cholesterol efflux and suppresses foam cell formation in patients with type 2 diabetes mellitus. Atherosclerosis 2013, 229, 62–70. [Google Scholar] [CrossRef]
- Clarenbach, J.; Vega, G.; Adams-Huet, B.; Considine, R.; Ricks, M.; Sumner, A. Variability in Postheparin Hepatic Lipase Activity Is Associated with Plasma Adiponectin Levels in African Americans. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2007, 55, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; von Eynatten, M.; Schiekofer, S.; Nawroth, P.P.; Dugi, K.A. Low Plasma Adiponectin Levels Are Associated With Increased Hepatic Lipase Activity In Vivo: Response to Kobayashi et al. Diabetes Care 2006, 29, 181. [Google Scholar] [CrossRef]
- Magge, S.N.; Stettler, N.; Koren, D.; Levitt Katz, L.E.; Gallagher, P.R.; Mohler, E.R.; Rader, D.J. Adiponectin is associated with favorable lipoprotein profile, independent of BMI and insulin resistance, in adolescents. J. Clin. Endocrinol. Metab. 2011, 96, 1549–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.; Otvos, J.D.; Flyvbjerg, A.; Miserez, A.R.; Frystyk, J.; Sinnreich, R.; Kark, J.D. Adiponectin and Lipoprotein Particle Size. Diabetes Care 2009, 32, 1317–1319. [Google Scholar] [CrossRef] [Green Version]
- Tsubakio-Yamamoto, K.; Sugimoto, T.; Nishida, M.; Okano, R.; Monden, Y.; Kitazume-Taneike, R.; Yamashita, T.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; et al. Serum adiponectin level is correlated with the size of HDL and LDL particles determined by high performance liquid chromatography. Metabolism 2012, 61, 1763–1770. [Google Scholar] [CrossRef]
- Shin, M.J.; Kim, O.Y. Plasma adiponectin is associated with less atherogenic lipoprotein phenotype. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 770–775. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kusunoki, M.; Murase, Y.; Kawashiri, M.; Higashikata, T.; Miwa, K.; Katsuda, S.; Takata, M.; Asano, A.; Nohara, A.; et al. Relationship of Lipoprotein Lipase and Hepatic Triacylglycerol Lipase Activity to Serum Adiponectin Levels in Japanese Hyperlipidemic Men. Horm. Metab. Res. 2005, 37, 505–509. [Google Scholar] [CrossRef]
- Von Eynatten, M.; Schneider, J.G.; Humpert, P.M.; Rudofsky, G.; Schmidt, N.; Barosch, P.; Hamann, A.; Morcos, M.; Kreuzer, J.; Bierhaus, A.; et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: An association independent of systemic inflammation and insulin resistance. Diabetes Care 2004, 27, 2925–2929. [Google Scholar] [CrossRef] [Green Version]
- Van Greevenbroek, M.M.J.; Schalkwijk, C.G.; Stehouwer, C.D.A. Obesity-associated low-grade inflammation in type 2 diabetes mellitus: Causes and consequences. Neth. J. Med. 2013, 71, 174–187. [Google Scholar]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef]
- Kaser, S.; Tatarczyk, T.; Stadlmayr, A.; Ciardi, C.; Ress, C.; Tschoner, A.; Sandhofer, A.; Paulweber, B.; Ebenbichler, C.F.; Patsch, J.R. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur. J. Clin. Investig. 2008, 38, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.K.; Ciaraldi, T.P.; Henry, R.R.; Wittgrove, A.C.; Phillips, S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2013, 2, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Tchkonia, T.; Jensen, M.D. Sphingolipid content of human adipose tissue: Relationship to adiponectin and insulin resistance. Obesity 2012, 20, 2341–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Sato, K.; Kuwabara, A.; Tomura, H.; Ishiwara, M.; Kobayashi, I.; Ui, M.; Okajima, F. Sphingosine 1-phosphate may be a major component of plasma lipoproteins responsible for the cytoprotective actions in human umbilical vein endothelial cells. J. Biol. Chem. 2001, 276, 31780–31785. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, K.; Lee, Y.-M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Gazit, S.L.; Mariko, B.; Thérond, P.; Decouture, B.; Xiong, Y.; Couty, L.; Bonnin, P.; Baudrie, V.; Le Gall, S.M.; Dizier, B.; et al. Platelet and Erythrocyte Sources of S1P Are Redundant for Vascular Development and Homeostasis, but Both Rendered Essential After Plasma S1P Depletion in Anaphylactic Shock. Circ. Res. 2016, 119, e110–e126. [Google Scholar] [CrossRef]
- Lee, M.J.; Van Brocklyn, J.R.; Thangada, S.; Liu, C.H.; Hand, A.R.; Menzeleev, R.; Spiegel, S.; Hla, T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998, 279, 1552–1555. [Google Scholar] [CrossRef]
- Kowalski, G.M.; Carey, A.L.; Selathurai, A.; Kingwell, B.A.; Bruce, C.R. Plasma sphingosine-1-phosphate is elevated in obesity. PLoS ONE 2013, 8, e72449. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, I.; Mastrandrea, L.D. Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 2012, 41, 442–449. [Google Scholar] [CrossRef]
- Green, C.L.; Mitchell, S.E.; Derous, D.; Wang, Y.; Chen, L.; Han, J.-D.J.; Promislow, D.E.L.; Lusseau, D.; Douglas, A.; Speakman, J.R. The effects of graded levels of calorie restriction: IX. Global metabolomic screen reveals modulation of carnitines, sphingolipids and bile acids in the liver of C57BL/6 mice. Aging Cell 2017, 16, 529–540. [Google Scholar] [CrossRef]
- Silva, V.R.R.; Micheletti, T.O.; Pimentel, G.D.; Katashima, C.K.; Lenhare, L.; Morari, J.; Mendes, M.C.S.; Razolli, D.S.; Rocha, G.Z.; de Souza, C.T.; et al. Hypothalamic S1P/S1PR1 axis controls energy homeostasis. Nat. Commun. 2014, 5, 4859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christoffersen, C.; Federspiel, C.K.; Borup, A.; Christensen, P.M.; Madsen, A.N.; Heine, M.; Nielsen, C.H.; Kjaer, A.; Holst, B.; Heeren, J.; et al. The Apolipoprotein M/S1P Axis Controls Triglyceride Metabolism and Brown Fat Activity. Cell Rep. 2018, 22, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlbäck, B. Lean ApoM-/-Mice with Hyperactive Brown Adipose Tissue. Trends Endocrinol. Metab. 2018, 29, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Frej, C.; Mendez, A.J.; Ruiz, M.; Castillo, M.; Hughes, T.A.; Dahlbäck, B.; Goldberg, R.B. A Shift in ApoM/S1P Between HDL-Particles in Women With Type 1 Diabetes Mellitus Is Associated with Impaired Anti-Inflammatory Effects of the ApoM/S1P Complex. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Brolin, R.E. Bariatric surgery and long-term control of morbid obesity. JAMA 2002, 288, 2793–2796. [Google Scholar] [CrossRef]
- Steinbrook, R. Surgery for severe obesity. N. Engl. J. Med. 2004, 350, 1075–1079. [Google Scholar] [CrossRef]
- Kuno, T.; Tanimoto, E.; Morita, S.; Shimada, Y.J. Effects of Bariatric Surgery on Cardiovascular Disease: A Concise Update of Recent Advances. Front. Cardiovasc. Med. 2019, 6. [Google Scholar] [CrossRef]
- Benraoune, F.; Litwin, S.E. Reductions in Cardiovascular Risk after Bariatric Surgery. Curr. Opin. Cardiol. 2011, 26, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Lorkowski, S.W.; Brubaker, G.; Rotroff, D.M.; Kashyap, S.R.; Bhatt, D.L.; Nissen, S.E.; Schauer, P.R.; Aminian, A.; Smith, J.D. Bariatric Surgery Improves HDL Function Examined by ApoA1 Exchange Rate and Cholesterol Efflux Capacity in Patients with Obesity and Type 2 Diabetes. Biomolecules 2020, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Raffaelli, M.; Guidone, C.; Callari, C.; Iaconelli, A.; Bellantone, R.; Mingrone, G. Effect of gastric bypass versus diet on cardiovascular risk factors. Ann. Surg. 2014, 259, 694–699. [Google Scholar] [CrossRef]
- Christou, N.V.; Sampalis, J.S.; Liberman, M.; Look, D.; Auger, S.; McLean, A.P.H.; MacLean, L.D. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann. Surg. 2004, 240, 416–424. [Google Scholar] [CrossRef]
- Williams, D.B.; Hagedorn, J.C.; Lawson, E.H.; Galanko, J.A.; Safadi, B.Y.; Curet, M.J.; Morton, J.M. Gastric bypass reduces biochemical cardiac risk factors. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2007, 3, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zlabek, J.A.; Grimm, M.S.; Larson, C.J.; Mathiason, M.A.; Lambert, P.J.; Kothari, S.N. The effect of laparoscopic gastric bypass surgery on dyslipidemia in severely obese patients. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2005, 1, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, M.M.; Austrheim-Smith, I.T.; Stanhope, K.L.; Van Loan, M.D.; Ali, M.R.; Wolfe, B.M.; Havel, P.J. Circulating concentrations of high-molecular-weight adiponectin are increased following Roux-en-Y gastric bypass surgery. Diabetologia 2006, 49, 2552–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopp, H.-P.; Krzyzanowska, K.; Möhlig, M.; Spranger, J.; Pfeiffer, A.F.H.; Schernthaner, G. Effects of marked weight loss on plasma levels of adiponectin, markers of chronic subclinical inflammation and insulin resistance in morbidly obese women. Int. J. Obes. 2005, 29, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, C.D.; Lissner, L.; Wedel, H.; Sjöström, L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: The SOS Intervention Study. Obes. Res. 1999, 7, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric Surgery versus Intensive Medical Therapy in Obese Patients with Diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Brethauer, S.A.; Navaneethan, S.D.; Aminian, A.; Pothier, C.E.; Kim, E.S.H.; Nissen, S.E.; et al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N. Engl. J. Med. 2014, 370, 2002–2013. [Google Scholar] [CrossRef] [Green Version]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet Lond. Engl. 2015, 386, 964–973. [Google Scholar] [CrossRef]
- Lorkowski, S.W.; Brubaker, G.; Li, L.; Li, X.S.; Hazen, S.L.; Smith, J.D. A Novel Cell-Free Fluorescent Assay for HDL Function: Low Apolipoprotein A1 Exchange Rate Associated with Increased Incident Cardiovascular Events. J. Appl. Lab. Med. 2020, 5, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Heffron, S.P.; Lin, B.-X.; Parikh, M.; Scolaro, B.; Adelman, S.J.; Collins, H.L.; Berger, J.S.; Fisher, E.A. Changes in High-Density Lipoprotein Cholesterol Efflux Capacity After Bariatric Surgery Are Procedure Dependent. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zvintzou, E.; Skroubis, G.; Chroni, A.; Petropoulou, P.-I.; Gkolfinopoulou, C.; Sakellaropoulos, G.; Gantz, D.; Mihou, I.; Kalfarentzos, F.; Kypreos, K.E. Effects of bariatric surgery on HDL structure and functionality: Results from a prospective trial. J. Clin. Lipidol. 2014, 8, 408–417. [Google Scholar] [CrossRef]
- Longitudinal Assessment of Bariatric Surgery (LABS) Consortium; Flum, D.R.; Belle, S.H.; King, W.C.; Wahed, A.S.; Berk, P.; Chapman, W.; Pories, W.; Courcoulas, A.; McCloskey, C.; et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N. Engl. J. Med. 2009, 361, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coimbra, S.; Reis, F.; Ferreira, C.; Nunes, S.; Viana, S.; Catarino, A.; Rocha-Pereira, P.; Belo, L.; Monteiro, L.; Catarino, C.; et al. Weight loss achieved by bariatric surgery modifies high-density lipoprotein subfractions and low-density lipoprotein oxidation towards atheroprotection. Clin. Biochem. 2019, 63, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kjellmo, C.A.; Karlsson, H.; Nestvold, T.K.; Ljunggren, S.; Cederbrant, K.; Marcusson-Ståhl, M.; Mathisen, M.; Lappegård, K.T.; Hovland, A. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J. Clin. Lipidol. 2018, 12, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osto, E.; Doytcheva, P.; Corteville, C.; Bueter, M.; Dörig, C.; Stivala, S.; Buhmann, H.; Colin, S.; Rohrer, L.; Hasballa, R.; et al. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: Role of glucagon-like peptide-1. Circulation 2015, 131, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Huang, H.; Xu, Y.; Zhu, H.; Zhong, C. MiR-222 in Cardiovascular Diseases: Physiology and Pathology. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Taïbi, F.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. miR-223: An inflammatory oncomiR enters the cardiovascular field. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2014, 1842, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.H.; Ong, K.L.; Torres, L.F.C.; Liu, Y.; Adam, S.; Iqbal, Z.; Dhage, S.; Ammori, B.J.; Syed, A.A.; Rye, K.-A.; et al. High-density lipoprotein-associated miRNA is increased following Roux-en-Y gastric bypass surgery for severe obesity. J. Lipid Res. 2020, jlr.RA120000963. [Google Scholar] [CrossRef]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Mauer, E.A.; Shukla, A.P.; Rathi, S.; Aronne, L.J. Low adoption of weight loss medications: A comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity 2016, 24, 1955–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Cho, H.-J.; Kang, H.-C.; Youn, B.-B.; Lee, K.-R. Effects on Weight Reduction and Safety of Short-Term Phentermine Administration in Korean Obese People. Yonsei Med. J. 2006, 47, 614–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef]
- Davidson, M.H.; Tonstad, S.; Oparil, S.; Schwiers, M.; Day, W.W.; Bowden, C.H. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m(2). Am. J. Cardiol. 2013, 111, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): A randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Mittendorfer, B.; Ostlund, R.E.; Patterson, B.W.; Klein, S. Orlistat Inhibits Dietary Cholesterol Absorption. Obes. Res. 2001, 9, 599–604. [Google Scholar] [CrossRef]
- Berne, C.; Orlistat Swedish Type 2 diabetes Study Group. A randomized study of orlistat in combination with a weight management programme in obese patients with Type 2 diabetes treated with metformin. Diabet. Med. J. Br. Diabet. Assoc. 2005, 22, 612–618. [Google Scholar] [CrossRef]
- Hanefeld, M.; Sachse, G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: A randomized, placebo-controlled trial. Diabetes Obes. Metab. 2002, 4, 415–423. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.E.; Bray, G.A.; Pi-Sunyer, F.X.; Klein, S.; Hill, J.; Miles, J.; Hollander, P. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin-treated type 2 diabetes: A 1-year randomized controlled trial. Diabetes Care 2002, 25, 1033–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollander, P.A.; Elbein, S.C.; Hirsch, I.B.; Kelley, D.; McGill, J.; Taylor, T.; Weiss, S.R.; Crockett, S.E.; Kaplan, R.A.; Comstock, J.; et al. Role of Orlistat in the Treatment of Obese Patients With Type 2 Diabetes: A 1-year randomized double-blind study. Diabetes Care 1998, 21, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Miles, J.M.; Leiter, L.; Hollander, P.; Wadden, T.; Anderson, J.W.; Doyle, M.; Foreyt, J.; Aronne, L.; Klein, S. Effect of Orlistat in Overweight and Obese Patients With Type 2 Diabetes Treated With Metformin. Diabetes Care 2002, 25, 1123–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar] [CrossRef] [Green Version]
- Langsted, A.; Freiberg, J.J.; Nordestgaard, B.G. Fasting and nonfasting lipid levels: Influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008, 118, 2047–2056. [Google Scholar] [CrossRef] [Green Version]
- Fidler, M.C.; Sanchez, M.; Raether, B.; Weissman, N.J.; Smith, S.R.; Shanahan, W.R.; Anderson, C.M.; BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: The BLOSSOM trial. J. Clin. Endocrinol. Metab. 2011, 96, 3067–3077. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, P.M.; Smith, S.R.; Weissman, N.J.; Fidler, M.C.; Sanchez, M.; Zhang, J.; Raether, B.; Anderson, C.M.; Shanahan, W.R. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM study. Obesity 2012, 20, 1426–1436. [Google Scholar] [CrossRef]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Mathew, H.; Paschou, S.A.; Perakakis, N.; Koniaris, A.; Kelesidis, T.; Mantzoros, C.S. Lorcaserin treatment decreases body weight and improves cardiometabolic risk factors of obese adults: A 6-month-long, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes. Metab. 2019, 21, 1487–1492. [Google Scholar] [CrossRef]
- Burcelin, R.; Gourdy, P. Harnessing glucagon-like peptide-1 receptor agonists for the pharmacological treatment of overweight and obesity. Obes. Rev. 2017, 18, 86–98. [Google Scholar] [CrossRef]
- Christou, G.A.; Katsiki, N.; Kiortsis, D.N. The Current Role of Liraglutide in the Pharmacotherapy of Obesity. Curr. Vasc. Pharmacol. 2016, 14, 201–207. [Google Scholar] [CrossRef]
- Engelbrechtsen, L.; Lundgren, J.; Wewer Albrechtsen, N.J.; Mahendran, Y.; Iepsen, E.W.; Finocchietto, P.; Jonsson, A.E.; Madsbad, S.; Holst, J.J.; Vestergaard, H.; et al. Treatment with liraglutide may improve markers of CVD reflected by reduced levels of apoB. Obes. Sci. Pract. 2017, 3, 425–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity–A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Calorie restriction and cardiometabolic health. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Snel, M.; Jonker, J.T.; Hammer, S.; Lamb, H.J.; de Roos, A.; Meinders, A.E.; Pijl, H.; Romijn, J.A.; Smit, J.W.A.; et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases plasma CETP and increases apolipoprotein AI levels without improving the cholesterol efflux properties of HDL. Diabetes Care 2011, 34, 2576–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maroofi, M.; Nasrollahzadeh, J. Effect of intermittent versus continuous calorie restriction on body weight and cardiometabolic risk markers in subjects with overweight or obesity and mild-to-moderate hypertriglyceridemia: A randomized trial. Lipids Health Dis. 2020, 19, 216. [Google Scholar] [CrossRef]
- Liang, K.-W.; Lee, W.-J.; Lee, I.-T.; Lee, W.-L.; Lin, S.-Y.; Hsu, S.-L.; Wan, C.-J.; Yu, C.-Y.; Tsai, I.-C.; Fu, C.-P.; et al. Persistent elevation of paraoxonase-1 specific enzyme activity after weight reduction in obese non-diabetic men with metabolic syndrome. Clin. Chim. Acta 2011, 412, 1835–1841. [Google Scholar] [CrossRef]
- Kotani, K.; Sakane, N.; Sano, Y.; Tsuzaki, K.; Matsuoka, Y.; Egawa, K.; Yoshimura, M.; Horikawa, C.; Kitagawa, Y.; Kiso, Y.; et al. Changes on the physiological lactonase activity of serum paraoxonase 1 by a diet intervention for weight loss in healthy overweight and obese women. J. Clin. Biochem. Nutr. 2009, 45, 329–334. [Google Scholar] [CrossRef]
- Shoji, T.; Nishizawa, Y.; Koyama, H.; Hagiwara, S.; Aratani, H.; Sasao, K.; Kishimoto, H.; Tanishita, H.; Morii, H. High-density-lipoprotein metabolism during a very-low-calorie diet. Am. J. Clin. Nutr. 1992, 56, 297S–298S. [Google Scholar] [CrossRef]
- Hietanen, E.; Laitinen, M.; Ahonen, E.; Marniemi, J.; Nousiainen, U. Changes in Serum Lipids and Lecithin: Cholesterol Acyltransferase Enzyme during 1 Week Weight Reduction of Women on a Low-Calorie Diet. Enzyme 1983, 29, 160–166. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Bardagjy, A.S.; Steinberg, F.M. Relationship between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019, 11, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, A.V.; Li, L.; Byun, J.; Guo, Y.; Michailidis, G.; Jaiswal, M.; Chen, Y.E.; Pop-Busui, R.; Pennathur, S. Therapeutic Lifestyle Changes Improve HDL Function by Inhibiting Myeloperoxidase-Mediated Oxidation in Patients With Metabolic Syndrome. Diabetes Care 2018. [Google Scholar] [CrossRef] [Green Version]
| HDL Subclass | Size | Shape | Abundant Components | Important Functions |
|---|---|---|---|---|
| Pre-β HDL | 9.6 nm diameter, 4.7 nm thickness | discoidal | ApoA-I, phospholipids | ABCA1-Cholesterol efflux |
| HDL3 | 7.5 nm, 175 kDa | spherical | Protein:lipid ratio 55:45 PON1, ApoA-II, ApoM, S1P | Anti-oxidative activity Anti-inflammatory activity ABCA1-Cholesterol efflux |
| HDL2 | 10 nm, 350 kDa | spherical | Protein:lipid ratio 40:60 | ABCG1- Cholesterol Efflux |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985. https://doi.org/10.3390/ijms21238985
Stadler JT, Marsche G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. International Journal of Molecular Sciences. 2020; 21(23):8985. https://doi.org/10.3390/ijms21238985
Chicago/Turabian StyleStadler, Julia T., and Gunther Marsche. 2020. "Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function" International Journal of Molecular Sciences 21, no. 23: 8985. https://doi.org/10.3390/ijms21238985






