Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib
Abstract
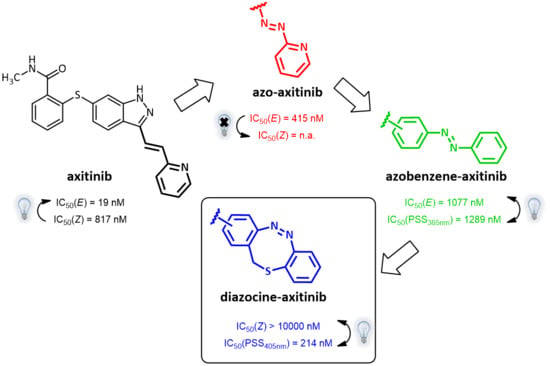
:1. Introduction
Axitinib as Template for Photoresponsive Kinase Inhibitors
2. Results
2.1. Molecular Modeling
2.1.1. Molecular Modeling of Azobenzene Derivatives 3 and 4 in the ATP Binding Pocket of VEGFR-2
2.1.2. Molecular Modeling of Diazocine Derivatives 5–7 in the ATP Binding Pocket of VEGFR-2
2.2. Synthesis
2.3. Photochemical Characterization of the Photoswitchable Compounds 3–7
2.3.1. Photochemical Characterization of Azobenzene Derivatives 3 and 4
2.3.2. Photochemical Characterization of Diazocine Derivatives 5–7
2.4. Biological Evaluation
2.4.1. VEGFR-2 Kinase Assays
VEGFR-2 Kinase Assays of Azobenzene Derivatives 3 and 4
VEGFR-2 Kinase Assay of Diazocine Derivatives 5–7
2.4.2. Kinome Profiling (PamGene) of Sulfur–diazocine Derivative 5
3. Discussion
4. Materials and Methods
4.1. Computational Chemistry
4.2. Synthesis
4.3. Photochemical Characterization
4.4. Kinase Assays
4.5. Kinase Selectivity Profiling (PamGene)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| DBPO | Dibenzoyl peroxide |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DTT | Dithiothreitol |
| FDA | Food and Drug Administration |
| HUVEC | Human umbilical vein endothelial cell |
| LED | Light-emitting diode |
| NBS | N-Bromosuccinimide |
| pdb | Protein Data Bank |
| PKC | Protein kinase C |
| PSS | Photostationary state |
| RMSD | Root mean square deviation |
| RT | Room temperature |
| THF | Tetrahydrofuran |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond proof of principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. Engl. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A roadmap to success in photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Hüll, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. [Google Scholar] [CrossRef] [PubMed]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bournez, C.; Carles, F.; Peyrat, G.; Aci-Sèche, S.; Bourg, S.; Meyer, C.; Bonnet, P. Comparative Assessment of Protein Kinase Inhibitors in Public Databases and in PKIDB. Molecules 2020, 25, 3226. [Google Scholar] [CrossRef] [PubMed]
- Carles, F.; Bourg, S.; Meyer, C.; Bonnet, P. PKIDB: A Curated, Annotated and Updated Database of Protein Kinase Inhibitors in Clinical Trials. Molecules 2018, 23, 908. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Fleming, C.L.; Grøtli, M.; Andréasson, J. On-Command Regulation of Kinase Activity using Photonic Stimuli. ChemPhotoChem 2019, 3, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.; Nilsson, J.R.; Solano, C.; Andréasson, J.; Grøtli, M. Design, Synthesis and Inhibitory Activity of Photoswitchable RET Kinase Inhibitors. Sci. Rep. 2015, 5, 9769. [Google Scholar] [CrossRef] [Green Version]
- Schehr, M.; Ianes, C.; Weisner, J.; Heintze, L.; Müller, M.P.; Pichlo, C.; Charl, J.; Brunstein, E.; Ewert, J.; Lehr, M.; et al. 2-Azo-, 2-diazocine-thiazols and 2-azo-imidazoles as photoswitchable kinase inhibitors: Limitations and pitfalls of the photoswitchable inhibitor approach. Photochem. Photobiol. Sci. 2019, 18, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Hoorens, M.W.H.; Ourailidou, M.E.; Rodat, T.; van der Wouden, P.E.; Kobauri, P.; Kriegs, M.; Peifer, C.; Feringa, B.L.; Dekker, F.J.; Szymanski, W. Light-controlled inhibition of BRAFV600E kinase. Eur. J. Med. Chem. 2019, 179, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Li, J.W.; Branda, N.R. Visible-Light-Triggered Activation of a Protein Kinase Inhibitor. ChemMedChem 2017, 12, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Rodat, T.; Heintze, L.; Weber, J.; Horbert, R.; Girreser, U.; Raeker, T.; Bußmann, L.; Kriegs, M.; Hartke, B.; et al. Axitinib: A Photoswitchable Approved Tyrosine Kinase Inhibitor. ChemMedChem 2018, 13, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Wood, L.S.; Rini, B.I. Axitinib in Metastatic Renal Cell Carcinoma. Biol. Ther. 2012, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Wei, N.; Liang, J.; Peng, S.; Sun, Q.; Dai, Q.; Dong, M. Design, Synthesis, and Biological Evaluation of Axitinib Derivatives. Molecules 2018, 23, 747. [Google Scholar] [CrossRef] [Green Version]
- Weston, C.E.; Richardson, R.D.; Fuchter, M.J. Photoswitchable basicity through the use of azoheteroarenes. Chem. Commun. (Camb) 2016, 52, 4521–4524. [Google Scholar] [CrossRef] [Green Version]
- Leippe, P. Tethered Photopharmacology. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2018. [Google Scholar]
- Simeth, N.A.; Crespi, S.; Fagnoni, M.; König, B. Tuning the Thermal Isomerization of Phenylazoindole Photoswitches from Days to Nanoseconds. J. Am. Chem. Soc. 2018, 140, 2940–2946. [Google Scholar] [CrossRef]
- Otsuki, J.; Suwa, K.; Sarker, K.K.; Sinha, C. Photoisomerization and thermal isomerization of arylazoimidazoles. J. Phys. Chem. A 2007, 111, 1403–1409. [Google Scholar] [CrossRef]
- Crespi, S.; Simeth, N.A.; Bellisario, A.; Fagnoni, M.; König, B. Unraveling the Thermal Isomerization Mechanisms of Heteroaryl Azoswitches: Phenylazoindoles as Case Study. J. Phys. Chem. A 2019, 123, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Rustler, K.; Nitschke, P.; Zahnbrecher, S.; Zach, J.; Crespi, S.; König, B. Photochromic Evaluation of 3(5)-Arylazo-1H-pyrazoles. J. Org. Chem. 2020, 85, 4079–4088. [Google Scholar] [CrossRef] [PubMed]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly efficient reversible Z-E photoisomerization of a bridged azobenzene with visible light through resolved S(1)(n pi*) absorption bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef]
- Samanta, S.; Qin, C.; Lough, A.J.; Woolley, G.A. Bidirectional photocontrol of peptide conformation with a bridged azobenzene derivative. Angew. Chem. Int. Ed. Engl. 2012, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Eljabu, F.; Dhruval, J.; Yan, H. Incorporation of cyclic azobenzene into oligodeoxynucleotides for the photo-regulation of DNA hybridization. Bioorg. Med. Chem. Lett. 2015, 25, 5594–5596. [Google Scholar] [CrossRef]
- Trads, J.B.; Hüll, K.; Matsuura, B.S.; Laprell, L.; Fehrentz, T.; Görldt, N.; Kozek, K.A.; Weaver, C.D.; Klöcker, N.; Barber, D.M.; et al. Sign Inversion in Photopharmacology: Incorporation of Cyclic Azobenzenes in Photoswitchable Potassium Channel Blockers and Openers. Angew. Chem. Int. Ed. Engl. 2019, 58, 15421–15428. [Google Scholar] [CrossRef]
- Thapaliya, E.R.; Zhao, J.; Ellis-Davies, G.C.R. Locked-Azobenzene: Testing the Scope of a Unique Photoswitchable Scaffold for Cell Physiology. ACS Chem. Neurosci. 2019, 10, 2481–2488. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef] [Green Version]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerich, M.; Schütt, C.; Stähler, C.; Lentes, P.; Röhricht, F.; Höppner, R.; Herges, R. Heterodiazocines: Synthesis and Photochromic Properties, Trans to Cis Switching within the Bio-optical Window. J. Am. Chem. Soc. 2016, 138, 13111–13114. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Chekal, B.P.; Guinness, S.M.; Lillie, B.M.; McLaughlin, R.W.; Palmer, C.W.; Post, R.J.; Sieser, J.E.; Singer, R.A.; Sluggett, G.W.; Vaidyanathan, R.; et al. Development of an Efficient Pd-Catalyzed Coupling Process for Axitinib. Org. Process Res. Dev. 2013, 18, 266–274. [Google Scholar] [CrossRef]
- Zhai, L.-H.; Guo, L.-H.; Luo, Y.-H.; Ling, Y.; Sun, B.-W. Effective Laboratory-Scale Preparation of Axitinib by Two CuI-Catalyzed Coupling Reactions. Org. Process Res. Dev. 2015, 19, 849–857. [Google Scholar] [CrossRef]
- Correa, A.; Tellitu, I.; Domínguez, E.; SanMartin, R. Novel Alternative for the N − S Bond Formation and Its Application to the Synthesis of Benzisothiazol-3-ones. Org. Lett. 2006, 8, 4811–4813. [Google Scholar] [CrossRef]
- Sridhara, M.B.; Srinivasa, G.R.; Channe Gowda, D. Ammonium chloride mediated reduction of azo compounds to hydrazo compounds. J. Chem. Res. 2004, 74–75. [Google Scholar] [CrossRef]
- Koźlecki, T.; Syper, L.; Wilk, K.A. 4-Lithio-4’-alkylazobenzenes as Convenient Intermediates for the Preparation of Azobenzene Derivatives. Synthesis 1997, 681–684. [Google Scholar] [CrossRef]
- Strueben, J.; Gates, P.J.; Staubitz, A. Tin-functionalized azobenzenes as nucleophiles in Stille cross-coupling reactions. J. Org. Chem. 2014, 79, 1719–1728. [Google Scholar] [CrossRef]
- Strueben, J.; Lipfert, M.; Springer, J.-O.; Gould, C.A.; Gates, P.J.; Sönnichsen, F.D.; Staubitz, A. High-yield lithiation of azobenzenes by tin-lithium exchange. Chemistry 2015, 21, 11165–11173. [Google Scholar] [CrossRef] [Green Version]
- Schehr, M.; Hugenbusch, D.; Moje, T.; Näther, C.; Herges, R. Synthesis of mono-functionalized S-diazocines via intramolecular Baeyer–Mills reactions. Beilstein J. Org. Chem. 2018, 14, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Moormann, W.; Langbehn, D.; Herges, R. Synthesis of functionalized diazocines for application as building blocks in photo- and mechanoresponsive materials. Beilstein J. Org. Chem. 2019, 15, 727–732. [Google Scholar] [CrossRef]
- Moormann, W.; Langbehn, D.; Herges, R. Solvent-Free Synthesis of Diazocine. Synthesis 2017, 49, 3471–3475. [Google Scholar] [CrossRef]
- Maier, M.S.; Hüll, K.; Reynders, M.; Matsuura, B.S.; Leippe, P.; Ko, T.; Schäffer, L.; Trauner, D. Oxidative Approach Enables Efficient Access to Cyclic Azobenzenes. J. Am. Chem. Soc. 2019, 141, 17295–17304. [Google Scholar] [CrossRef]
- Bléger, D.; Hecht, S. Visible-Light-Activated Molecular Switches. Angew. Chem. Int. Ed. Engl. 2015, 54, 11338–11349. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, A.; Røe, K.; Ree, A.H.; de Wijn, R.; Risberg, K.; Busch, C.; Lønning, P.E.; Kristensen, V.; Geisler, J. Differential inhibition of ex-vivo tumor kinase activity by vemurafenib in BRAF(V600E) and BRAF wild-type metastatic malignant melanoma. PLoS ONE 2013, 8, e72692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arni, S.; Le, T.H.N.; de Wijn, R.; Garcia-Villegas, R.; Dankers, M.; Weder, W.; Hillinger, S. Ex vivo multiplex profiling of protein tyrosine kinase activities in early stages of human lung adenocarcinoma. Oncotarget 2017, 8, 68599–68613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baharani, A.; Trost, B.; Kusalik, A.; Napper, S. Technological advances for interrogating the human kinome. Biochem. Soc. Trans. 2017, 45, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Lentes, P.; Stadler, E.; Röhricht, F.; Brahms, A.; Gröbner, J.; Sönnichsen, F.D.; Gescheidt, G.; Herges, R. Nitrogen Bridged Diazocines: Photochromes Switching within the Near-Infrared Region with High Quantum Yields in Organic Solvents and in Water. J. Am. Chem. Soc. 2019, 141, 13592–13600. [Google Scholar] [CrossRef]
- Labots, M.; Gotink, K.J.; Dekker, H.; Azijli, K.; van der Mijn, J.C.; Huijts, C.M.; Piersma, S.R.; Jiménez, C.R.; Verheul, H.M.W. Evaluation of a tyrosine kinase peptide microarray for tyrosine kinase inhibitor therapy selection in cancer. Exp. Mol. Med. 2016, 48, e279. [Google Scholar] [CrossRef] [Green Version]















 | |||||
|---|---|---|---|---|---|
| # | Residue (R) | λexc (nm) | PSS (E/Z%), DMSO (NMR) | t1/2 (h), 37 °C, DMSO (UV/VIS) | IC50 VEGFR-2 (nM) |
| 1 |  | 385 (ZE) 365 (EZ) | 88/12 (ZE) 51/49 (EZ) | >>12 * | E: 19 Z: 817 PSS 385 nm: 29 |
| 2 |  | n/a | n/a | n/a | E: 415 |
| 3 |  | 365 | 17/83 | 43.1 | E: 1077 ** PSS 365 nm: 1289 |
| 4 |  | 385 | 20/80 | 5.7 | E: 1020 ** PSS 385 nm: 1435 |
| 5 |  | 405 | 47/53 | 7.3 | Z: >10,000 PSS 405 nm: 214 |
| 6 |  | 405 | 25/75 | 3.7 | Z: >10,000 PSS 405 nm: 251 |
| 7 |  | 405 | 60/40 | 1.5 | Z: n/a ** PSS 405 nm: 493 |
 | |||||
|---|---|---|---|---|---|
| # | Residue (R) | Glide Score | Induced-Fit Score | ||
| E-Isomer a | Z-Isomer | E-Isomer a | Z-Isomer | ||
| 5 |  | −14.1 | 🗶 | −16.2 | 🗶 |
| 6 |  | −14.0 | 🗶 | −15.5 | 🗶 |
| 7 |  | −14.3 | 🗶 | −16.4 | −16.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heintze, L.; Schmidt, D.; Rodat, T.; Witt, L.; Ewert, J.; Kriegs, M.; Herges, R.; Peifer, C. Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib. Int. J. Mol. Sci. 2020, 21, 8961. https://doi.org/10.3390/ijms21238961
Heintze L, Schmidt D, Rodat T, Witt L, Ewert J, Kriegs M, Herges R, Peifer C. Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib. International Journal of Molecular Sciences. 2020; 21(23):8961. https://doi.org/10.3390/ijms21238961
Chicago/Turabian StyleHeintze, Linda, Dorian Schmidt, Theo Rodat, Lydia Witt, Julia Ewert, Malte Kriegs, Rainer Herges, and Christian Peifer. 2020. "Photoswitchable Azo- and Diazocine-Functionalized Derivatives of the VEGFR-2 Inhibitor Axitinib" International Journal of Molecular Sciences 21, no. 23: 8961. https://doi.org/10.3390/ijms21238961