Discovery of Novel Agents on Spindle Assembly Checkpoint to Sensitize Vinorelbine-Induced Mitotic Cell Death against Human Non-Small Cell Lung Cancers
Abstract
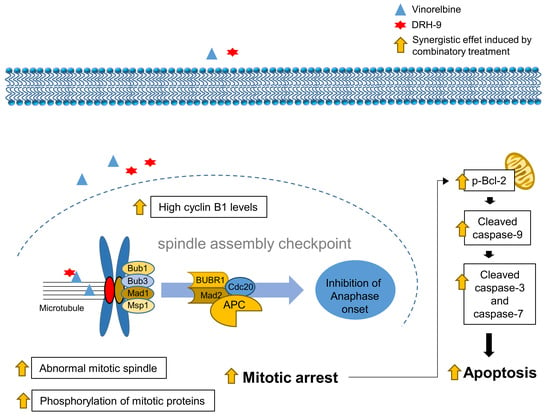
:1. Introduction
2. Results
2.1. YL-9 Shows Less Anti-PDE5 Activity But Reserves Sensitizing Capability to Vinorelbine-Induced Anti-NSCLC Effect
2.2. YL-9 Sensitizes Vinorelbine on Inducing Caspase Activation and Apoptosis in NCI-H460 Cells
2.3. YL-9 Synergistically Potentiates Vinorelbine-Induced Mitotic Spindle Defects and Mitotic Arrest of the Cell Cycle
2.4. YL-9 Potentiates Vinorelbine-Induced Phosphorylation and Cleavage of BUBR1
2.5. YL-9 Sensitizes Vinorelbine-Induced Increase of Mitotic Protein Expression and Phosphorylation
2.6. YL-9 Potentiates Vinorelbine-Induced Tubulin Acetylation on Lys40
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. PDE5A1 Activity Assay
4.4. SRB and Clonogenic Assays
4.5. Cell Proliferation Assay with CFSE Staining
4.6. Cell Cycle Progression Analysis with PI Staining
4.7. Nucleosomal DNA Fragmentation Assay
4.8. Western Bolting
4.9. Immunofluorescence Confocal Microscopy Analysis
4.10. In Vitro Tubulin Polymerization Assay
4.11. Microtubule Assembly Assay
4.12. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CFSE | Carboxyfluorescein succinimidyl ester |
| CI | Combination index |
| FBS | Fetal bovine serum |
| NSCLC | Non-small cell lung cancer |
| PDE5 | Phosphodiesterase type 5 |
| PI | Propidium iodide |
| PMSF | Phenylmethylsulfonyl fluorid |
| ROS | Reactive oxygen species |
| SAC | Spindle assembly checkpoint |
| SRB | Sulforhodamine B |
References
- Piccirillo, M.C.; Daniele, G.; Di Maio, M.; Bryce, J.; De Feo, G.; Del Giudice, A.; Perrone, F.; Morabito, A. Vinorelbine for non-small cell lung cancer. Expert Opin. Drug Saf. 2010, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, J.O.; Jung, S.S.; Park, H.S.; Chung, C.; Park, D.; Lee, J.E. Efficacy of Vinorelbine Monotherapy as Third-or Further-Line Therapy in Patients with Advanced Non-Small-Cell Lung Cancer. Oncology 2019, 97, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, G.; Boland, A.; Brown, T.; Oyee, J.; Bagust, A.; Dickson, R. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015, 70, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffo, O.; Dipasquale, M.; Murgia, V.; Veccia, A.; Galligioni, E. An evaluation of the pharmacokinetics and clinical use of vinorelbine for NSCLC treatment. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1037–1051. [Google Scholar] [CrossRef]
- Domvri, K.; Zarogoulidis, K.; Zogas, N.; Zarogoulidis, P.; Petanidis, S.; Porpodis, K.; Kioseoglou, E.; Hohenforst-Schmidt, W. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J. Cancer 2017, 8, 3648–3656. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Son, J.Y.; Lee, B.M.; Kim, H.S.; Yoon, S. Aging-Related Repositioned Drugs, Donepezil and Sildenafil Citrate, Increase Apoptosis of Anti-mitotic Drug-resistant KBV20C Cells Through Different Molecular Mechanisms. Anticancer Res. 2018, 38, 5149–5157. [Google Scholar] [CrossRef]
- Chang, J.F.; Hsu, J.L.; Sheng, Y.H.; Leu, W.J.; Yu, C.C.; Chan, S.H.; Chan, M.L.; Hsu, L.C.; Liu, S.P.; Guh, J.H. Phosphodiesterase Type 5 (PDE5) Inhibitors Sensitize Topoisomerase II Inhibitors in Killing Prostate Cancer Through PDE5-Independent Impairment of HR and NHEJ DNA Repair Systems. Front. Oncol. 2019, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, Y.; Becher, A.; Diepold, K.; Schmid, E.; Fehn, A.; Brunner, C.; Rouhi, A.; Chiosis, G.; Cronauer, M.; et al. Sildenafil triggers tumor lethality through altered expression of HSP90 and degradation of PKD2. Carcinogenesis 2020. [Google Scholar] [CrossRef]
- Dar, M.I.; Jan, S.; Reddy, G.L.; Wani, R.; Syed, M.; Dar, M.J.; Sawant, S.D.; Vishwakarma, R.A.; Syed, S.H. Differentiation of human neuroblastoma cell line IMR-32 by sildenafil and its newly discovered analogue IS00384. Cell Signal. 2020, 65. [Google Scholar] [CrossRef]
- Li, Q.; Shu, Y. Pharmacological modulation of cytotoxicity and cellular uptake of anti-cancer drugs by PDE5 inhibitors in lung cancer cells. Pharm. Res. 2014, 31, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Catalano, S.; Panza, S.; Augimeri, G.; Giordano, C.; Malivindi, R.; Gelsomino, L.; Marsico, S.; Giordano, F.; Győrffy, B.; Bonofiglio, D.; et al. Phosphodiesterase 5 (PDE5) Is Highly Expressed in Cancer-Associated Fibroblasts and Enhances Breast Tumor Progression. Cancers 2019, 11, 1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.Q.; Qureshi, A.A.; Robinson, K.C.; Han, J. Sildenafil use and increased risk of incident melanoma in US men: A prospective cohort study. JAMA Intern. Med. 2014, 174, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Duan, X.; Liu, B.; Lan, Y.; Cai, C.; Zhang, T.; Zhu, W.; Mai, Z.; Wu, W.; Zeng, G. Association between phosphodiesterase type 5 inhibitors use and risk of melanoma: A meta-analysis. Neoplasma 2018, 65, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhou, L.; Liu, Q.; He, Q.; Liao, B.; Wei, X.; Li, H.; Wang, K.; Zhu, Y. Are phosphodiesterase type 5 inhibitors associated with increased risk of melanoma? A systematic review and meta-analysis. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Lian, Y.; Yin, H.; Pollak, M.N.; Carrier, S.; Platt, R.W.; Suissa, S.; Azoulay, L. Phosphodiesterase Type 5 Inhibitors and the Risk of Melanoma Skin Cancer. Eur. Urol. 2016, 70, 808–815. [Google Scholar] [CrossRef]
- Pottegård, A.; Schmidt, S.A.; Olesen, A.B.; Achacoso, N.; Van Den Eeden, S.K.; Hallas, J.; Sørensen, H.T.; Friis, S.; Habel, L.A. Use of sildenafil or other phosphodiesterase inhibitors and risk of melanoma. Br. J. Cancer 2016, 115, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Dhayade, S.; Kaesler, S.; Sinnberg, T.; Dobrowinski, H.; Peters, S.; Naumann, U.; Liu, H.; Hunger, R.E.; Thunemann, M.; Biedermann, T.; et al. Sildenafil Potentiates a cGMP-Dependent Pathway to Promote Melanoma Growth. Cell Rep. 2016, 14, 2599–2610. [Google Scholar] [CrossRef] [Green Version]
- Arozarena, I.; Sanchez-Laorden, B.; Packer, L.; Hidalgo-Carcedo, C.; Hayward, R.; Viros, A.; Sahai, E.; Marais, R. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell 2011, 19, 45–57. [Google Scholar] [CrossRef]
- Kloner, R.A. Pharmacology and drug interaction effects of the phosphodiesterase 5 inhibitors: Focus on alpha-blocker interactions. Am. J. Cardiol. 2005, 96, 42M–46M. [Google Scholar] [CrossRef]
- Bolanos-Garcia, V.M.; Blundell, T.L. BUB1 and BUBR1: Multifaceted kinases of the cell cycle. Trends Biochem. Sci. 2011, 36, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Fu, X.; Hua, Y.; Han, Y.; Lu, Y.; Wang, J. The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, B.; Lindsey, A.; Li, N.; Lee, K.; Ramirez-Alcantara, V.; Canzoneri, J.C.; Fajardo, A.; Madeira da Silva, L.; Thomas, M.; Piazza, J.T. Phosphodiesterase 10A is overexpressed in lung tumor cells and inhibitors selectively suppress growth by blocking β-catenin and MAPK signaling. Oncotarget 2017, 8, 69264–69280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Toso, R.J.; Jordan, M.A.; Farrell, K.W.; Matsumoto, B.; Wilson, L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry 1993, 32, 1285–1293. [Google Scholar] [PubMed]
- Guo, Y.; Kim, C.; Ahmad, S.; Zhang, J.; Mao, Y. CENP-E—Dependent BubR1 autophosphorylation enhances chromosome alignment and the mitotic checkpoint. J. Cell Biol. 2012, 198, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Liang, X.W.; Zhang, Q.H.; Li, M.; Yuan, J.; Li, S.; Sun, S.C.; Ouyang, Y.C.; Schatten, H.; Sun, Q.Y. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle 2010, 9, 1112–1121. [Google Scholar] [CrossRef]
- Eichhorn, J.M.; Sakurikarm, N.; Alford, S.E.; Chu, R.; Chambers, T.C. Critical role of anti-apoptotic Bcl-2 protein phosphorylation in mitotic death. Cell Death Dis. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, G.J.; Corfe, B.M.; Savory, P.; Leech, S.; Esposti, M.D.; Hickman, J.A.; Dive, C. Cellular damage signals promote sequential changes at the N-terminus and BH-1 domain of the pro-apoptotic protein Bak. Oncogene 2001, 20, 7668–7676. [Google Scholar] [CrossRef] [Green Version]
- Gadde, S.; Heald, R. Mechanisms and molecules of the mitotic spindle. Curr. Biol. 2004, 14, R797–R805. [Google Scholar] [CrossRef] [Green Version]
- Westermann, S.; Weber, K. Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell Biol. 2003, 4, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Mohan, R.; Panda, D. Kinetic stabilization of microtubule dynamics by estramustine is associated with tubulin acetylation, spindle abnormalities, and mitotic arrest. Cancer Res. 2008, 68, 6181–6189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skibbens, R.V.; Skeen, V.P.; Salmon, E.D. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: A push-pull mechanism. J. Cell Biol. 1993, 122, 859–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngan, V.K.; Bellman, K.; Panda, D.; Hill, B.T.; Jordan, M.A.; Wilson, L. Novel actions of the antitumor drugs vinflunine and vinorelbine on microtubules. Cancer Res. 2000, 60, 5045–5051. [Google Scholar] [PubMed]
- Eshun-Wilson, L.; Zhang, R.; Portran, D.; Nachury, M.V.; Toso, D.B.; Löhr, T.; Vendruscolo, M.; Bonomi, M.; Fraser, J.S.; Nogales, E. Effects of α-tubulin acetylation on microtubule structure and stability. Proc. Natl. Acad. Sci. USA 2019, 116, 10366–10371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinchcliffe, E.H.; Sluder, G. “It takes two to tango”: Understanding how centrosome duplication is regulated throughout the cell cycle. Genes Dev. 2001, 15, 1167–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, W.S.; Shuster, M.; Huang, X.; Gharaibeh, B.; Enyenihi, A.H.; Petersen, I.; Gollin, S.M. Chromosomal instability and cytoskeletal defects in oral cancer cells. Proc. Natl. Acad. Sci. USA 2000, 97, 303–308. [Google Scholar] [CrossRef] [Green Version]
- Quintyne, N.J.; Reing, J.E.; Hoffelder, D.R.; Gollin, S.M.; Saunders, W.S. Spindle multipolarity is prevented by centrosomal clustering. Science 2005, 307, 127–129. [Google Scholar] [CrossRef]
- Saunders, W. Centrosomal amplification and spindle multipolarity in cancer cells. Semin. Cancer Biol. 2005, 15, 25–32. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, W.K.; Lok, T.M.; Ma, H.T.; Poon, R.Y.C. Imbalance of the spindle-assembly checkpoint promotes spindle poison-mediated cytotoxicity with distinct kinetics. Cell Death Dis. 2019, 10, 314. [Google Scholar] [CrossRef] [Green Version]
- Brito, D.A.; Yang, Z.; Rieder, C.L. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J. Cell Biol. 2008, 182, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.Y.; Ahn, J.H.; Cho, M.G.; Lee, J.H. ATP depletion during mitotic arrest induces mitotic slippage and APC/CCdh1-dependent cyclin B1 degradation. Exp. Mol. Med. 2018, 50, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balachandran, R.S.; Kipreos, E.T. Addressing a weakness of anticancer therapy with mitosis inhibitors: Mitotic slippage. Mol. Cell Oncol. 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonski, S.A.; Chan, G.K.; Cooke, C.A.; Earnshaw, W.C.; Yen, T.J. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 1998, 107, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, Z.; Wu, H.; Wu, S.; Meadows, J.; Chen, J.; Zhu, V.; Dai, W. BUBR1 phosphorylation is regulated during mitotic checkpoint activation. Cell Growth Differ. 1999, 10, 769–775. [Google Scholar]
- Ditchfield, C.; Johnson, V.L.; Tighe, A.; Ellston, R.; Haworth, C.; Johnson, T.; Mortlock, A.; Keen, N.; Taylor, S.S. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 2003, 161, 267–280. [Google Scholar] [CrossRef]
- Matsumura, S.; Toyoshima, F.; Nishida, E. Polo-like kinase 1 facilitates chromosome alignment during prometaphase through BubR1. J. Biol. Chem. 2007, 282, 15217–15227. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Hittle, J.; Zappacosta, F.; Annan, R.S.; Hershko, A.; Yen, T.J. Phosphorylation sites in BubR1 that regulate kinetochore attachment, tension, and mitotic exit. J. Cell Biol. 2008, 183, 667–680. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Murphy, K.; Liu, F.; Parker, S.E.; Dowling, M.L.; Baff, W.; Kao, G.D. Caspase-Mediated specific cleavage of BubR1 is a determinant of mitotic progression. Mol. Cell Biol. 2005, 25, 9232–9248. [Google Scholar] [CrossRef] [Green Version]







| YL-9 (mM) | Vinorelbine (nM) | F | CI |
|---|---|---|---|
| 10 | 1.3 | 0.08 | 0.66 |
| 10 | 2.5 | 0.34 | 0.46 |
| 10 | 5 | 0.69 | 0.38 |
| 10 | 10 | 0.86 | 0.43 |
| 10 | 20 | 0.92 | 0.55 |
| 30 | 1.3 | 0.06 | 0.79 |
| 30 | 2.5 | 0.5 | 0.31 |
| 30 | 5 | 0.78 | 0.29 |
| 30 | 10 | 0.92 | 0.29 |
| 30 | 20 | 0.96 | 0.39 |
| 50 | 1.3 | 0.12 | 0.49 |
| 50 | 2.5 | 0.54 | 0.28 |
| 50 | 5 | 0.8 | 0.27 |
| 50 | 10 | 0.94 | 0.25 |
| 50 | 20 | 0.94 | 0.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Tseng, Y.-L.; Leu, W.-J.; Du, C.-M.; Jiang, Y.-H.; Hsu, L.-C.; Hsu, J.-L.; Hou, D.-R.; Guh, J.-H. Discovery of Novel Agents on Spindle Assembly Checkpoint to Sensitize Vinorelbine-Induced Mitotic Cell Death against Human Non-Small Cell Lung Cancers. Int. J. Mol. Sci. 2020, 21, 5608. https://doi.org/10.3390/ijms21165608
Chang Y-C, Tseng Y-L, Leu W-J, Du C-M, Jiang Y-H, Hsu L-C, Hsu J-L, Hou D-R, Guh J-H. Discovery of Novel Agents on Spindle Assembly Checkpoint to Sensitize Vinorelbine-Induced Mitotic Cell Death against Human Non-Small Cell Lung Cancers. International Journal of Molecular Sciences. 2020; 21(16):5608. https://doi.org/10.3390/ijms21165608
Chicago/Turabian StyleChang, Ya-Ching, Yu-Ling Tseng, Wohn-Jenn Leu, Chi-Min Du, Yi-Huei Jiang, Lih-Ching Hsu, Jui-Ling Hsu, Duen-Ren Hou, and Jih-Hwa Guh. 2020. "Discovery of Novel Agents on Spindle Assembly Checkpoint to Sensitize Vinorelbine-Induced Mitotic Cell Death against Human Non-Small Cell Lung Cancers" International Journal of Molecular Sciences 21, no. 16: 5608. https://doi.org/10.3390/ijms21165608