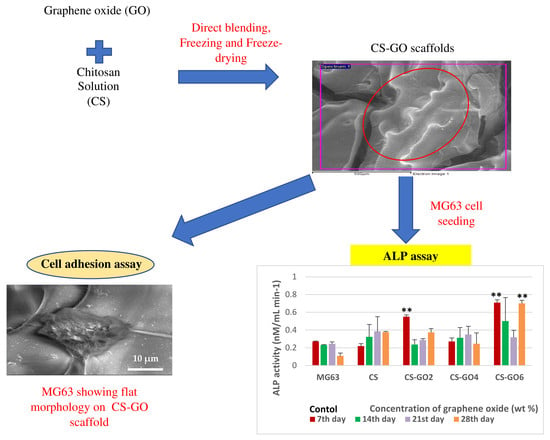
Preliminary In Vitro Evaluation of Chitosan–Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation
Abstract
:1. Introduction
2. Results
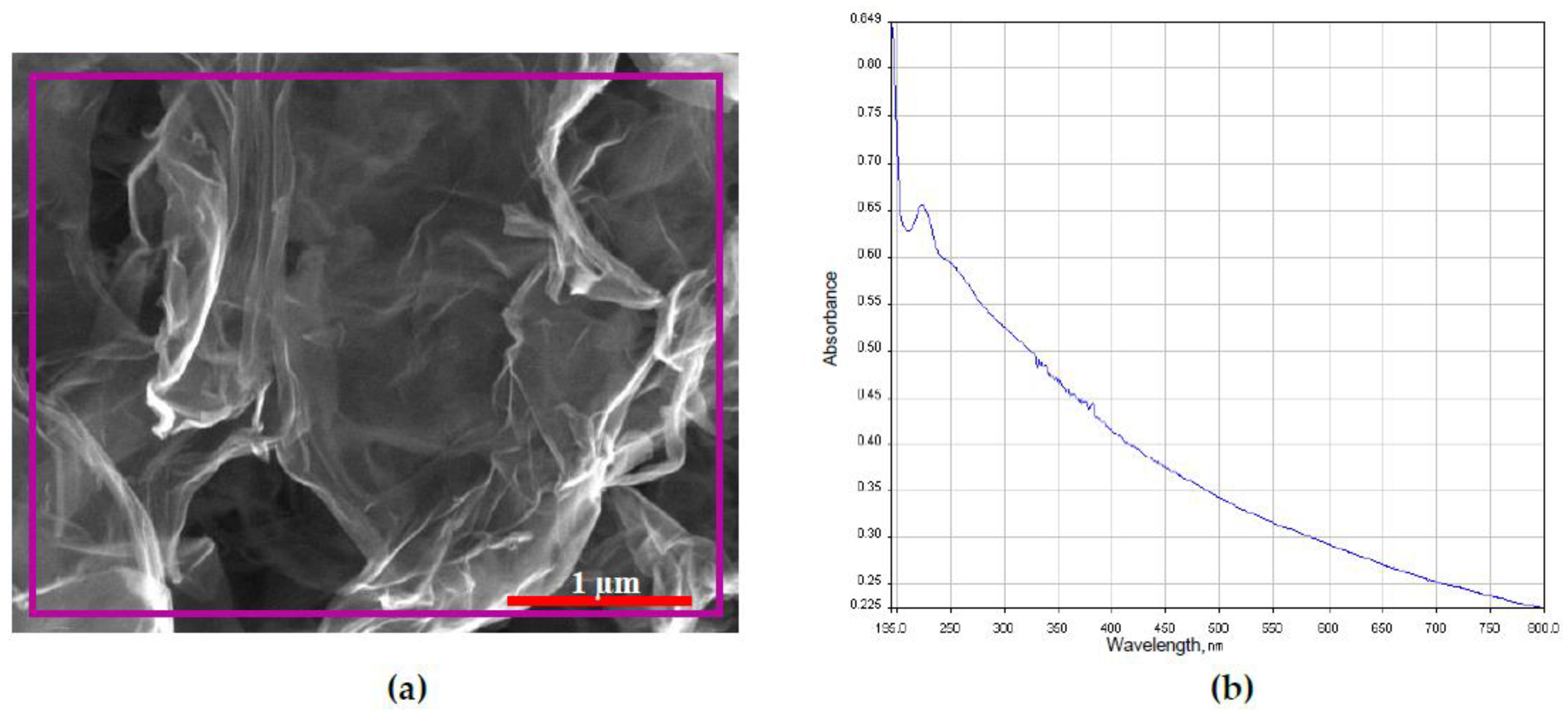
2.1. Characterization of GO
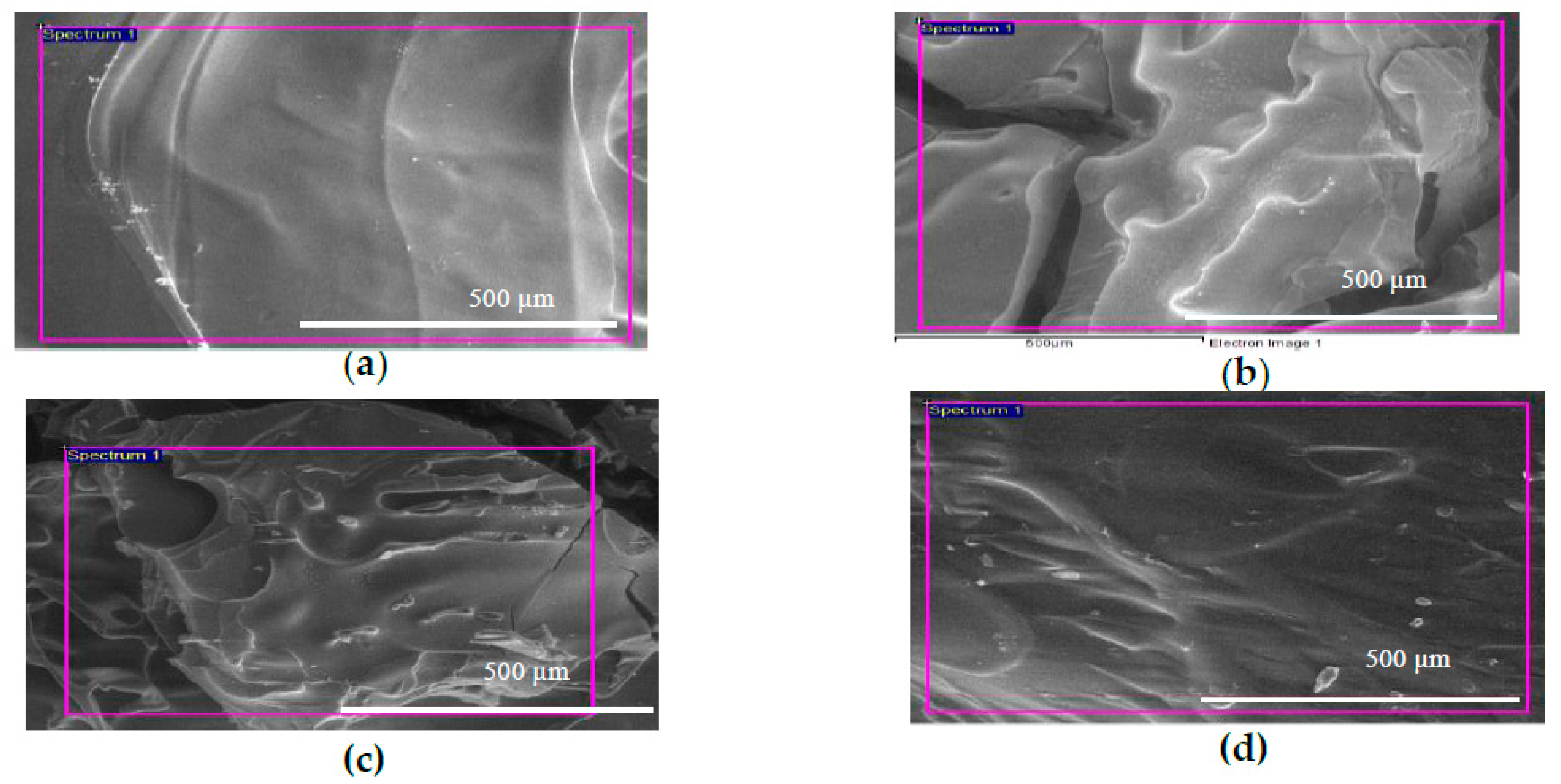
2.2. Morphology Study of GTA Crosslinked CS-GO Scaffolds
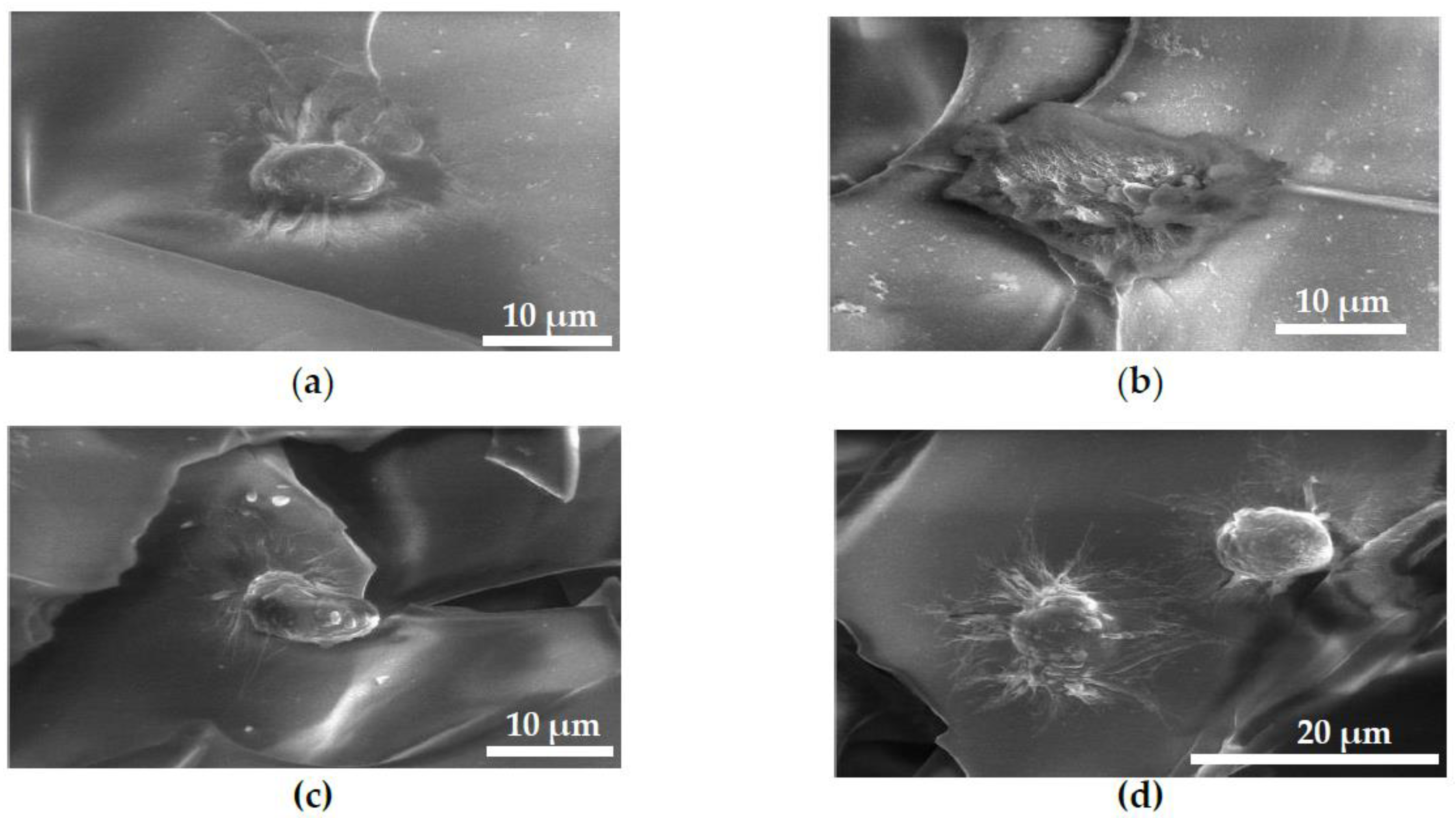
2.3. FESEM Images of MG63 Cells Fixed on Scaffolds for Cell Adhesion Study
2.4. MTT Assay
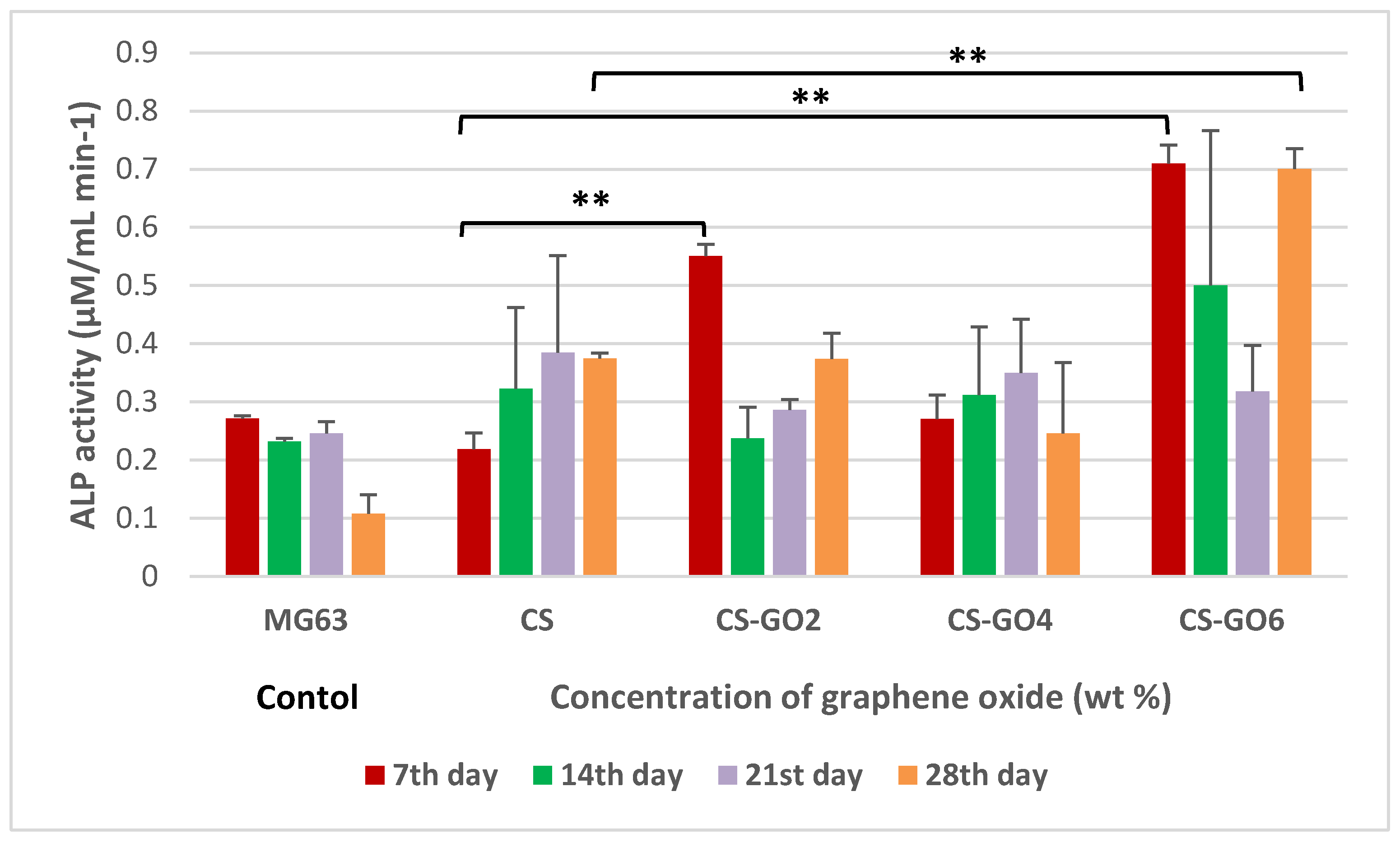
2.5. ALP Assay
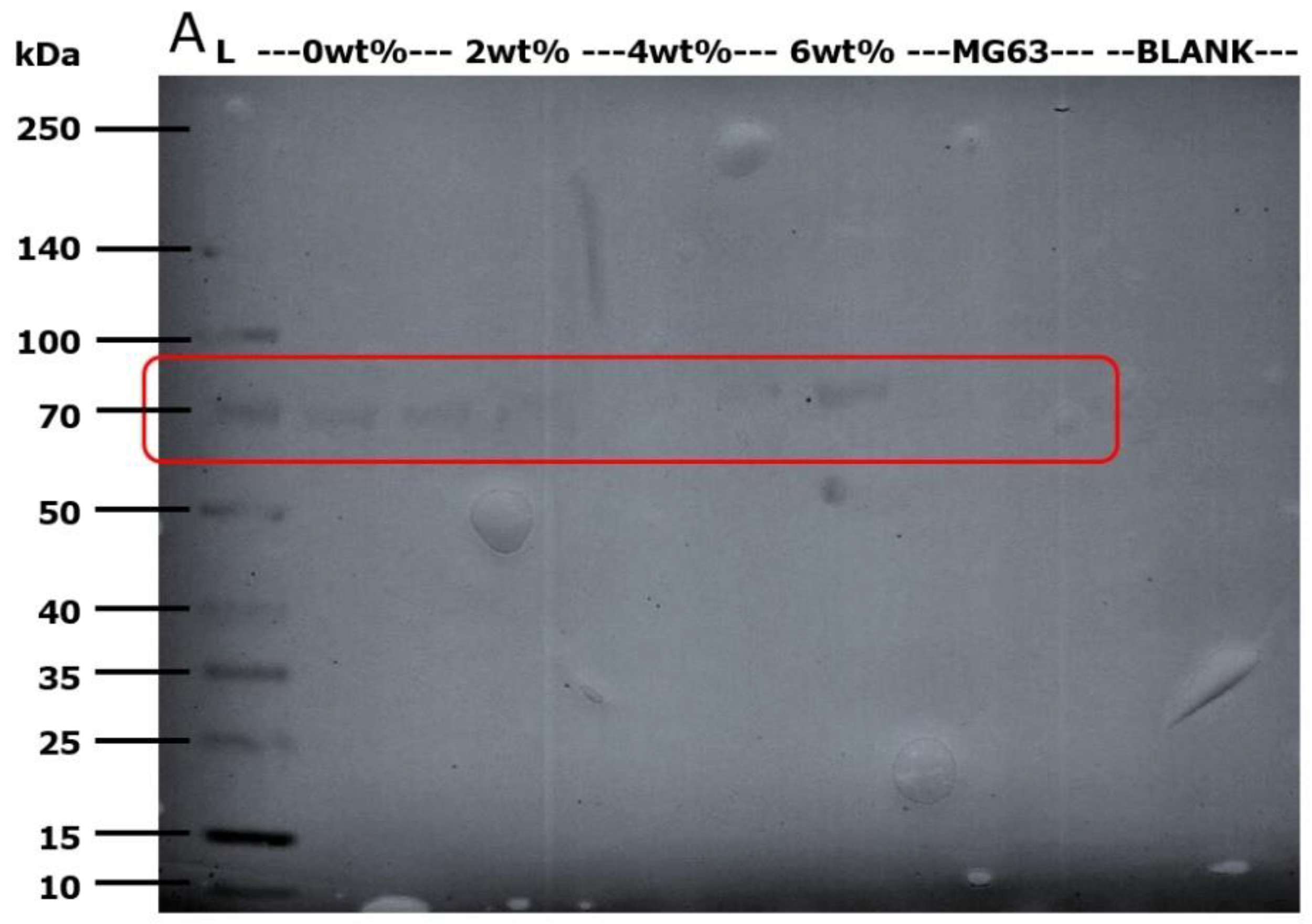
2.6. Western Blot Detection of Alkaline Phosphatase (ALP)
3. Discussion
4. Materials and Methods
4.1. Characterization of GO
4.2. Fabrication of GTA Crosslinked CS-GO Scaffolds
4.3. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-Ray Spectrometry (EDX) Examination on CS and CS-GO Scaffolds
4.4. Cell Seeding on GTA Crosslinked CS and CS-GO Scaffolds
4.5. Cell Adhesion Assay
4.6. MTT Assay
4.7. ALP Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| BTE | bone tissue engineering |
| CS | chitosan |
| Da | Dalton |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrices |
| EDX | Energy dispersive X-ray |
| FBS | fetal bovine serum |
| FESEM | field emission scanning electron microscope |
| GAGs | glycosaminoglycans |
| GO | graphene oxide |
| GTA | glutaraldehyde |
| hBMSC | human bone marrow mesenchymal stem cells |
| HMDS | hexamethyldisilazane |
| MEM | minimum essential medium |
| MG63 | human osteosarcoma cell line |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PBS | phosphate buffered saline |
| PBS-T | phosphate buffered saline-0.05% Tween-20 |
| pNp | p-nitrophenol |
| pNpp | p-nitrophenyl phosphate |
| SDS PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| TNSALP | tissue non-specific type |
| UV–VIS | ultraviolet-visible |
| wt % | weight percent |
References
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue Engineering of Bone: Material and Matrix Considerations. J. Bone Jt. Surgery 2008, 90, 36–42. [Google Scholar] [CrossRef]
- Folkman, J.; Haudenschild, C. Angiogenesis in vitro. Nature 1980, 288, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestwich, G.D.; E Healy, K. Why regenerative medicine needs an extracellular matrix. Expert Opin. Boil. Ther. 2014, 15, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-S.; Mooney, D. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Chapekar, M.S. Tissue Engineering: Challenges and Opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Yang, J.; Long, T.; He, N.-F.; Guo, Y.-P.; Zhu, Z.-A.; Ke, Q.-F. Fabrication of a chitosan/bioglass three-dimensional porous scaffold for bone tissue engineering applications. J. Mater. Chem. B 2014, 2, 6611–6618. [Google Scholar] [CrossRef]
- Dutta, P.K.; Rinki, K.; Dutta, J. Chitosan: A Promising Biomaterial for Tissue Engineering Scaffolds. Fortschr. Hochpolym.-Forsch. 2011, 244, 45–79. [Google Scholar]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Lee, D.W.; Lim, H.; Na Chong, H.; Shim, W.S. Advances in Chitosan Material and its Hybrid Derivatives: A Review. Open Biomater. J. 2009, 1, 10–20. [Google Scholar] [CrossRef]
- Kumar, A.S.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, L.J.; Ni, J.; Weng, J.; Yue, C.Y. Manufacture and evaluation of bioactive and biodegradable materials and scaffolds for tissue engineering. J. Mater. Sci. Mater. Electron. 2004, 12, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Shin, S.H.; Kim, Y.D.; Lee, J.Y.; Baek, Y.J.; Yoon, S.Y.; Kim, H.S. Comparative study of chitosan/fibroin-hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: Micro-computed tomography analysis. Int. J. Oral Sci. 2014, 6, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Stegemann, J.P. Thermogelling Chitosan and Collagen Composite Hydrogels Initiated with β-glycerophosphate for Bone Tissue Engineeirng. Biomaterials 2010, 31, 3976–3985. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2012, 10, S96–S101. [Google Scholar]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Shi, D.; Li, Y.; Dong, H.; Li, Y. Graphene-based nanovehicles for photodynamic medical therapy. Int. J. Nanomed. 2015, 10, 2451–2459. [Google Scholar] [CrossRef] [Green Version]
- Bitounis, D.; Hong, B.H.; Min, D.-H.; Kostarelos, K.; Ali-Boucetta, H. Prospects and Challenges of Graphene in Biomedical Applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Aidun, A.; Firoozabady, A.S.; Moharrami, M.; Ahmadi, A.; Haghighipour, N.; Bonakdar, S.; Faghihi, S. Graphene oxide incorporated polycaprolactone/chitosan/collagen electrospun scaffold: Enhanced osteogenic properties for bone tissue engineering. Artif. Organs 2019, 43, E264–E281. [Google Scholar] [CrossRef] [PubMed]
- Grausova, L.; Vacik, J.; Bilkova, P.; Vorlicek, V.; Svorcik, V.; Soukup, D.; Bacakova, M.; Lisa, V.; Bacakova, L. Regionally-selective Adhesion and Growth of Human Osteoblast-like MG63 Cells on Micropatterned Fullerence C60 Layers. Optoelectron. Adv. Mat. 2008, 10, 2071–2076. [Google Scholar]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin-alginate-graphene oxide for bone tissue engineering. Int. J. Boil. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Makita, S.; Al-Shawafi, H.A.; Sultana, S.; Sohda, M.; Nomura, S.; Oda, K. A dimerization defect caused by a glycine substitution at position 420 by serine in tissue-nonspecific alkaline phosphatase associated with perinatal hypophosphatasia. FEBS J. 2012, 279, 4327–4337. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Sultana, N.; Wang, M. Fabrication of Tissue Engineering Scaffolds Using the Emulsion Freezing/Freeze-drying Technique and Characteristics of the Scaffolds. In Integrated Biomaterials in Tissue Engineering; Scrivener Publishing LLC: Beverly, MA, USA, 2012; pp. 63–90. [Google Scholar]
- Lim, S.S.; Chai, C.Y.; Loh, H.-S. In vitro evaluation of osteoblast adhesion, proliferation and differentiation on chitosan-TiO2 nanotubes scaffolds with Ca2+ ions. Mater. Sci. Eng. C 2017, 76, 144–152. [Google Scholar] [CrossRef]
- Bačáková, L.; Grausova, L.; Vacik, J.; Kromka, A.; Biederman, H.; Choukourov, A.; Starý, V. Nanocomposite and Nanostructured Carbon-based Films as Growth Substrates for Bone Cells. In Advances in Diverse Industrial Applications of Nanocomposites; InTech: London, UK, 2011; pp. 371–408. [Google Scholar]
- Depan, D.; Girase, B.; Shah, J.; Misra, R. Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.; Qu, S.; Zhang, X. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Jirka, I.; Vandrovcová, M.; Frank, A.O.; Tolde, Z.; Plšek, J.; Luxbacher, T.; Bačáková, L.; Starý, V. On the role of Nb-related sites of an oxidized β-TiNb alloy surface in its interaction with osteoblast-like MG-63 cells. Mater. Sci. Eng. C 2013, 33, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Farani, M.R.; Dehghani, M.; Tamjid, E.; Simchi, A. Physicochemical and biological properties of electrodeposited graphene oxide/chitosan films with drug-eluting capacity. Carbon 2015, 84, 91–102. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Wang, Y.B.; Cheng, Y.; Zheng, Y.; Xi, T.F.; Wei, S. Cell response of nanographene platelets to human osteoblast-like MG63 cells. J. Biomed. Mater. Res. Part A 2013, 102, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Elkhenany‡, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D.; et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2014, 35, 367–374. [Google Scholar] [CrossRef]
- Nair, M.; Nancy, D.; Krishnan, A.G.; Anjusree, G.S.; Vadukumpully, S.; Nair, S.V. Graphene oxide nanoflakes incorporated gelatin–hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology 2015, 26, 161001. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Shi, X.; Chang, H.; Chen, S.; Lai, C.; Khademhosseini, A.; Wu, H. Regulating Cellular Behavior on Few-Layer Reduced Graphene Oxide Films with Well-Controlled Reduction States. Adv. Funct. Mater. 2011, 22, 751–759. [Google Scholar] [CrossRef]
- Miyaji, H.; Kanayama, I.; Takita, H.; Nishida, E.; Tsuji, M.; Fugetsu, B.; Sun, L.; Inoue, K.; Ibara, A.; Akasaka, T.; et al. Comparative study of bioactivity of collagen scaffolds coated with graphene oxide and reduced graphene oxide. Int. J. Nanomed. 2014, 9, 3363–3373. [Google Scholar] [CrossRef] [Green Version]
- Madihally, S.V.; Matthew, H. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Beagin, E.E.; Merkel, B.J. Cleavage of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) by HT2 and CTLL-2 Cells. Bios 2002, 73, 80–85. [Google Scholar]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]

| Element | Atomic % |
|---|---|
| Carbon | 72.80 |
| Oxygen | 27.09 |
| Sulfur | 0.11 |
| Scaffold | Wt % of GO |
|---|---|
| CS | 0 |
| CS-GO2 | 2 |
| CS-GO4 | 4 |
| CS-GO6 | 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, S.H.M.; Lim, S.S.; Tiong, T.J.; Show, P.L.; Zaid, H.F.M.; Loh, H.-S. Preliminary In Vitro Evaluation of Chitosan–Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation. Int. J. Mol. Sci. 2020, 21, 5202. https://doi.org/10.3390/ijms21155202
Wong SHM, Lim SS, Tiong TJ, Show PL, Zaid HFM, Loh H-S. Preliminary In Vitro Evaluation of Chitosan–Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation. International Journal of Molecular Sciences. 2020; 21(15):5202. https://doi.org/10.3390/ijms21155202
Chicago/Turabian StyleWong, Sonia How Ming, Siew Shee Lim, Timm Joyce Tiong, Pau Loke Show, Hayyiratul Fatimah Mohd Zaid, and Hwei-San Loh. 2020. "Preliminary In Vitro Evaluation of Chitosan–Graphene Oxide Scaffolds on Osteoblastic Adhesion, Proliferation, and Early Differentiation" International Journal of Molecular Sciences 21, no. 15: 5202. https://doi.org/10.3390/ijms21155202