Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo
Abstract
:1. Recent Breakthroughs in Immunotherapy of Cancer
2. Response Markers in the Tumor Microenvironment (TME)
3. Preclinical Data from Rodent Animal Models of Cancer and Exercise
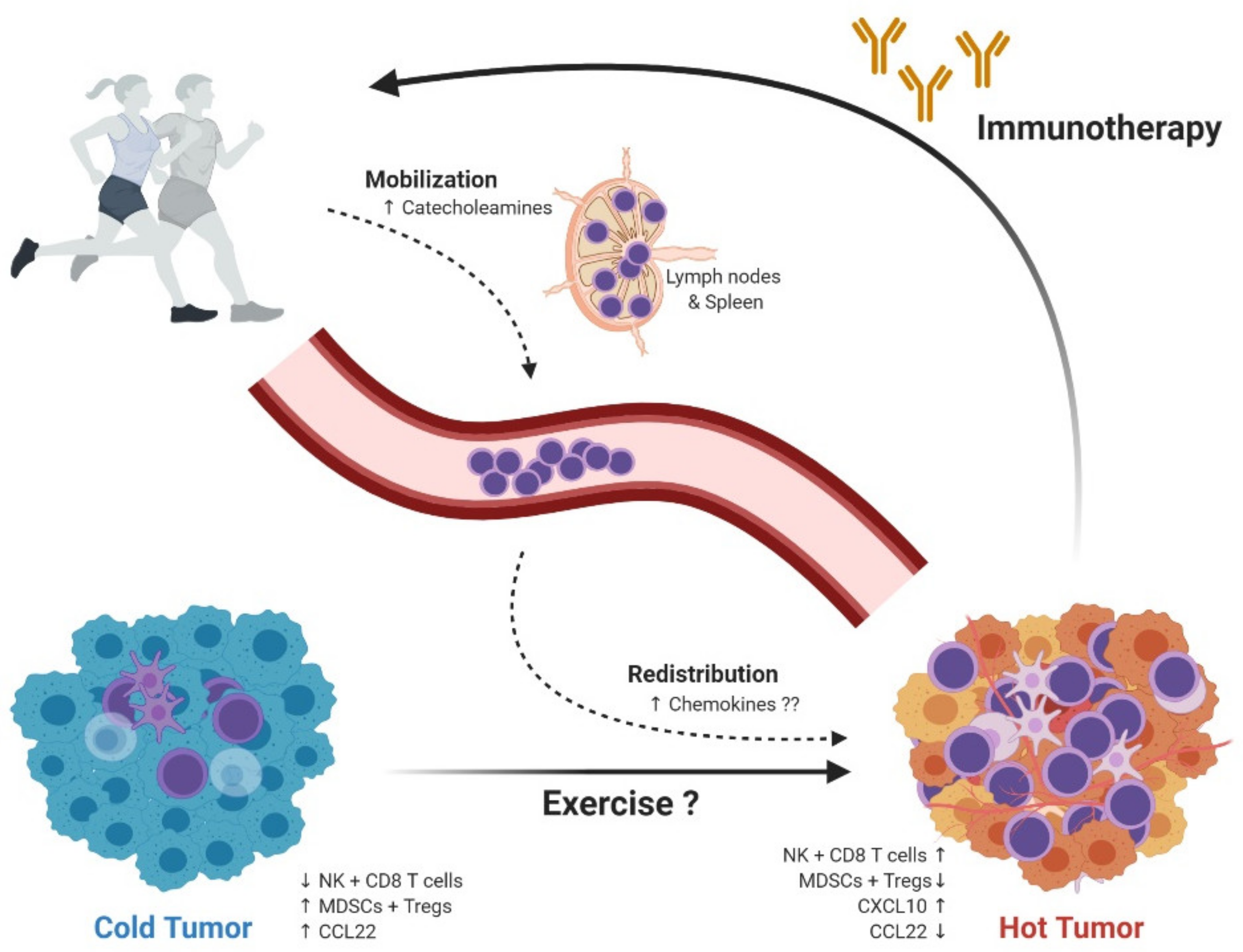
4. Mechanisms Behind Exercise-Induced Tumor Growth Control
5. Exercise Oncology; Focus on the Immune System; From Mouse to Man
6. Exercise Oncology in the Clinic; Current Status and Future Prospects
7. Exercise Oncology in the Clinic; HI AIM
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma-An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reck, M.; Schenker, M.; Lee, K.H.; Provencio, M.; Nishio, M.; Lesniewski-Kmak, K.; Sangha, R.; Ahmed, S.; Raimbourg, J.; Feeney, K.; et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: Patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur. J. Cancer 2019, 116, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.D.L.M.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Tang, J.; Shalabi, A.; Hubbard-Lucey, V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018, 29, 84–91. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Xu, J.; Du, C.; Wu, Y.; Xia, D.; Lv, W.; Hu, J. The Predictive Value of Tumor Mutation Burden on Efficacy of Immune Checkpoint Inhibitors in Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; Elder, D.E.; Guerry, D.; Braitman, L.E.; Trock, B.J.; Schultz, D.; Synnestvedt, M.; Halpern, A.C. Model predicting survival in stage I melanoma based on tumor progression. J. Natl. Cancer Inst. 1989, 81, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galon, J.; Coleno-Costes, A.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Zinzindohoué, F.; Bruneval, P.; Cugnenc, P.-H.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [Green Version]
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Séguier, S.; Tran, T.; Paolini, L.; Lebbe, C.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Perea, F.; Bernal, M.; Carretero, J.; Torres, C.; Bayarri, C.; Gómez-Morales, M.; Garrido, F.; Ruiz-Cabello, F.; Sánchez-Palencia, A. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int. J. Cancer 2017, 140, 888–899. [Google Scholar] [CrossRef]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment—The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef] [Green Version]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Parry, T.L.; Hayward, R. Exercise Protects against Cancer-induced Cardiac Cachexia. Med. Sci. Sports Exerc. 2018, 50, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [Green Version]
- Gershbein, L.L.; Benuck, I.; Shurrager, P.S. Influence of stress of lesion growth and on survival of animals bearing parenteral and intracerebral leukemia L1210 and Walker tumors. Oncology 1974, 30, 429–435. [Google Scholar] [CrossRef]
- De Lima, C.; Alves, L.E.; Iagher, F.; Machado, A.F.; Bonatto, S.J.R.; Kuczera, D.; De Souza, C.F.; Pequito, D.C.; Muritiba, A.L.; Nunes, E.; et al. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur. J. Appl. Physiol. 2008, 104, 957–964. [Google Scholar] [CrossRef]
- De Lima, C.; Alves, L.; Iagher, F.; Machado, A.F.; Kryczyk, M.; Yamazaki, R.K.; Brito, G.A.P.; Nunes, E.; Naliwaiko, K.; Fernandes, L.C. Tumor growth reduction in walker 256 tumorbearing rats performing anaerobic exercise: Participation of Bcl-2, Bax, apoptosis, and peroxidation. Appl. Physiol. Nutr. Metab. 2011, 36, 533–538. [Google Scholar] [CrossRef]
- Moreira, V.M.; Almeida, D.; Franco, C.C.D.S.; Gomes, R.M.; Palma-Rigo, K.; Prates, K.V.; Tófolo, L.P.; Malta, A.; Francisco, F.A.; Pavanello, A.; et al. Moderate exercise training since adolescence reduces Walker 256 tumour growth in adult rats. J. Physiol. 2019, 597, 3905–3925. [Google Scholar] [CrossRef]
- Rincón-Castanedo, C.; Morales, J.S.; Martín-Ruiz, A.; Valenzuela, P.L.; Ramírez, M.; Santos-Lozano, A.; Lucia, A.; Fiuza-Luces, C. Physical exercise effects on metastasis: A systematic review and meta-analysis in animal cancer models. Cancer Metastasis Rev. 2020, 39, 91–114. [Google Scholar] [CrossRef]
- DeMarzo, M.M.P.; Garcia, S.B. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004, 216, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, L.; Ji, B.; Li, L.; Qi, Z.; Ding, S. Endurance training but not high-intensity interval training reduces liver carcinogenesis in mice with hepatocellular carcinogen diethylnitrosamine. Exp. Gerontol. 2020, 133, 110853. [Google Scholar] [CrossRef] [PubMed]
- LaVoy, E.C.; Hussain, M.; Reed, J.; Kunz, H.; Pistillo, M.; Bigley, A.B.; Simpson, R.J. T-cell redeployment and intracellular cytokine expression following exercise: Effects of exercise intensity and cytomegalovirus infection. Physiol. Rep. 2017, 5, e13070. [Google Scholar] [CrossRef] [PubMed]
- Hagar, A.; Wang, Z.; Koyama, S.; Aponte-Serrano, J.O.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Ryen, S.V.D.D.T.; Deldicque, L. The regulation of the metastatic cascade by physical activity: A narrative review. Cancers 2020, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Hoffman-Goetz, L.; MacNeil, B.; Arumugam, Y.; Simpson, J. Differential effects of exercise and housing condition on murine natural killer cell activity and tumor growth. Int. J. Sports Med. 1992, 13, 167–171. [Google Scholar] [CrossRef]
- Buss, L.A.; Ang, A.D.; Hock, B.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effect of post-implant exercise on tumour growth rate, perfusion and hypoxia in mice. PLoS ONE 2020, 15, e0229290. [Google Scholar] [CrossRef]
- Tank, A.W.; Wong, D.L. Peripheral and central effects of circulating catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, F.; Yang, R.; Zheng, X.; Gao, H.; Zhang, P. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS ONE 2013, 8, e61435. [Google Scholar] [CrossRef] [Green Version]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissen, M.D.; Sloan, E.K.; Mattarollo, S.R. β-Adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol. Res. 2018, 6, 98–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhi, X.; Li, B.; Li, Z.; Zhang, J.; Yu, J.; Zhang, L.; Xu, Z. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int. J. Oncol. 2019, 54, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Park, D.; Lee, G.Y.; Curran, W.J.; Deng, X. Exercise-induced lung cancer regression: Mechanistic findings from a mouse model. Cancer 2014, 120, 3302–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; DeMaria, S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef] [Green Version]
- Bacurau, A.V.N.; Belmonte, M.A.; Navarro, F.; Moraes, M.R.; Pontes, F.L.; Pesquero, J.; Araujo, R.; Bacurau, R.F.P. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor bearing rats. Exp. Boil. Med. 2007, 232, 1289–1299. [Google Scholar] [CrossRef]
- Bacuau, R.F.P.; Belmonte, M.A.; Seelaender, M.C.L.; Bacurau, R.F.P.; Coast Rosa, L.F.B.P. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem. Funct. 2000, 18, 249–258. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef]
- Almeida, P.W.M.; Gomes-Filho, A.; Ferreira, A.J.; Rodrigues, C.E.M.; Dias-Peixoto, M.F.; Russo, R.C.; Teixeira, M.M.; Cassali, G.D.; Ferreira, E.; Santos, I.C.; et al. Swim training suppresses tumor growth in mice. J. Appl. Physiol. 2009, 107, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, J.L.; Steiner, J.L.; Day, S.D.; Enos, R.; Davis, M.J.; Singh, U.P.; Murphy, E.A. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int. J. Oncol. 2014, 45, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, R.J.; Bigley, A.B.; Agha, N.; Hanley, P.J.; Bollard, C.M. Mobilizing Immune Cells with Exercise for Cancer Immunotherapy. Exerc. Sport Sci. Rev. 2017, 45, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocr. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Graff, R.M.; Kunz, H.E.; Agha, N.H.; Baker, F.L.; Laughlin, M.; Bigley, A.B.; Markofski, M.M.; LaVoy, E.C.; Katsanis, E.; Bond, R.A.; et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain, Behav. Immun. 2018, 74, 143–153. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef] [Green Version]
- Ngo, S.T.; Steyn, F.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocr. 2014, 35, 347–369. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Timmons, B.W.; Hamadeh, M.; Devries, M.C.; Tarnopolsky, M.A. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J. Appl. Physiol. 2005, 99, 979–985. [Google Scholar] [CrossRef] [Green Version]
- Aiello, A.; Farzaneh, F.; Candore, G.; Malavolta, M.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [Green Version]
- Malavolta, M.; Accardi, G.; Virruso, C.; Candore, G. Sex, gender and immunosenescence: A key to understand the different lifespan between men and women? Immun. Ageing 2013, 10, 20. [Google Scholar] [CrossRef] [Green Version]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fülöp, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2018, 41, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gounant, V.; Lavolé, A.; Quoix, E. Ongoing challenges of using immunotherapy in special populations: Poor performance status patients, elderly patients, and people living with HIV. Lung Cancer 2020, 145, 71–75. [Google Scholar] [CrossRef]
- Rooney, B.V.; Bigley, A.B.; LaVoy, E.C.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018, 194, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.F.; DeLisa, J.A.; Warren, C.G.; DeLateur, B.J.; Bryant, P.L.; Nicholson, C.G. Cancer rehabilitation: Assessment of need, development, and evaluation of a model of care. Arch. Phys. Med. Rehabilitation 1978, 59, 410–419. [Google Scholar]
- Winningham, M.L.; MacVicar, M.G.; Bondoc, M.; Anderson, J.I.; Minton, J.P. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol. Nurs. Forum 1989, 16, 683–689. [Google Scholar]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef] [PubMed]
- DiMeo, F.; Fetscher, S.; Lange, W.; Mertelsmann, R.; Keul, J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood 1997, 90, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A systematic review. Curr. Oncol. 2017, 24, e290–e315. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 40. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.; Arem, H.; De Gonzalez, A.B.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef] [Green Version]
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Paciorek, A.; Carroll, P.R.; Chan, J.M. Physical activity after diagnosis and risk of prostate cancer progression: Data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011, 71, 3889–3895. [Google Scholar] [CrossRef] [Green Version]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Hvid, T.; Lindegaard, B.; Winding, K.; Iversen, P.; Brasso, K.; Solomon, T.P.J.; Pedersen, B.K.; Hojman, P. Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control. 2016, 27, 165–174. [Google Scholar] [CrossRef]
- De Rezende, L.F.M.; De Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.-M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770,000 cancer cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.U.; Kenfield, S.A.; Hart, N.H.; Chan, J.M.; Courneya, K.S.; Catto, J.W.; Finn, S.; Greenwood, R.; Hughes, D.C.; Mucci, L.; et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): A multicentre, randomised, controlled phase III study protocol. BMJ Open 2018, 8, e022899. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Friedenreich, C.M.; Campbell, K.L.; Prapavessis, H.; Crawford, J.J.; O’Brien, P.; Dhillon, H.M.; Jonker, D.J.; et al. Effects of a Structured Exercise Program on Physical Activity and Fitness in Colon Cancer Survivors: One Year Feasibility Results from the CHALLENGE Trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courneya, K.S.; Booth, C.; Gill, S.; O’Brien, P.; Vardy, J.; Friedenreich, C.; Au, H.; Brundage, M.; Tu, D.; Dhillon, H.; et al. The Colon Health and Life-Long Exercise Change trial: A randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr. Oncol. 2008, 15, 271–278. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.P.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Govindan, R.; Anders, R.A.; Antonia, S.J.; Bonerigo, S.; Davies, M.J.; Dubinett, S.; Ferris, A.; Gandhi, L.; Garon, E.B.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Chiang, A.C.; Herbst, R.S. Frontline immunotherapy for NSCLC—The tale of the tail. Nat. Rev. Clin. Oncol. 2020, 17, 73–74. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Plesca, I.; Tunger, A.; Müller, L.; Wehner, R.; Lai, X.; Grimm, M.-O.; Rutella, S.; Bachmann, M.; Schmitz, M. Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2020, 11, 364. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Ferrone, S. HLA Class I Antigen Processing Machinery Defects in Cancer Cells-Frequency, Functional Significance, and Clinical Relevance with Special Emphasis on Their Role in T Cell-Based Immunotherapy of Malignant Disease. Methods Mol. Biol. 2020, 2055, 325–350. [Google Scholar] [CrossRef]
- Rosenthal, R.; The TRACERx Consortium; Cadieux, E.L.; Salgado, R.; Al Bakir, M.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanic, M.; Reading, J.L.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Perea, F.; Sánchez-Palencia, A.; Gómez-Morales, M.; Bernal, M.; Concha, Á.; García, M.M.; Gonzalez-Ramirez, A.R.; Kerick, M.; Martin, J.; Garrido, F.; et al. HLA class I loss and PD-L1 expression in lung cancer: Impact on T-cell infiltration and immune escape. Oncotarget 2018, 9, 4120–4133. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmen Olofsson, G.; Jensen, A.W.P.; Idorn, M.; thor Straten, P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. Int. J. Mol. Sci. 2020, 21, 3816. https://doi.org/10.3390/ijms21113816
Holmen Olofsson G, Jensen AWP, Idorn M, thor Straten P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. International Journal of Molecular Sciences. 2020; 21(11):3816. https://doi.org/10.3390/ijms21113816
Chicago/Turabian StyleHolmen Olofsson, Gitte, Agnete Witness Praest Jensen, Manja Idorn, and Per thor Straten. 2020. "Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo" International Journal of Molecular Sciences 21, no. 11: 3816. https://doi.org/10.3390/ijms21113816




