Hypercholesterolemia Interferes with Induction of miR-125b-1-3p in Preconditioned Hearts
Abstract
:1. Introduction
2. Results
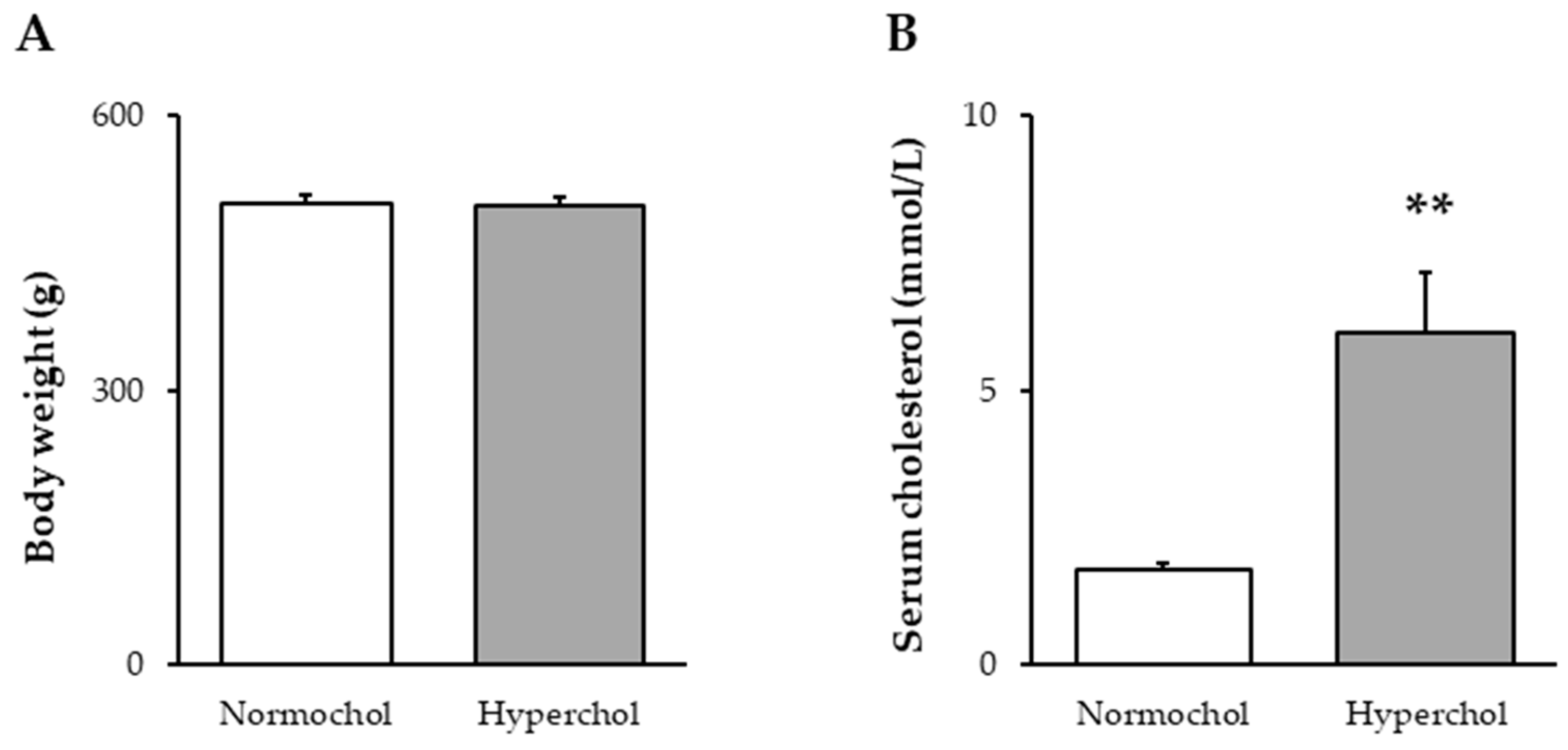
2.1. Verification of Hypercholesterolemia
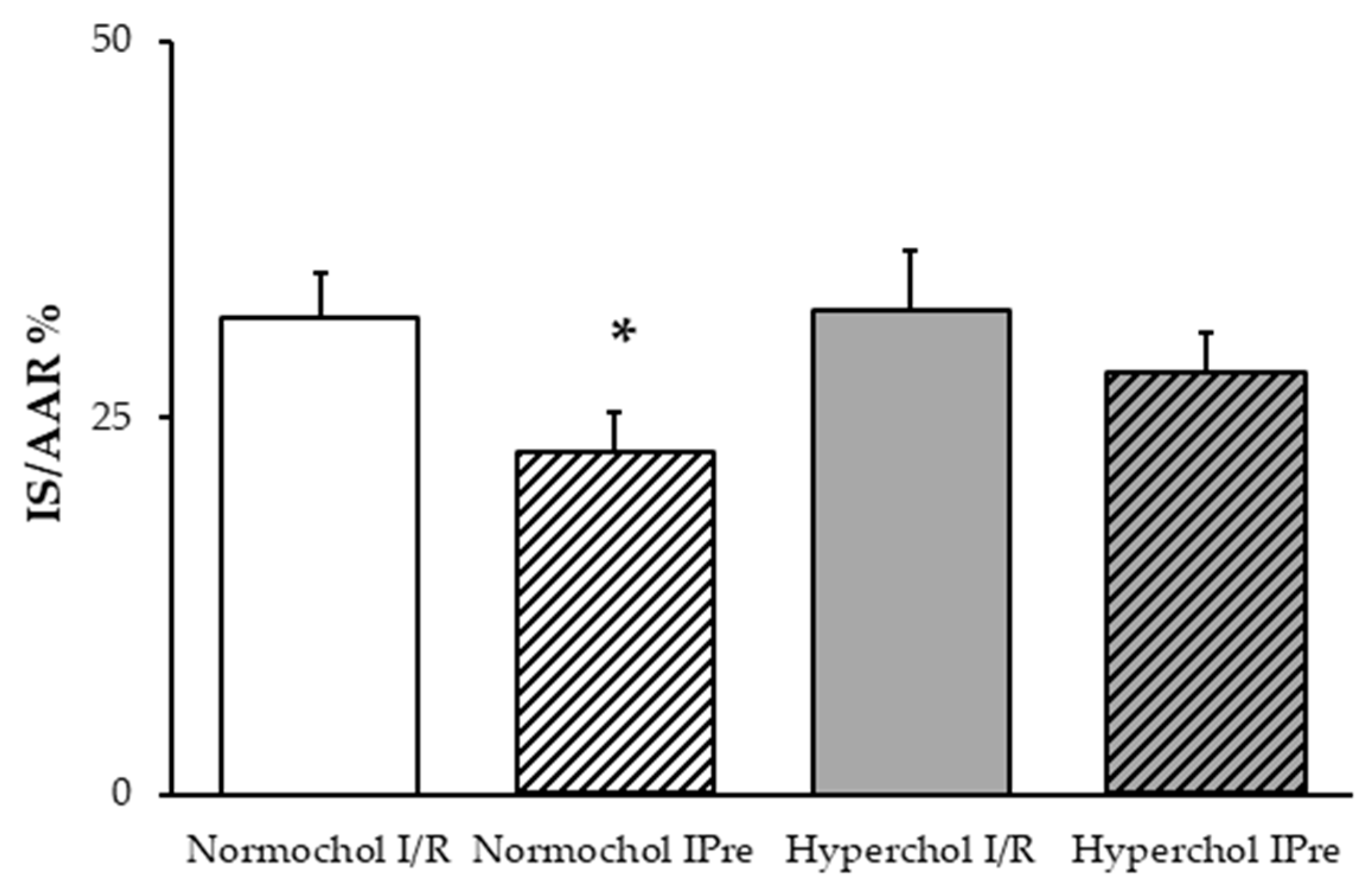
2.2. Hypercholesterolemia Attenuates the Infarct Size-Limiting Effect of Ischemic Preconditioning
2.3. Upregulation of miR-125b-1-3p Induced by Preconditioning is Lost in Settings of Hypercholesterolemia
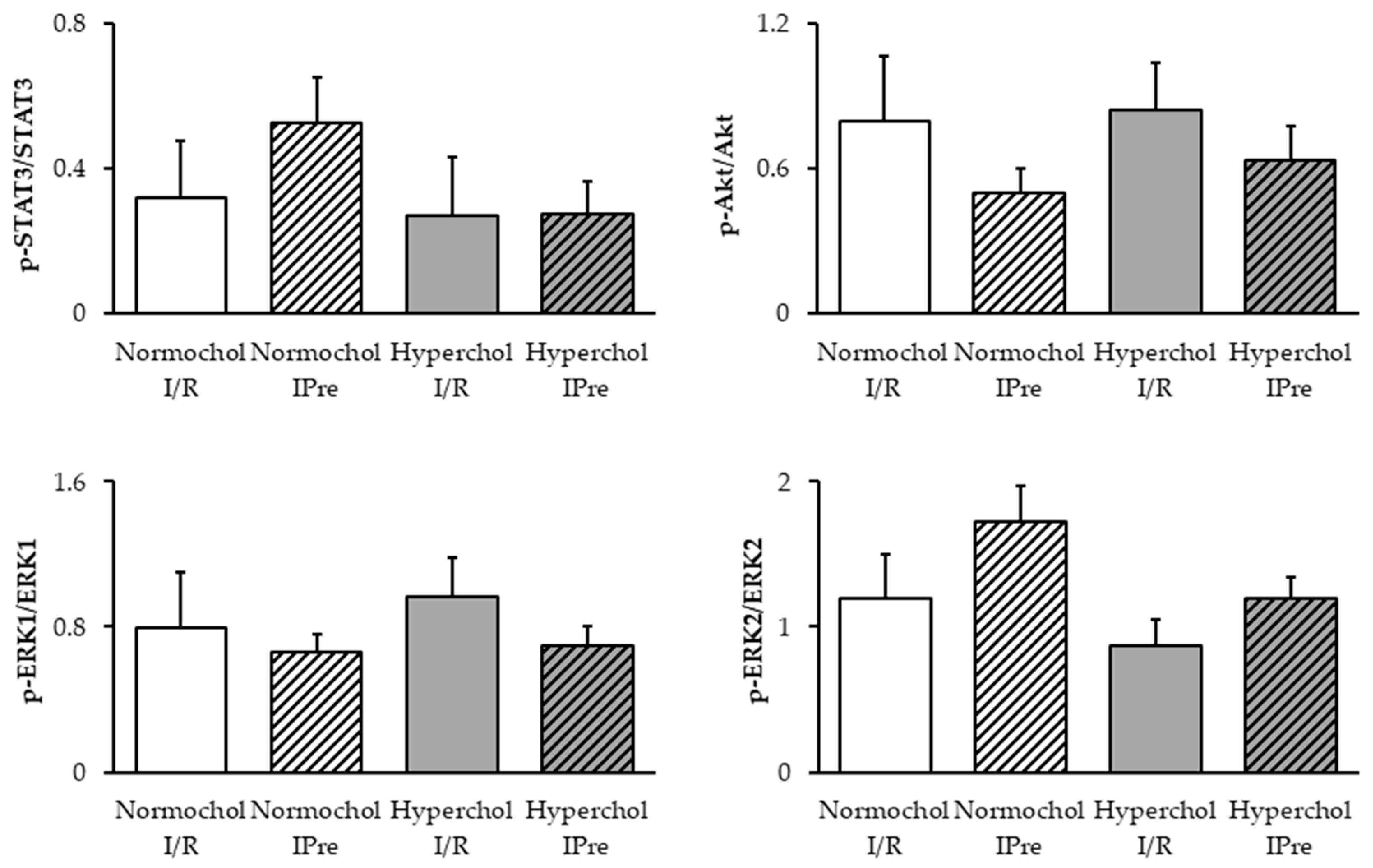
2.4. Ischemic Preconditioning Failed to Affect the RISK and SAFE Pathways at the End of Reperfusion
3. Discussion
4. Materials and Methods
4.1. Animals
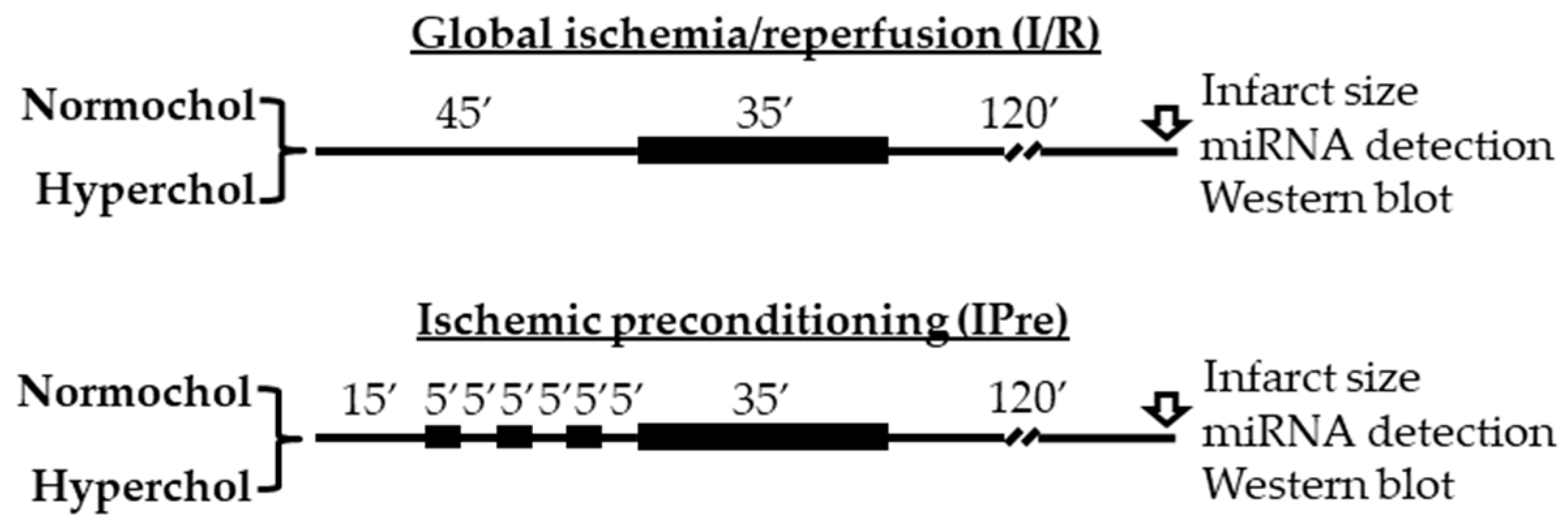
4.2. Experimental Setup
4.3. Serum Total Cholesterol Measurement
4.4. Infarct Size Determination
4.5. Measurement of Cardiac miR-125b-1-3p Level
4.6. Bioinformatic Analysis
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| I/R | Ischemia/Reperfusion |
| IPre | Ischemic Preconditioning |
| miRNA PKG RISK | microRNA Protein kinase G Reperfusion Injury Salvage Kinases |
| SAFE | Survivor Activating Factor Enhancement pathway |
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality From Ischemic Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellon, D.M.; Baxter, G.F.; Garcia-Dorado, D.; Heusch, G.; Sumeray, M.S. Ischaemic preconditioning: Present position and future directions. Cardiovasc. Res. 1998, 37, 21–33. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Salloum, F.N.; Kukreja, R.C. A novel role of microRNA in late preconditioning: Upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ. Res. 2009, 104, 572–575. [Google Scholar] [CrossRef]
- Varga, Z.V.; Zvara, A.; Faragó, N.; Kocsis, G.F.; Pipicz, M.; Gáspár, R.; Bencsik, P.; Görbe, A.; Csonka, C.; Puskás, L.G.; et al. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: ProtectomiRs. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H216–H227. [Google Scholar] [CrossRef] [Green Version]
- Csonka, C.; Murlasits, Z.; Ferdinandy, P.; Csont, T. Ischemic stress adaptation of the myocardium in the disease states: Role of hyperlipidemia. In Advances in Cardiomyocyte Research; Nánási, P., Ed.; Transworld Research Network: Kerala, India, 2009; pp. 245–265. [Google Scholar]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxid. Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef]
- Csont, T.; Bereczki, E.; Bencsik, P.; Fodor, G.; Görbe, A.; Zvara, A.; Csonka, C.; Puskás, L.G.; Sántha, M.; Ferdinandy, P. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc. Res. 2007, 76, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Varga, Z.V.; Kupai, K.; Szűcs, G.; Gáspár, R.; Pálóczi, J.; Faragó, N.; Zvara, A.; Puskás, L.G.; Rázga, Z.; Tiszlavicz, L.; et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J. Mol. Cell. Cardiol. 2013, 62, 111–121. [Google Scholar] [CrossRef]
- Yang, J.-S.; Phillips, M.D.; Betel, D.; Mu, P.; Ventura, A.; Siepel, A.C.; Chen, K.C.; Lai, E.C. Widespread regulatory activity of vertebrate microRNA* species. RNA N. Y. N 2011, 17, 312–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohanian, M.; Humphreys, D.T.; Anderson, E.; Preiss, T.; Fatkin, D. A heterozygous variant in the human cardiac miR-133 gene, MIR133A2, alters miRNA duplex processing and strand abundance. BMC Genet. 2013, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Qin, M.; Li, T.; Gu, Z.; Tan, Q.; Huang, P.; Ren, L. Rutin protects against pirarubicin-induced cardiotoxicity by adjusting microRNA-125b-1-3p-mediated JunD signaling pathway. Mol. Cell. Biochem. 2020, 466, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, T.; Zou, J.; Ren, D.; Liu, L.; Zhang, X.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc. Res. 2014, 102, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Bayoumi, A.S.; Park, K.-M.; Wang, Y.; Teoh, J.-P.; Aonuma, T.; Tang, Y.; Su, H.; Weintraub, N.L.; Kim, I.-M. A carvedilol-responsive microRNA, miR-125b-5p protects the heart from acute myocardial infarction by repressing pro-apoptotic bak1 and klf13 in cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 114, 72–82. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, K.; Xu, Y.; Hu, H.; Zhang, N.; Wang, Y.; Zhong, Z.; Zhao, J.; Li, Q.; Zhu, D.; et al. Transplanted Mesenchymal Stem Cells Reduce Autophagic Flux in Infarcted Hearts via the Exosomal Transfer of miR-125b. Circ. Res. 2018, 123, 564–578. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Tian, T.; Wang, J.-Y.; He, J.-N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.-X.; Qiu, X.-T.; Li, C.-C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Bie, Z.-D.; Sun, L.-Y.; Geng, C.-L.; Meng, Q.-G.; Lin, X.-J.; Wang, Y.-F.; Wang, X.-B.; Yang, J. MiR-125b regulates SFRP5 expression to promote growth and activation of cardiac fibroblasts. Cell Biol. Int. 2016, 40, 1224–1234. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Lu, S.; Yan, L.; Hu, F.; Wang, Z.; Cheng, B. Androgen receptor regulates cardiac fibrosis in mice with experimental autoimmune myocarditis by increasing microRNA-125b expression. Biochem. Biophys. Res. Commun. 2018, 506, 130–136. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Szilvássy, Z.; Horváth, L.I.; Csont, T.; Csonka, C.; Nagy, E.; Szentgyörgyi, R.; Nagy, I.; Koltai, M.; Dux, L. Loss of pacing-induced preconditioning in rat hearts: Role of nitric oxide and cholesterol-enriched diet. J. Mol. Cell. Cardiol. 1997, 29, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Ungi, I.; Ungi, T.; Ruzsa, Z.; Nagy, E.; Zimmermann, Z.; Csont, T.; Ferdinandy, P. Hypercholesterolemia attenuates the anti-ischemic effect of preconditioning during coronary angioplasty. Chest 2005, 128, 1623–1628. [Google Scholar] [CrossRef] [PubMed]
- Giricz, Z.; Lalu, M.M.; Csonka, C.; Bencsik, P.; Schulz, R.; Ferdinandy, P. Hyperlipidemia attenuates the infarct size-limiting effect of ischemic preconditioning: Role of matrix metalloproteinase-2 inhibition. J. Pharmacol. Exp. Ther. 2006, 316, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, Y.; Kitakaze, M.; Komamura, K.; Minamino, T.; Asanuma, H.; Sato, H.; Kuzuya, T.; Takeda, H.; Hori, M. Pravastatin restored the infarct size-limiting effect of ischemic preconditioning blunted by hypercholesterolemia in the rabbit model of myocardial infarction. J. Am. Coll. Cardiol. 1999, 34, 2120–2125. [Google Scholar] [CrossRef] [Green Version]
- Iliodromitis, E.K.; Zoga, A.; Vrettou, A.; Andreadou, I.; Paraskevaidis, I.A.; Kaklamanis, L.; Kremastinos, D.T. The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis 2006, 188, 356–362. [Google Scholar] [CrossRef]
- Heusch, G.; Musiolik, J.; Gedik, N.; Skyschally, A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ. Res. 2011, 109, 1302–1308. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, H.; Wang, S.; Mao, X.; Yan, D.; Wong, S.S.; Xia, Z.; Irwin, M.G. Repeated Non-Invasive Limb Ischemic Preconditioning Confers Cardioprotection Through PKC-Ԑ/STAT3 Signaling in Diabetic Rats. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 45, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bian, H.-J.; Li, X.-X.; Liu, X.-B.; Sun, J.-P.; Li, N.; Zhang, Y.; Ji, X.-P. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol. Med. Camb. Mass 2010, 16, 307–315. [Google Scholar] [CrossRef]
- Chen, P.-J.; Shang, A.-Q.; Yang, J.-P.; Wang, W.-W. microRNA-874 inhibition targeting STAT3 protects the heart from ischemia-reperfusion injury by attenuating cardiomyocyte apoptosis in a mouse model. J. Cell. Physiol. 2019, 234, 6182–6193. [Google Scholar] [CrossRef]
- Bellis, A.; Castaldo, D.; Trimarco, V.; Monti, M.G.; Chivasso, P.; Sadoshima, J.; Trimarco, B.; Morisco, C. Cross-talk between PKA and Akt protects endothelial cells from apoptosis in the late ischemic preconditioning. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1207–1212. [Google Scholar] [CrossRef]
- Yang, C.; Talukder, M.A.H.; Varadharaj, S.; Velayutham, M.; Zweier, J.L. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine1176 phosphorylation. Cardiovasc. Res. 2013, 97, 33–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdinandy, P.; Csonka, C.; Csont, T.; Szilvássy, Z.; Dux, L. Rapid pacing-induced preconditioning is recaptured by farnesol treatment in hearts of cholesterol-fed rats: Role of polyprenyl derivatives and nitric oxide. Mol. Cell. Biochem. 1998, 186, 27–34. [Google Scholar] [CrossRef]
- Pipicz, M.; Demján, V.; Sárközy, M.; Csont, T. Effects of Cardiovascular Risk Factors on Cardiac STAT3. Int. J. Mol. Sci. 2018, 19, 3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csonka, C.; Kupai, K.; Bencsik, P.; Görbe, A.; Pálóczi, J.; Zvara, A.; Puskás, L.G.; Csont, T.; Ferdinandy, P. Cholesterol-enriched diet inhibits cardioprotection by ATP-sensitive K+ channel activators cromakalim and diazoxide. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H405–H413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, G.F.; Sárközy, M.; Bencsik, P.; Pipicz, M.; Varga, Z.V.; Pálóczi, J.; Csonka, C.; Ferdinandy, P.; Csont, T. Preconditioning protects the heart in a prolonged uremic condition. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1229–H1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipicz, M.; Varga, Z.V.; Kupai, K.; Gáspár, R.; Kocsis, G.F.; Csonka, C.; Csont, T. Rapid ventricular pacing-induced postconditioning attenuates reperfusion injury: Effects on peroxynitrite, RISK and SAFE pathways. Br. J. Pharmacol. 2015, 172, 3472–3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csonka, C.; Kupai, K.; Kocsis, G.F.; Novák, G.; Fekete, V.; Bencsik, P.; Csont, T.; Ferdinandy, P. Measurement of myocardial infarct size in preclinical studies. J. Pharmacol. Toxicol. Methods 2010, 61, 163–170. [Google Scholar] [CrossRef]
- Csont, T.; Sárközy, M.; Szűcs, G.; Szűcs, C.; Bárkányi, J.; Bencsik, P.; Gáspár, R.; Földesi, I.; Csonka, C.; Kónya, C.; et al. Effect of a multivitamin preparation supplemented with phytosterol on serum lipids and infarct size in rats fed with normal and high cholesterol diet. Lipids Health Dis. 2013, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gáspár, R.; Pipicz, M.; Hawchar, F.; Kovács, D.; Djirackor, L.; Görbe, A.; Varga, Z.V.; Kiricsi, M.; Petrovski, G.; Gácser, A.; et al. The cytoprotective effect of biglycan core protein involves Toll-like receptor 4 signaling in cardiomyocytes. J. Mol. Cell. Cardiol. 2016, 99, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Siska, A.; Kővári, B.; Szűcs, G.; Márványkövi, F.; Kovács, M.G.; Diószegi, P.; Bodai, L.; et al. Chronic kidney disease induces left ventricular overexpression of the pro-hypertrophic microRNA-212. Sci. Rep. 2019, 9, 1302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sárközy, M.; Gáspár, R.; Zvara, Á.; Kiscsatári, L.; Varga, Z.; Kővári, B.; Kovács, M.G.; Szűcs, G.; Fábián, G.; Diószegi, P.; et al. Selective Heart Irradiation Induces Cardiac Overexpression of the Pro-hypertrophic miR-212. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, M.R.; Gáspár, R.; Pipicz, M.; Zsindely, N.; Diószegi, P.; Sárközy, M.; Bodai, L.; Csont, T. Hypercholesterolemia Interferes with Induction of miR-125b-1-3p in Preconditioned Hearts. Int. J. Mol. Sci. 2020, 21, 3744. https://doi.org/10.3390/ijms21113744
Szabó MR, Gáspár R, Pipicz M, Zsindely N, Diószegi P, Sárközy M, Bodai L, Csont T. Hypercholesterolemia Interferes with Induction of miR-125b-1-3p in Preconditioned Hearts. International Journal of Molecular Sciences. 2020; 21(11):3744. https://doi.org/10.3390/ijms21113744
Chicago/Turabian StyleSzabó, Márton R., Renáta Gáspár, Márton Pipicz, Nóra Zsindely, Petra Diószegi, Márta Sárközy, László Bodai, and Tamás Csont. 2020. "Hypercholesterolemia Interferes with Induction of miR-125b-1-3p in Preconditioned Hearts" International Journal of Molecular Sciences 21, no. 11: 3744. https://doi.org/10.3390/ijms21113744





