Evolutionary Plasticity in Detoxification Gene Modules: The Preservation and Loss of the Pregnane X Receptor in Chondrichthyes Lineages
Abstract
:1. Introduction
2. Results
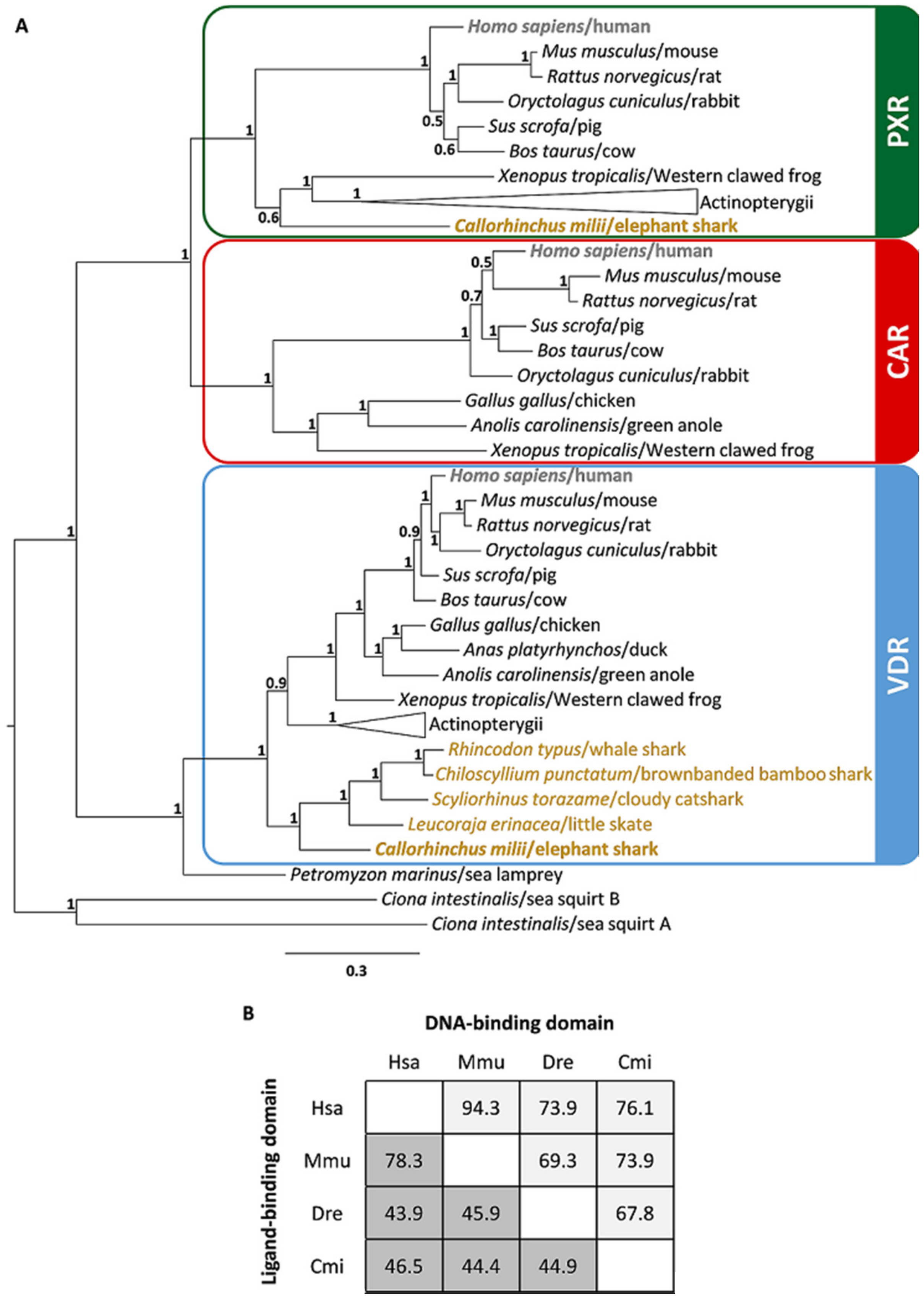
2.1. Identification of nuclear receptor subfamily 1 group I (NR1I) Ortholog Genes in Chondrichthyes
2.2. Synteny Analysis of NR1I Ortholog Genes
2.3. Gene Expression Analysis of the Elephant Shark Pregnane X Receptor (PXR)
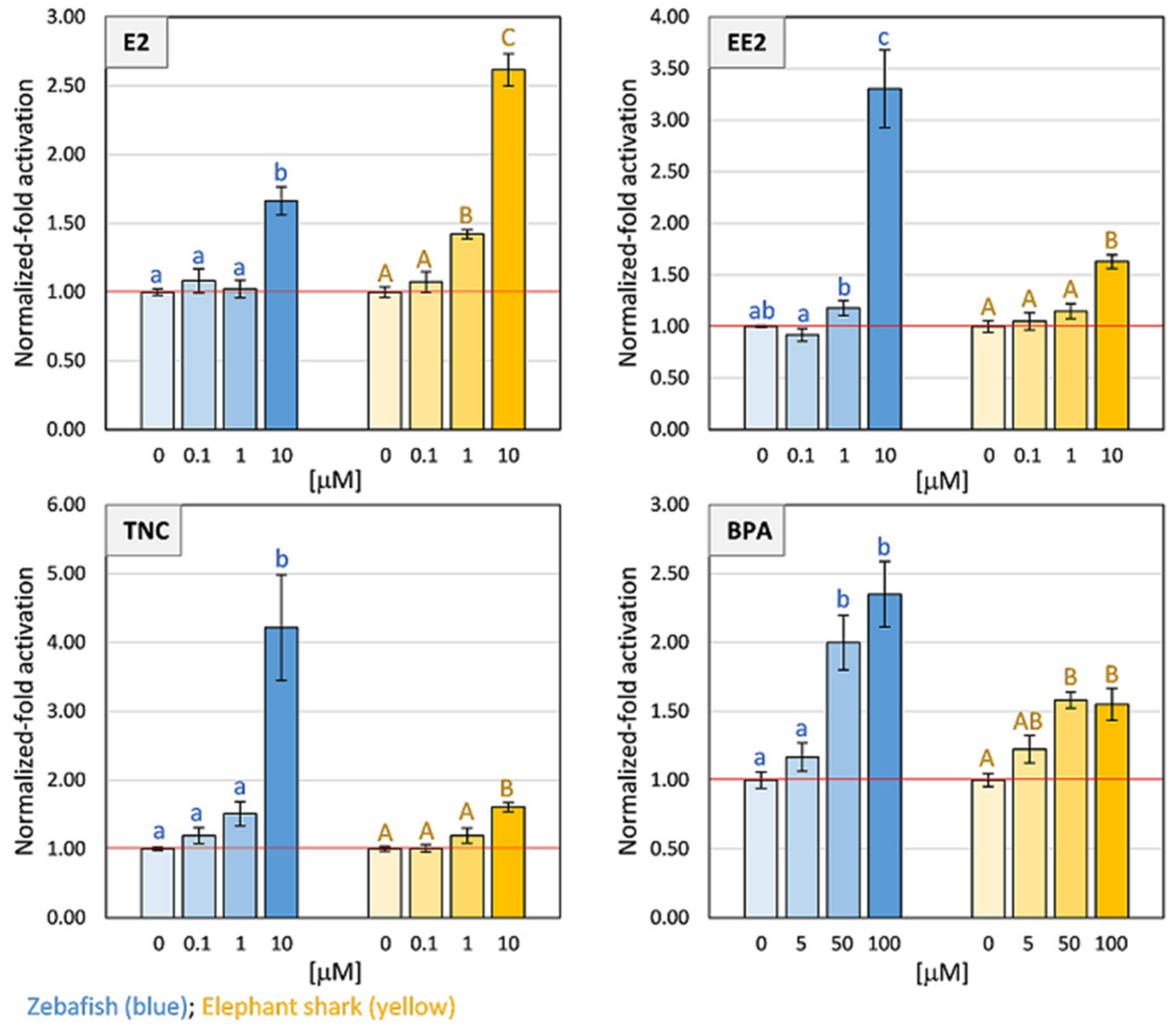
2.4. Transactivation Assays of CmiPXR
3. Discussion
4. Material and Methods
4.1. Sequence Retrieval and Phylogenetic Analysis
4.2. Synteny Analysis
4.3. Construction of Plasmid Vectors
4.4. Gene Expression
4.5. Transfection and Transactivation Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bookout, A.; Jeong, Y.; Downes, M.; Yu, R.; Evans, R.M.; Mangelsdorf, D. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 2006, 126, 789–799. [Google Scholar] [CrossRef]
- Capitão, A.; Lopes-Marques, M.; Ishii, Y.; Ruivo, R.; Fonseca, E.; Páscoa IJorge, R.P.; Barbosa, M.A.G.; Hiromori, Y.; Miyagi, T.; et al. Evolutionary Exploitation of Vertebrate Peroxisome Proliferator-Activated Receptor γ by Organotins. Environ. Sci. Technol. 2018, 52, 13951–13959. [Google Scholar] [CrossRef]
- Santos, M.; Ruivo, R.; Capitão, A.; Fonseca, E.; Castro, L. Identifying the gaps: Resources and perspectives on the use of nuclear receptor based-assays to improve hazard assessment of emerging contaminants. J. Hazard Mater. 2018, 358, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, J.; Hamdoun, A.; Cole, B.; Howard-Ashby, M.; Nebert, D.; Scally, M.; Dean, M.; Epel, D.; Hahn, M.E.; Stegeman, J.J. The chemical defensome: Environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev. Biol. 2006, 300, 366–384. [Google Scholar] [PubMed] [Green Version]
- Di Masi, A.; de Marinis, E.; Ascenzi, P.; Marino, M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol. Asp. Med. 2009, 30, 297–343. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, W.; Hu, L.; Lv, J.; Wang, H.; Zhou, H.; Fan, L. Role of pregnane X receptor in chemotherapeutic treatment. Cancer Chemother. Pharmacol. 2014, 74, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolson, A.; Wang, H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv. Drug Deliv. Rev. 2010, 62, 1238–1249. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Lopes-Marques, M.; Ruivo, R.; Rodrigues-Oliveira, N.; Santos, M.; Rocha, M.; Rocha, E.; Castro, L.F. A mollusk VDR/PXR/CAR-like (NR1J) nuclear receptor provides insight into ancient detoxification mechanisms. Aquat. Toxicol. 2016, 174, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, H.; Hwang, D.; Kim, H.; Hagiwara, A.; Lee, J.; Jeong, C.B. Genome-wide identification of nuclear receptor (NR) genes and the evolutionary significance of the NR1O subfamily in the monogonont rotifer Brachionus spp. Gen. Comp. Endocrinol. 2017, 252, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Mathäs, M.; Burk, O.; Qiu, H.; Nusshag, C.; Gödtel-Armbrust, U.; Baranyai, D.; Deng, S.; Römer, K.; Nem, D.; Windshügel, B.; et al. Evolutionary history and functional characterization of the amphibian xenosensor CAR. Mol. Endocrinol. 2012, 26, 14–26. [Google Scholar] [CrossRef]
- Eide, M.; Rydbeck, H.; Tørresen, O.; Lille-Langøy, R.; Puntervoll, P.; Goldstone, J.; Jakobsen, K.; Stegeman, J.; Goksøyr, A.; Karlsen, O. Independent losses of a xenobiotics receptor across teleost evolution. Sci. Rep. 2018, 8, 10404. [Google Scholar] [CrossRef] [PubMed]
- Kollitz, E.; Zhang, G.; Hawkins, M.; Whitfield, G.; Reif, D.; Kullman, S. Molecular cloning, functional characterization, and evolutionary analysis of vitamin D receptors isolated from basal vertebrates. PLoS ONE 2015, 10, e0122853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, K.; Giesy, J.; Hu, J. Families of nuclear receptors in vertebrate models: Characteristic and comparative toxicological perspective. Sci. Rep. 2015, 5, 8554. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Venkatesh, B. The divergent genomes of teleosts. Annu. Rev. Anim. Biosci. 2018, 6, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, S.; Brunet, F.; Escriva, H.; Parmentier, G.; Laudet, V.; Robinson-Rechavi, M. Evolutionary genomics of nuclear receptors: From twenty-five ancestral genes to derived endocrine systems. Mol. Biol. Evol. 2004, 21, 1923–1937. [Google Scholar] [CrossRef]
- Chimaeras, D.D. The Living Marine Resources of the Western Central Atlantic Volume 1: Introduction, Mollusks, Crustaceans, Hagfishes, Sharks, Batoid Fishes, and Chimaeras [Internet]; FAO Species Identification Guide for Fishery Purposes and American Society of Ichthyologists and Herpetologists Special Publication No. 5; Carpenter, K.E., Ed.; FAO: Rome, Italy, 2002; pp. 592–599. Available online: http://www.fao.org/tempref/docrep/fao/009/y4160e/y4160e41.pdf. (accessed on 1 February 2019).
- Walker, T.; Stevens, J.; Braccini, J.; Daley, R.; Huveneers, C.; Irvine, S.; Bell, J.; Tovar-Ávila, J.; Trinnie, F.; Phillips, D.; et al. Rapid Assessment of Sustainability for Ecological Risk of Shark and Other Chondrichthyan Bycatch Species Taken in the Southern and Eastern Scalefish and Shark Fishery. [Internet]; Fisheries Research and Development Corporation: Queenscliff, Australia, 2008; Available online: http://frdc.com.au/Archived-Reports/FRDC Projects/2002-033-DLD.pdf. (accessed on 1 February 2019).
- King, B.; Gillis, J.; Carlisle, H.; Dahn, R. A natural deletion of the HoxC cluster in elasmobranch fishes. Science 2011, 334, 1517. [Google Scholar] [CrossRef] [PubMed]
- Wyffels, J.; King, B.; Vincent, J.; Chen, C.; Wu, C.; Polson, S. SkateBase, an elasmobranch genome project and collection of molecular resources for chondrichthyan fishes. F1000Research 2014, 3, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Read, T.; Petit, R.; Joseph, S.; Alam, M.; Weil, M.; Ahmad, M.; Bhimani, R.; Vuong, J.S.; Haase, C.P.; Webb, D.H.; et al. Draft sequencing and assembly of the genome of the world’s largest fish, the whale shark: Rhincodon typus Smith 1828. BMC Genom. 2017, 18, 532. [Google Scholar]
- Venkatesh, B.; Lee, A.; Ravi, V.; Maurya, A.; Lian, M.; Swann, J.; Ohta, Y.; Flajnik, M.F.; Sutoh, Y.; Kasahara, M.; et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 2014, 505, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y.; Yamaguchi, K.; Onimaru, K.; Kadota, M.; Koyanagi, M.; Keeley, S.; Tanaka, K.; Tatsumi, K.; Motone, F.; Kageyama, Y.; et al. Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat. Ecol. Evol. 2018, 2, 1761–1771. [Google Scholar] [CrossRef]
- Bainy, A.; Kubota, A.; Goldstone, J.; Lille-Langøy, R.; Karchner, S.; Celander, M.; Tatsumi, K.; Tanaka, K.; Motone, F.; Kageyama, Y.; et al. Functional characterization of a full length pregnane X receptor, expression in vivo, and identification of PXR alleles, in zebrafish (Danio rerio). Aquat. Toxicol. 2013, 142–143, 447–457. [Google Scholar] [CrossRef]
- Krasowski, M.; Yasuda, K.; Hagey, L.; Schuetz, E. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors). Nucl. Recept. 2005, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, B.; Gauthier, K.; Umetani, M.; Watson, M.; Lochansky, M.; Collins, J.; Leitersdorf, E.; Mangelsdorf, D.J.; Kliewer, S.A.; Repa, J.J. Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 223–228. [Google Scholar] [CrossRef]
- Kliewer, S.; Moore, J.; Wade, L.; Staudinger, J.; Watson, M.; Jones, S.; McKee, D.D.; Oliver, B.B.; Willson, T.M.; Zetterström, R.H. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Krasowski, M.; Yasuda, K.; Hagey, L.; Schuetz, E. Evolution of the pregnane X receptor: Adaptation to cross-species differences in biliary bile salts. Mol. Endocrinol. 2005, 19, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; McKee, D.; Watson, M.; Willson, T.; Moore, J.; Kliewer, S. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 1998, 102, 1016–1023. [Google Scholar] [CrossRef]
- Moore, L.; Parks, D.; Jones, S.; Bledsoe, R.; Consler, T.; Stimmel, J.; Goodwin, B.; Liddle, C.; Blanchard, S.G.; Willson, T.M.; et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000, 275, 15122–15127. [Google Scholar] [CrossRef]
- Handschin, C.; Podvinec, M.; Meyer, U. CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR). Proc. Natl. Acad. Sci. USA 2000, 97, 10769–10774. [Google Scholar] [CrossRef]
- Moore, L.B.; Maglich, J.; McKee, D.; Wisely, B.; Willson, T.; Kliewer, S.; Lambert, M.H.; Moore, J.T. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 2002, 16, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Milnes, M.; Garcia, A.; Grossman, E.; Grün, F.; Shiotsugu, J.; Tabb, M.; Kawashima, Y.; Katsu, Y.; Watanabe, H.; Iguchi, T.; et al. Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ. Health Perspect. 2008, 116, 880–885. [Google Scholar] [CrossRef]
- Lille-Langøy, R.; Goldstone, J.; Rusten, M.; Milnes, M.; Male, R.; Stegeman, J.; Blumberg, B.; Goksøyr, A. Environmental contaminants activate human and polar bear (Ursus maritimus) pregnane X receptors (PXR, NR1I2) differently. Toxicol. Appl. Pharmacol. 2015, 284, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Scheer, N.; Ross, J.; Kapelyukh, Y.; Rode, A.; Wolf, C. In vivo responses of the human and murine pregnane X receptor to dexamethasone in mice. Drug Metab. Dispos. 2010, 38, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Ai, N.; Park, S.; Rios-Pilier, J.; Perkins, J.; Welsh, W.; Zhou, C. Bisphenol A and its analogues activate human pregnane X receptor. Environ. Health Perspect. 2010, 120, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Elentner, A.; Schmuth, M.; Yannoutsos, N.; Eichmann, T.; Gruber, R.; Radner, F.; Hermann, M.; del Frari, B.; Dubrac, S. Epidermal Overexpression of Xenobiotic Receptor PXR Impairs the Epidermal Barrier and Triggers Th2 Immune Response. J. Investig. Dermatol. 2018, 138, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Schmuth, M.; Moosbrugger-Martinz, V.; Blunder, S.; Dubrac, S. Role of PPAR, LXR, and PXR in epidermal homeostasis and inflammation. Biochim. Biophys. Acta 2014, 1841, 463–473. [Google Scholar] [CrossRef]
- Shah, M.; Maibach, H. Estrogen and skin. An overview. Am. J. Clin. Dermatol. 2001, 2, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Frazier-Jessen, M.; Mott, F.; Witte, P.; Kovacs, E. Estrogen suppression of connective tissue deposition in a murine model of peritoneal adhesion formation. J. Immunol. 1996, 156, 3036–3042. [Google Scholar] [PubMed]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.; Schwartz, T.; Pickett, B.; He, S.; Klem, E.; Scheuermann, R.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for Access to Phylogenetic Tools via the CIPRES Science Gateway. Evol. Bioinform. Online 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Nascimento, F.; Reis, M.; Yang, Z. A biologist’s guide to Bayesian phylogenetic analysis. Nat. Ecol. Evol. 2017, 1, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, E.; Ruivo, R.; Lopes-Marques, M.; Zhang, H.; Santos, M.; Venkatesh, B.; Castro, L.F.C. LXRα and LXRβ Nuclear Receptors Evolved in the Common Ancestor of Gnathostomes. Genome Biol. Evol. 2017, 9, 222–230. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, E.S.S.; Ruivo, R.; Machado, A.M.; Conrado, F.; Tay, B.-H.; Venkatesh, B.; Santos, M.M.; Castro, L.F.C. Evolutionary Plasticity in Detoxification Gene Modules: The Preservation and Loss of the Pregnane X Receptor in Chondrichthyes Lineages. Int. J. Mol. Sci. 2019, 20, 2331. https://doi.org/10.3390/ijms20092331
Fonseca ESS, Ruivo R, Machado AM, Conrado F, Tay B-H, Venkatesh B, Santos MM, Castro LFC. Evolutionary Plasticity in Detoxification Gene Modules: The Preservation and Loss of the Pregnane X Receptor in Chondrichthyes Lineages. International Journal of Molecular Sciences. 2019; 20(9):2331. https://doi.org/10.3390/ijms20092331
Chicago/Turabian StyleFonseca, Elza S. S., Raquel Ruivo, André M. Machado, Francisca Conrado, Boon-Hui Tay, Byrappa Venkatesh, Miguel M. Santos, and L. Filipe C. Castro. 2019. "Evolutionary Plasticity in Detoxification Gene Modules: The Preservation and Loss of the Pregnane X Receptor in Chondrichthyes Lineages" International Journal of Molecular Sciences 20, no. 9: 2331. https://doi.org/10.3390/ijms20092331




