Polycystins and Mechanotransduction in Human Disease
Abstract
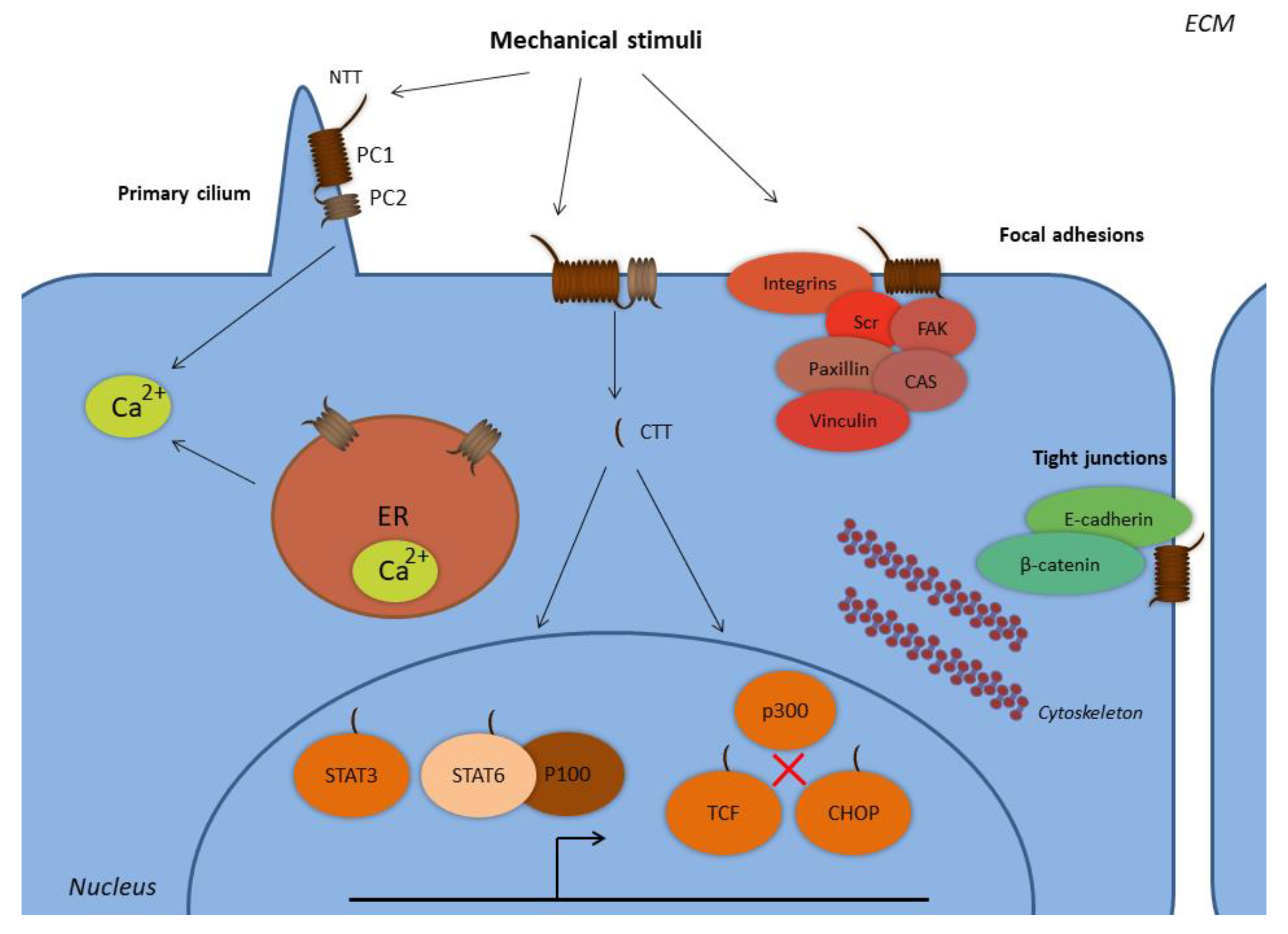
:1. Introduction
2. Structure and Function of Polycystins
3. Polycystins Govern Cyst Formation
4. Polycystins Participate in the Acquisition of Oncogenic Features in Cancer Cells
5. Polycystins in Bone Loss, Cardiomyopathies, and Other Pathophysiologies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal polycystic kidney disease |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein-1 |
| cAMP | Cyclic adenosine monophosphate |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CHOP | C/EBP homologous protein |
| CRC | Colorectal cancer |
| CTCs | Circulating tumor cells |
| CTT | C-terminal tail |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| GPCR | G-protein coupled receptor |
| GSK3β | Glycogen synthase kinase 3 beta |
| JAK2 | Janus kinase 2 |
| JNK | c-Jun N-terminal kinase |
| LTCC | L-type calcium channel |
| MAPK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| NFAT | Nuclear factor of activated T-cells |
| PC1 | Polycystin-1 |
| PC2 | Polycystin-2 |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PHD3 | Prolyl hydroxylase domain containing protein EGLN3 |
| PKD1 | Polycystic Kidney Disease 1 |
| PKD2 | Polycystic Kidney Disease 2 |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| Runx2 | Runt-related transcription factor 2 |
| STAT | Signal transducer and activator of transcription |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TCF | T-cell factor |
| YAP | Yes-associated protein 1 |
References
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.; Koenderink, G.H. A guide to mechanobiology: Where biology and physics meet. Biochim. Biophys. Acta 2015, 1853, 3043–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.S. Mechanotransduction—A field pulling together? J. Cell Sci. 2008, 121, 3285–3292. [Google Scholar] [CrossRef]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargalionis, A.N.; Papavassiliou, K.A.; Basdra, E.K.; Papavassiliou, A.G. Polycystins: Mechanosensors with Diagnostic and Prognostic Potential in Cancer. Trends Mol. Med. 2016, 22, 7–9. [Google Scholar] [CrossRef]
- Koulen, P.; Cai, Y.; Geng, L.; Maeda, Y.; Nishimura, S.; Witzgall, R.; Ehrlich, B.E.; Somlo, S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 2002, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Padovano, V.; Caplan, M.J. The Polycystin Complex Reveals Its Complexity. Biochemistry 2018. [Google Scholar] [CrossRef]
- Su, Q.; Hu, F.; Ge, X.; Lei, J.; Yu, S.; Wang, T.; Zhou, Q.; Mei, C.; Shi, Y. Structure of the human PKD1-PKD2 complex. Science 2018, 361. [Google Scholar] [CrossRef]
- Drummond, I.A. Polycystins, focal adhesions and extracellular matrix interactions. Biochim. Biophys. Acta 2011, 1812, 1322–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, D.H.; Cai, Y.; Chauvet, V.; Rajendran, V.; Zeltner, R.; Geng, L.; Avner, E.D.; Sweeney, W.; Somlo, S.; Caplan, M.J. Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J. Biol. Chem. 2003, 278, 36786–36793. [Google Scholar] [CrossRef]
- Scheffers, M.S.; van der Bent, P.; Prins, F.; Spruit, L.; Breuning, M.H.; Litvinov, S.V.; de Heer, E.; Peters, D.J. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum. Mol. Genet. 2000, 9, 2743–2750. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.D.; Geng, L.; Li, X.; Burrow, C.R. The PKD1 gene product, “polycystin-1”, is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab. Investig. 1999, 79, 1311–1323. [Google Scholar] [PubMed]
- Merrick, D.; Bertuccio, C.A.; Chapin, H.C.; Lal, M.; Chauvet, V.; Caplan, M.J. Polycystin-1 cleavage and the regulation of transcriptional pathways. Pediatr. Nephrol. 2014, 29, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Low, S.H.; Vasanth, S.; Larson, C.H.; Mukherjee, S.; Sharma, N.; Kinter, M.T.; Kane, M.E.; Obara, T.; Weimbs, T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev. Cell 2006, 10, 57–69. [Google Scholar] [CrossRef]
- Merrick, D.; Chapin, H.; Baggs, J.E.; Yu, Z.; Somlo, S.; Sun, Z.; Hogenesch, J.B.; Caplan, M.J. The gamma-secretase cleavage product of polycystin-1 regulates TCF and CHOP-mediated transcriptional activation through a p300-dependent mechanism. Dev. Cell 2012, 22, 197–210. [Google Scholar] [CrossRef]
- Padovano, V.; Kuo, I.Y.; Stavola, L.K.; Aerni, H.R.; Flaherty, B.J.; Chapin, H.C.; Ma, M.; Somlo, S.; Boletta, A.; Ehrlich, B.E.; et al. The polycystins are modulated by cellular oxygen-sensing pathways and regulate mitochondrial function. Mol. Biol. Cell 2017, 28, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Kurashige, M.; Liu, Y.; Terabayashi, T.; Ishimoto, Y.; Wang, T.; Choudhary, V.; Hobbs, R.; Liu, L.K.; Lee, P.H.; et al. A cleavage product of Polycystin-1 is a mitochondrial matrix protein that affects mitochondria morphology and function when heterologously expressed. Sci. Rep. 2018, 8, 2743. [Google Scholar] [CrossRef]
- Ong, A.C.; Harris, P.C. A polycystin-centric view of cyst formation and disease: The polycystins revisited. Kidney Int. 2015, 88, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Watnick, T.; He, N.; Wang, K.; Liang, Y.; Parfrey, P.; Germino, G.; St George-Hyslop, P. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1999, 10, 1524–1529. [Google Scholar]
- Watnick, T.J.; Torres, V.E.; Gandolph, M.A.; Qian, F.; Onuchic, L.F.; Klinger, K.W.; Landes, G.; Germino, G.G. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease. Mol. Cell 1998, 2, 247–251. [Google Scholar] [CrossRef]
- Hopp, K.; Ward, C.J.; Hommerding, C.J.; Nasr, S.H.; Tuan, H.F.; Gainullin, V.G.; Rossetti, S.; Torres, V.E.; Harris, P.C. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Investig. 2012, 122, 4257–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, S.; Kubly, V.J.; Consugar, M.B.; Hopp, K.; Roy, S.; Horsley, S.W.; Chauveau, D.; Rees, L.; Barratt, T.M.; van’t Hoff, W.G.; et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009, 75, 848–855. [Google Scholar] [CrossRef] [Green Version]
- Burtey, S.; Riera, M.; Ribe, E.; Pennekamp, P.; Passage, E.; Rance, R.; Dworniczak, B.; Fontes, M. Overexpression of PKD2 in the mouse is associated with renal tubulopathy. Nephrol. Dial. Transplant. 2008, 23, 1157–1165. [Google Scholar] [CrossRef]
- Thivierge, C.; Kurbegovic, A.; Couillard, M.; Guillaume, R.; Cote, O.; Trudel, M. Overexpression of PKD1 causes polycystic kidney disease. Mol. Cell Biol. 2006, 26, 1538–1548. [Google Scholar] [CrossRef]
- Malekshahabi, T.; Khoshdel Rad, N.; Serra, A.L.; Moghadasali, R. Autosomal dominant polycystic kidney disease: Disrupted pathways and potential therapeutic interventions. J. Cell Physiol. 2019. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F. Polycystins and partners: Proposed role in mechanosensitivity. J. Physiol. 2014, 592, 2453–2471. [Google Scholar] [CrossRef] [PubMed]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J.; et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Padovano, V.; Podrini, C.; Boletta, A.; Caplan, M.J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 2018, 14, 678–687. [Google Scholar] [CrossRef]
- Zhang, B.; Tran, U.; Wessely, O. Polycystin 1 loss of function is directly linked to an imbalance in G-protein signaling in the kidney. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Parnell, S.C.; Magenheimer, B.S.; Maser, R.L.; Pavlov, T.S.; Havens, M.A.; Hastings, M.L.; Jackson, S.F.; Ward, C.J.; Peterson, K.R.; Staruschenko, A.; et al. A mutation affecting polycystin-1 mediated heterotrimeric G-protein signaling causes PKD. Hum. Mol. Genet. 2018, 27, 3313–3324. [Google Scholar] [CrossRef] [PubMed]
- Delling, M.; Indzhykulian, A.A.; Liu, X.; Li, Y.; Xie, T.; Corey, D.P.; Clapham, D.E. Primary cilia are not calcium-responsive mechanosensors. Nature 2016, 531, 656–660. [Google Scholar] [CrossRef]
- Liu, X.; Vien, T.; Duan, J.; Sheu, S.H.; DeCaen, P.G.; Clapham, D.E. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Elife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.K.; Low, B.C.; Lim, C.T. Mechanobiology of Tumor Growth. Chem. Rev. 2018, 118, 6499–6515. [Google Scholar] [CrossRef]
- Aw Yong, K.M.; Sun, Y.; Merajver, S.D.; Fu, J. Mechanotransduction-Induced Reversible Phenotypic Switching in Prostate Cancer Cells. Biophys. J. 2017, 112, 1236–1245. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Kim, J.; Hong, H.; Lee, S.H.; Lee, J.K.; Jung, E.; Kim, J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2016, 35, 462–478. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Cancer mechanobiology: Effects and therapeutic perspectives. Int. J. Cancer 2018, 142, 1298–1299. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Tumor mechanosensing and its therapeutic potential. J. Cell Biochem. 2018, 119, 4304–4308. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.X.; Liao, W.; Farhoodi, H.P.; Wong, C.W.; Chen, C.C.; Segaliny, A.I.; Chacko, J.V.; Nguyen, L.P.; Lu, M.; et al. Mechanoresponsive stem cells to target cancer metastases through biophysical cues. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Seeger-Nukpezah, T.; Geynisman, D.M.; Nikonova, A.S.; Benzing, T.; Golemis, E.A. The hallmarks of cancer: Relevance to the pathogenesis of polycystic kidney disease. Nat. Rev. Nephrol. 2015, 11, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.M.; Chuang, Y.W.; Yu, M.C.; Chen, C.H.; Yang, C.K.; Huang, S.T.; Lin, C.L.; Shu, K.H.; Kao, C.H. Risk of cancer in patients with polycystic kidney disease: A propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol. 2016, 17, 1419–1425. [Google Scholar] [CrossRef]
- Xiao, Z.; Baudry, J.; Cao, L.; Huang, J.; Chen, H.; Yates, C.R.; Li, W.; Dong, B.; Waters, C.M.; Smith, J.C.; et al. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis. J. Clin. Investig. 2018, 128, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Korkolopoulou, P.; Farmaki, E.; Piperi, C.; Dalagiorgou, G.; Adamopoulos, C.; Levidou, G.; Saetta, A.; Fragkou, P.; Tsioli, P.; et al. Polycystin-1 and polycystin-2 are involved in the acquisition of aggressive phenotypes in colorectal cancer. Int. J. Cancer 2015, 136, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins in Colorectal Cancer. Int. J. Mol. Sci. 2018, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Gyparaki, M.T.; Basdra, E.K.; Papavassiliou, A.G. DNA methylation biomarkers as diagnostic and prognostic tools in colorectal cancer. J. Mol. Med. 2013, 91, 1249–1256. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zhou, H.; Gu, Y.; Bai, Y.; Yu, S.; An, R.; Qi, J. Identification of potential key genes and high-frequency mutant genes in prostate cancer by using RNA-Seq data. Oncol. Lett. 2018, 15, 4550–4556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Ye, C.; Zhou, Q.; Zheng, R.; Lv, X.; Chen, Y.; Hu, Z.; Guo, H.; Zhang, Z.; Wang, Y.; et al. PKD1 inhibits cancer cells migration and invasion via Wnt signaling pathway in vitro. Cell Biochem. Funct. 2007, 25, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, Z.; Lv, X.; Fan, J.; Chen, Y.; Wang, Y.; Tan, R.; Liu, Y.; Zhou, Q. Polycystin-1 induced apoptosis and cell cycle arrest in G0/G1 phase in cancer cells. Cell Biol. Int. 2008, 32, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.H.; Cao, G.; Lv, X.Y.; Li, Q.W.; Sun, H.; Xiao, Y.; Ai, J.Z.; Yang, Q.T.; Duan, J.J.; Wang, Y.D.; et al. Down-regulation of Pkd2 by siRNAs suppresses cell-cell adhesion in the mouse melanoma cells. Mol. Biol. Rep. 2010, 37, 2387–2395. [Google Scholar] [CrossRef]
- Adamopoulos, C.; Gargalionis, A.N.; Piperi, C.; Papavassiliou, A.G. Recent Advances in Mechanobiology of Osteosarcoma. J. Cell Biochem. 2017, 118, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins and mechanotransduction in bone. Oncotarget 2017, 8, 106159–106160. [Google Scholar] [CrossRef]
- Xiao, Z.; Quarles, L.D. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev. Endocr. Metab. Disord. 2015, 16, 115–129. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Zhang, S.; Magenheimer, B.S.; Luo, J.; Quarles, L.D. Polycystin-1 regulates skeletogenesis through stimulation of the osteoblast-specific transcription factor RUNX2-II. J. Biol. Chem. 2008, 283, 12624–12634. [Google Scholar] [CrossRef]
- Dalagiorgou, G.; Piperi, C.; Adamopoulos, C.; Georgopoulou, U.; Gargalionis, A.N.; Spyropoulou, A.; Zoi, I.; Nokhbehsaim, M.; Damanaki, A.; Deschner, J.; et al. Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis. Cell Mol. Life Sci. 2017, 74, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Dalagiorgou, G.; Piperi, C.; Georgopoulou, U.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Mechanical stimulation of polycystin-1 induces human osteoblastic gene expression via potentiation of the calcineurin/NFAT signaling axis. Cell Mol. Life Sci. 2013, 70, 167–180. [Google Scholar] [CrossRef]
- Merrick, D.; Mistry, K.; Wu, J.; Gresko, N.; Baggs, J.E.; Hogenesch, J.B.; Sun, Z.; Caplan, M.J. Polycystin-1 regulates bone development through an interaction with the transcriptional co-activator taz. Hum. Mol. Genet. 2018. [Google Scholar] [CrossRef] [PubMed]
- Karamesinis, K.; Spyropoulou, A.; Dalagiorgou, G.; Katsianou, M.A.; Nokhbehsaim, M.; Memmert, S.; Deschner, J.; Vastardis, H.; Piperi, C. Continuous hydrostatic pressure induces differentiation phenomena in chondrocytes mediated by changes in polycystins, SOX9, and RUNX2. J. Orofac. Orthop. 2017, 78, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, W.; Ma, J.; Pan, Y.; Wang, L.; Zhang, W. Polycystin-1 mediates mechanical strain-induced osteoblastic mechanoresponses via potentiation of intracellular calcium and Akt/beta-catenin pathway. PLoS ONE 2014, 9, e91730. [Google Scholar] [CrossRef]
- Xiao, Z.; Cao, L.; Liang, Y.; Huang, J.; Stern, A.R.; Dallas, M.; Johnson, M.; Quarles, L.D. Osteoblast-specific deletion of Pkd2 leads to low-turnover osteopenia and reduced bone marrow adiposity. PLoS ONE 2014, 9, e114198. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins in disease mechanobiology. J. Cell Biochem. 2018. [Google Scholar] [CrossRef]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef]
- Varela, A.; Piperi, C.; Sigala, F.; Agrogiannis, G.; Davos, C.H.; Andri, M.A.; Manopoulos, C.; Tsangaris, S.; Basdra, E.K.; Papavassiliou, A.G. Elevated expression of mechanosensory polycystins in human carotid atherosclerotic plaques: Association with p53 activation and disease severity. Sci. Rep. 2015, 5, 13461. [Google Scholar] [CrossRef]
- Sharif-Naeini, R.; Folgering, J.H.; Bichet, D.; Duprat, F.; Lauritzen, I.; Arhatte, M.; Jodar, M.; Dedman, A.; Chatelain, F.C.; Schulte, U.; et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 2009, 139, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, V.; Qian, F.; Boute, N.; Cai, Y.; Phakdeekitacharoen, B.; Onuchic, L.F.; Attie-Bitach, T.; Guicharnaud, L.; Devuyst, O.; Germino, G.G.; et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am. J. Pathol. 2002, 160, 973–983. [Google Scholar] [CrossRef]
- Pedrozo, Z.; Criollo, A.; Battiprolu, P.K.; Morales, C.R.; Contreras-Ferrat, A.; Fernandez, C.; Jiang, N.; Luo, X.; Caplan, M.J.; Somlo, S.; et al. Polycystin-1 Is a Cardiomyocyte Mechanosensor That Governs L-Type Ca2+ Channel Protein Stability. Circulation 2015, 131, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Cordova-Casanova, A.; Olmedo, I.; Riquelme, J.A.; Barrientos, G.; Sanchez, G.; Gillette, T.G.; Lavandero, S.; Chiong, M.; Donoso, P.; Pedrozo, Z. Mechanical stretch increases L-type calcium channel stability in cardiomyocytes through a polycystin-1/AKT-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 289–296. [Google Scholar] [CrossRef]
- Paavola, J.; Schliffke, S.; Rossetti, S.; Kuo, I.Y.; Yuan, S.; Sun, Z.; Harris, P.C.; Torres, V.E.; Ehrlich, B.E. Polycystin-2 mutations lead to impaired calcium cycling in the heart and predispose to dilated cardiomyopathy. J. Mol. Cell Cardiol. 2013, 58, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Kuo, I.Y.; Duong, S.L.; Nguyen, L.; Ehrlich, B.E. Decreased Polycystin 2 Levels Result in Non-Renal Cardiac Dysfunction with Aging. PLoS ONE 2016, 11, e0153632. [Google Scholar] [CrossRef]
- Malakou, L.S.; Gargalionis, A.N.; Piperi, C.; Papadavid, E.; Papavassiliou, A.G.; Basdra, E.K. Molecular mechanisms of mechanotransduction in psoriasis. Ann. Transl. Med. 2018, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Malakou, L.S.; Adamopoulos, C.; Piperi, C.; Theohari, I.; Nokhbehsaim, M.; Deschner, J.; Kokkalis, G.; Korkolopoulou, P.; Papadavid, E.; et al. Polycystin-1 downregulation induces ERK-dependent mTOR pathway activation in a cellular model of psoriasis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3468–3476. [Google Scholar] [CrossRef] [PubMed]

| Pathophysiology | Pathogenic Mechanism |
|---|---|
| Autosomal dominant polycystic kidney disease | PKD1 and PKD2 loss-of-function mutations Aberrant sensing of the urine flow Deregulated calcium influx Pathogenic signaling promotes cystogenesis |
| Deregulated cell metabolism | |
| Cancer | PC2 overexpression activates the mTOR pathway in CRC |
| PC1 overexpression promotes EMT, invasion and metastasis in CRC | |
| PKD1 among high-risk mutant genes in prostate cancer | |
| Bone formation | PKD1 and PKD2 downregulation inhibits osteoblastogenesis |
| PC1 under mechanical loading leads to increased osteoblastogenesis through various mechanisms | |
| Cardiovascular diseases | PC1 overexpression in endothelial cells follows disturbed shear stress and is associated with atherosclerosis |
| PKD1 and PKD2 downregulation is associated with cardiomyopathy | |
| Psoriasis | Loss of PC1 expression promotes psoriasis via ERK and mTOR activation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins and Mechanotransduction in Human Disease. Int. J. Mol. Sci. 2019, 20, 2182. https://doi.org/10.3390/ijms20092182
Gargalionis AN, Basdra EK, Papavassiliou AG. Polycystins and Mechanotransduction in Human Disease. International Journal of Molecular Sciences. 2019; 20(9):2182. https://doi.org/10.3390/ijms20092182
Chicago/Turabian StyleGargalionis, Antonios N., Efthimia K. Basdra, and Athanasios G. Papavassiliou. 2019. "Polycystins and Mechanotransduction in Human Disease" International Journal of Molecular Sciences 20, no. 9: 2182. https://doi.org/10.3390/ijms20092182





