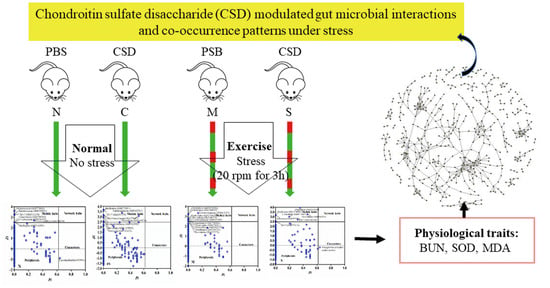
Microbial Co-Occurrence Patterns and Keystone Species in the Gut Microbial Community of Mice in Response to Stress and Chondroitin Sulfate Disaccharide
Abstract
:1. Introduction
2. Results
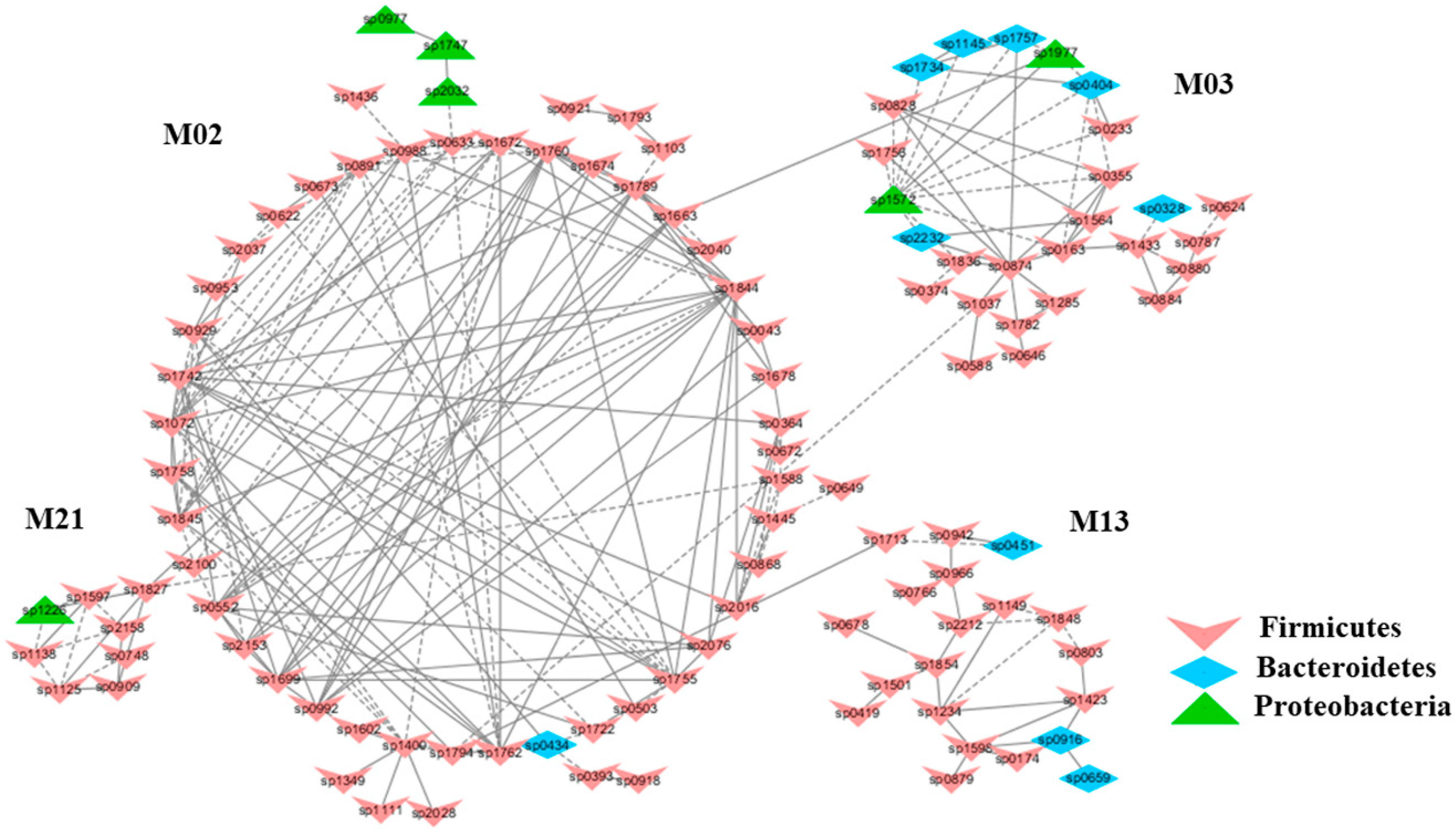
2.1. Stress from Exhaustive Exercise Induced a Distinctly Different Microbial Co-Occurrence Network in Mice
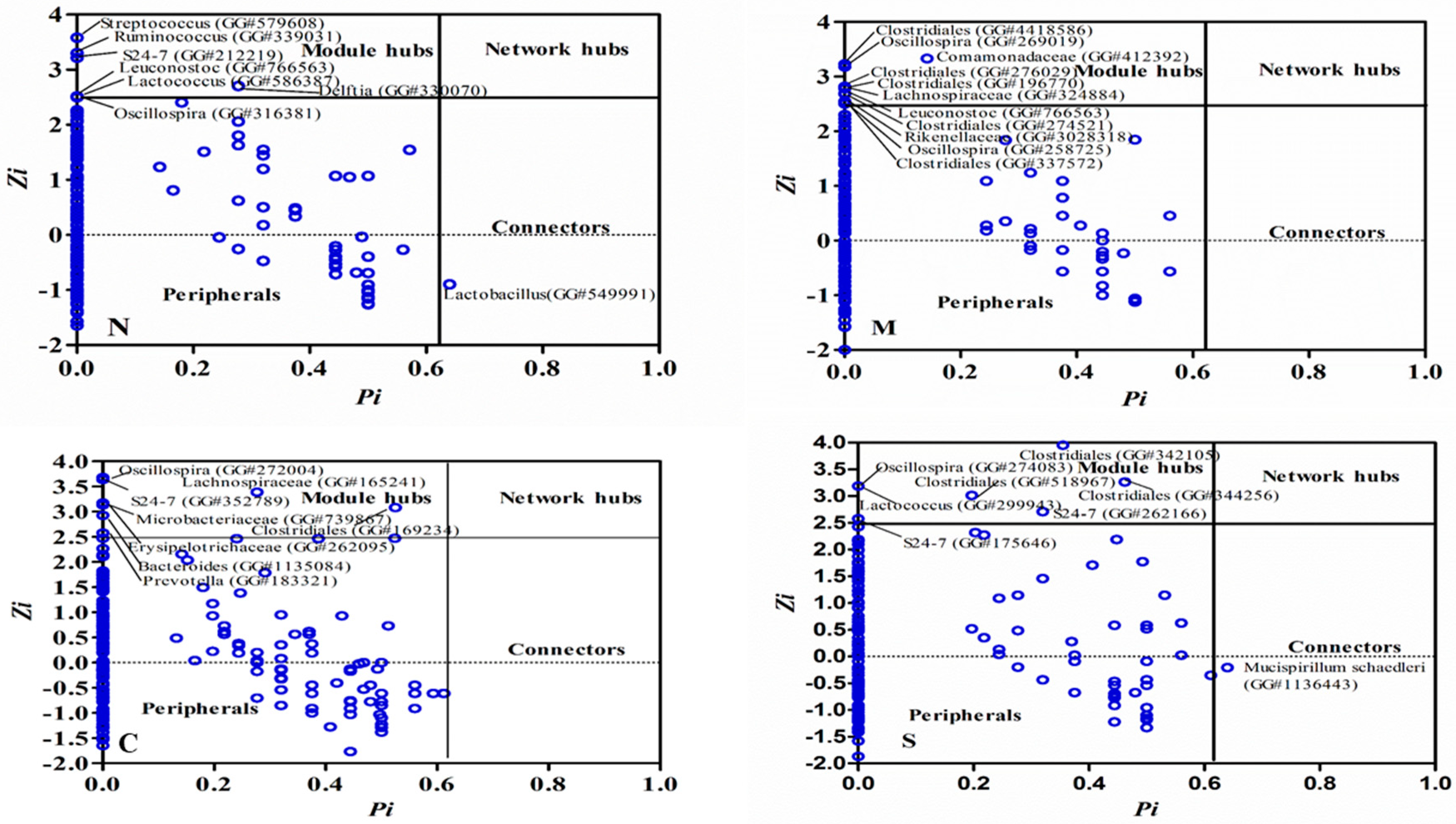
2.2. Keystone Species and their Possible Ecological Roles
2.3. The Correlations between Modules and Physiological Parameters
3. Discussion
4. Materials and Methods
4.1. Animals Experiment
4.2. Physiological Parameters
4.3. 16S rRNA Gene Sequencing and Data Analysis
4.4. Network Construction and Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, N.; Li, Z.; Wang, X.; Shi, H.; Xue, C.; Li, R.W.; Tang, Q. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci. Rep. 2017, 7, 6783. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Huang, S.Q.; Li, C.Q.; Xu, Q.; Zeng, Q.P. Akkermansia muciniphila May Determine Chondroitin Sulfate Ameliorating or Aggravating Osteoarthritis. Front. Microbiol. 2017, 8, 1955. [Google Scholar] [CrossRef] [Green Version]
- Shang, Q.; Shi, J.; Song, G.; Zhang, M.; Cai, C.; Hao, J.; Li, G.; Yu, G. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int. J. Biol. Macromol. 2016, 89, 489–498. [Google Scholar] [CrossRef]
- Shang, Q.; Yin, Y.; Zhu, L.; Li, G.; Yu, G.; Wang, X. Degradation of chondroitin sulfate by the gut microbiota of Chinese individuals. Int. J. Biol. Macromol. 2016, 86, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; White, E.; Kantor, E.D.; Zhang, Y.; Rho, J.; Song, X.; Milne, G.L.; Lampe, P.D.; Lampe, J.W. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PLoS ONE 2015, 10, e0117534. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.L.; Clarkson, P.M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Powers, S.K.; Lennon, S.L. Analysis of cellular responses to free radicals: Focus on exercise and skeletal muscle. Proc. Nutr. Soc. 1999, 58, 1025–1033. [Google Scholar] [CrossRef]
- Fielding, R.A.; Meydani, M. Exercise, free radical generation, and aging. Aging (Milano) 1997, 9, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.W.; Turk, J.R.; Laughlin, M.H. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1378–H1387. [Google Scholar] [CrossRef]
- Danao-Camara, T. Potential side effects of treatment with glucosamine and chondroitin. Arthritis. Rheum. 2000, 43, 2853. [Google Scholar] [CrossRef]
- Jujo, K.; Minami, Y.; Haruki, S.; Matsue, Y.; Shimazaki, K.; Kadowaki, H.; Ishida, I.; Kambayashi, K.; Arashi, H.; Sekiguchi, H.; et al. Persistent high blood urea nitrogen level is associated with increased risk of cardiovascular events in patients with acute heart failure. ESC Heart Fail. 2017, 4, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, K.; Minami, Y.; Sato, N.; Takano, T.; Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Serum sodium concentration, blood urea nitrogen, and outcomes in patients hospitalized for acute decompensated heart failure. Int. J. Cardiol. 2016, 222, 195–201. [Google Scholar] [CrossRef]
- You, S.; Zheng, D.; Zhong, C.; Wang, X.; Tang, W.; Sheng, L.; Zheng, C.; Cao, Y.; Liu, C.F. Prognostic Significance of Blood Urea Nitrogen in Acute Ischemic Stroke. Circ J. 2018, 82, 572–578. [Google Scholar] [CrossRef]
- Anders, H.J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Wakino, S.; Irie, J.; Tajima, T.; Hasegawa, K.; Kanda, T.; Tokuyama, H.; Hayashi, K.; Itoh, H. Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol. Dial. Transplant. 2016, 31, 401–412. [Google Scholar] [CrossRef]
- Tang, W.H.; Hazen, S.L. The Gut Microbiome and Its Role in Cardiovascular Diseases. Circulation 2017, 135, 1008–1010. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, S.; Xu, G. Targeting gut microbiota: A potential promising therapy for diabetic kidney disease. Am. J. Transl. Res. 2016, 8, 4009–4016. [Google Scholar]
- Williams, R.J.; Howe, A.; Hofmockel, K.S. Demonstrating microbial co-occurrence pattern analyses within and between ecosystems. Front. Microbiol. 2014, 5, 358. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. MBio 2011, 2, e00122-11. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef]
- Ruan, Q.; Dutta, D.; Schwalbach, M.S.; Steele, J.A.; Fuhrman, J.A.; Sun, F. Local similarity analysis reveals unique associations among marine bacterioplankton species and environmental factors. Bioinformatics 2006, 22, 2532–2538. [Google Scholar] [CrossRef] [Green Version]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef]
- Xia, L.C.; Ai, D.; Cram, J.; Fuhrman, J.A.; Sun, F. Efficient statistical significance approximation for local similarity analysis of high-throughput time series data. Bioinformatics 2013, 29, 230–237. [Google Scholar] [CrossRef]
- Weiss, S.; Van Treuren, W.; Lozupone, C.; Faust, K.; Friedman, J.; Deng, Y.; Xia, L.C.; Xu, Z.Z.; Ursell, L.; Alm, E.J.; et al. Correlation detection strategies in microbial data sets vary widely in sensitivity and precision. ISME J. 2016, 10, 1669–1681. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.A.; Koren, O.; Goodrich, J.K.; Johansson, M.E.; Nalbantoglu, I.; Aitken, J.D.; Su, Y.; Chassaing, B.; Walters, W.A.; Gonzalez, A.; et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 2012, 12, 139–152. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.; Schwab, C.; Milinovich, G.; Reichert, J.; Ben Mahfoudh, K.; Decker, T.; Engel, M.; Hai, B.; Hainzl, E.; Heider, S.; et al. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012, 6, 2091–2106. [Google Scholar] [CrossRef] [Green Version]
- Loy, A.; Pfann, C.; Steinberger, M.; Hanson, B.; Herp, S.; Brugiroux, S.; Gomes Neto, J.C.; Boekschoten, M.V.; Schwab, C.; Urich, T.; et al. Lifestyle and Horizontal Gene Transfer-Mediated Evolution of Mucispirillum schaedleri, a Core Member of the Murine Gut Microbiota. mSystems 2017, 2, e00171-16. [Google Scholar] [CrossRef]
- Raghavan, V.; Groisman, E.A. Species-specific dynamic responses of gut bacteria to a mammalian glycan. J. Bacteriol. 2015, 197, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, V.; Lowe, E.C.; Townsend, G.E., 2nd; Bolam, D.N.; Groisman, E.A. Tuning transcription of nutrient utilization genes to catabolic rate promotes growth in a gut bacterium. Mol. Microbiol. 2014, 93, 1010–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmagel, A.; Demmer, R.; Knights, D.; Butler, M.; Langsetmo, L.; Lane, N.E.; Ensrud, K. The Effects of Glucosamine and Chondroitin Sulfate on Gut Microbial Composition: A Systematic Review of Evidence from Animal and Human Studies. Nutrients 2019, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.P.; Peretz, A. Possible association between glucosamine treatment and renal toxicity: Comment on the letter by Danao-Camara. Arthritis. Rheum. 2001, 44, 2943–2944. [Google Scholar] [CrossRef] [Green Version]
- Li, R.W.; Li, W.; Sun, J.; Yu, P.; Baldwin, R.L.; Urban, J.F. The effect of helminth infection on the microbial composition and structure of the caprine abomasal microbiome. Sci. Rep. 2016, 6, 20606. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]




| Module | Physiological Parameters | r | p Value | Module Members |
|---|---|---|---|---|
| Global Network/Group: N | ||||
| N01 | BUN | 0.68 | 0.040 | 34 |
| N05 | BUN | 0.69 | 0.040 | 29 |
| N14 | CR | 0.76 | 0.002 | 12 |
| N09 | MDA | 0.67 | 0.050 | 40 |
| Global Network/Group: M | ||||
| M02 | BUN | 0.90 | 0.002 | 54 |
| M03 | BUN | −0.89 | 0.003 | 29 |
| M21 | BUN | 0.87 | 0.005 | 8 |
| M02 | CR | 0.90 | 0.002 | 54 |
| M21 | CR | 0.87 | 0.005 | 8 |
| M13 | CR | −0.65 | 0.080 | 20 |
| M27 | MDA | 0.67 | 0.070 | 8 |
| M01 | SOD | 0.71 | 0.050 | 38 |
| M14 | SOD | −0.72 | 0.050 | 22 |
| Global Network/Group: S | ||||
| S05 | BUN | −0.75 | 0.050 | 46 |
| S02 | CR | 0.68 | 0.090 | 32 |
| Global Network/Group: C | ||||
| C15 | BUN | 0.89 | 0.020 | 9 |
| C01 | CR | 0.73 | 0.100 | 77 |
| C03 | CR | 0.75 | 0.090 | 42 |
| C04 | CR | 0.77 | 0.080 | 31 |
| C07 | SOD | 0.76 | 0.080 | 20 |
| C10 | SOD | −0.94 | 0.006 | 8 |
| Treatment Group | Physiological Parameter | Taxon (Level) | r (Correlation Coefficient) | Significance (Probability) |
|---|---|---|---|---|
| N | BUN | Pseudomonodales (Order) | 0.8144 | 0.0020 |
| N | BUN | Porphyromonadaceae (Family) | 0.9165 | 0.0250 |
| N | BUN | Moraxellacease (Family) | 0.7874 | 0.0040 |
| C | CR | Pseudomonodales (Order) | 0.6137 | 0.0250 |
| C | BUN | Lactobacillacease (Family) | 0.3149 | 0.0460 |
| C | SOD | Lactobacillacease (Family) | 0.5234 | 0.0039 |
| M | SOD | Proteobacteria (Phylum) | 0.5663 | 0.0250 |
| S | CR | Pseudomonodales (Order) | 0.6167 | 0.0333 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Li, Z.; Wang, X.; Xue, C.; Tang, Q.; Li, R.W. Microbial Co-Occurrence Patterns and Keystone Species in the Gut Microbial Community of Mice in Response to Stress and Chondroitin Sulfate Disaccharide. Int. J. Mol. Sci. 2019, 20, 2130. https://doi.org/10.3390/ijms20092130
Liu F, Li Z, Wang X, Xue C, Tang Q, Li RW. Microbial Co-Occurrence Patterns and Keystone Species in the Gut Microbial Community of Mice in Response to Stress and Chondroitin Sulfate Disaccharide. International Journal of Molecular Sciences. 2019; 20(9):2130. https://doi.org/10.3390/ijms20092130
Chicago/Turabian StyleLiu, Fang, Zhaojie Li, Xiong Wang, Changhu Xue, Qingjuan Tang, and Robert W. Li. 2019. "Microbial Co-Occurrence Patterns and Keystone Species in the Gut Microbial Community of Mice in Response to Stress and Chondroitin Sulfate Disaccharide" International Journal of Molecular Sciences 20, no. 9: 2130. https://doi.org/10.3390/ijms20092130