Dysregulation of Circular RNAs in Myotonic Dystrophy Type 1
Abstract
:1. Introduction
2. Results
2.1. Identification of circRNA Expression in DM1 Skeletal Muscle by RNA-Sequencing
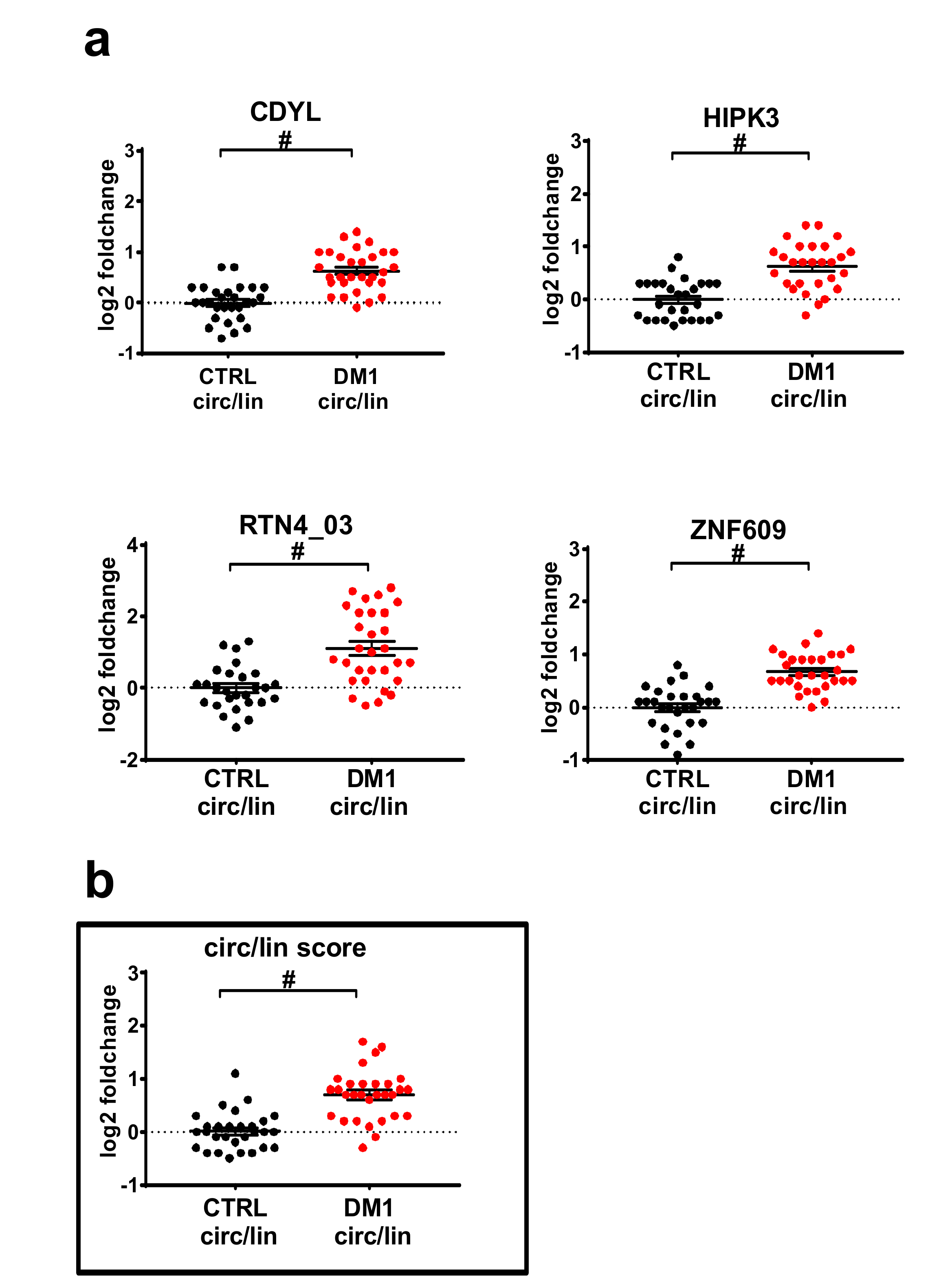
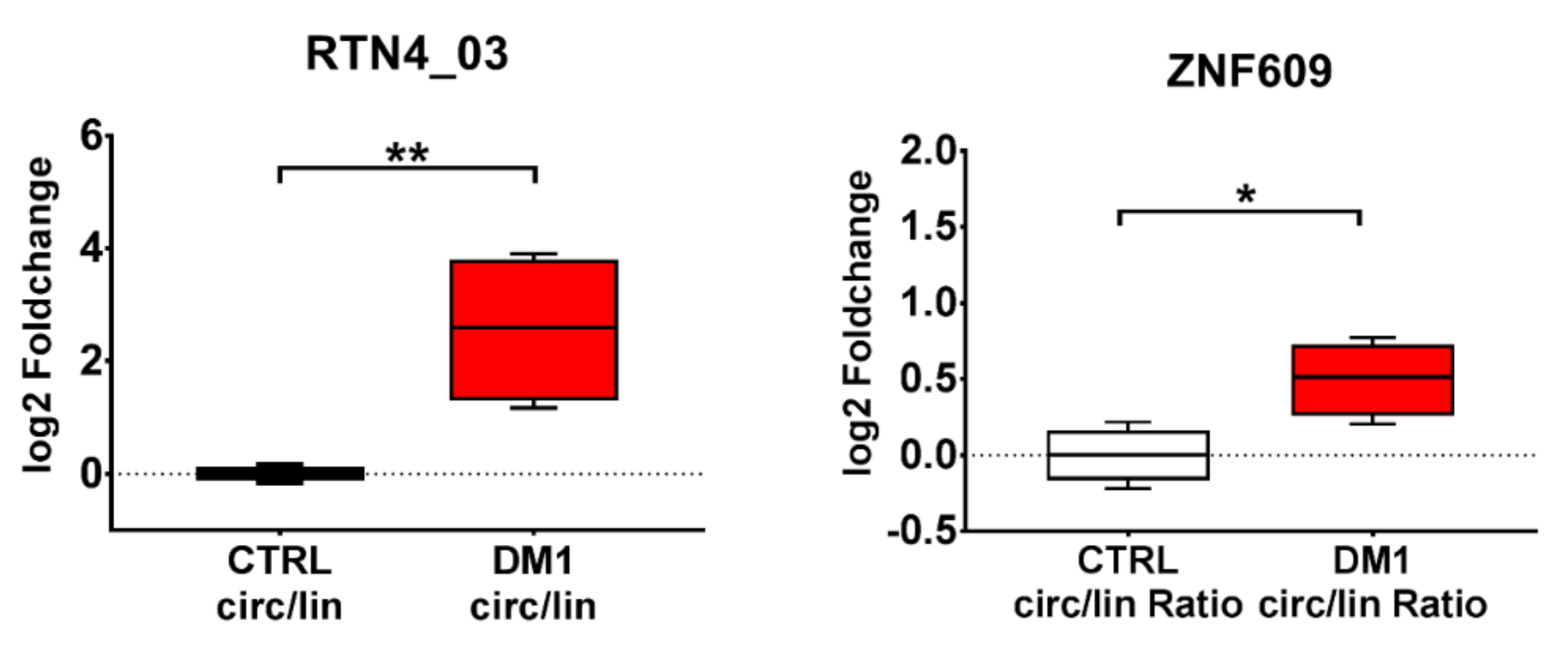
2.2. Validation by qPCR of Differentially Expressed circRNAs in DM1 Skeletal Muscles
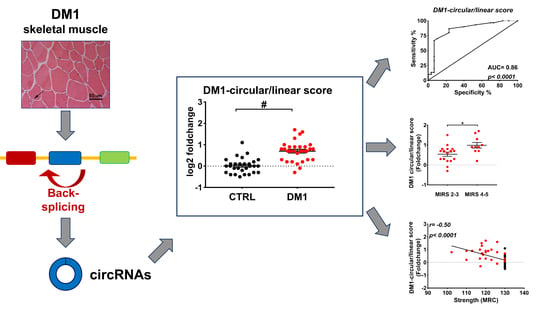
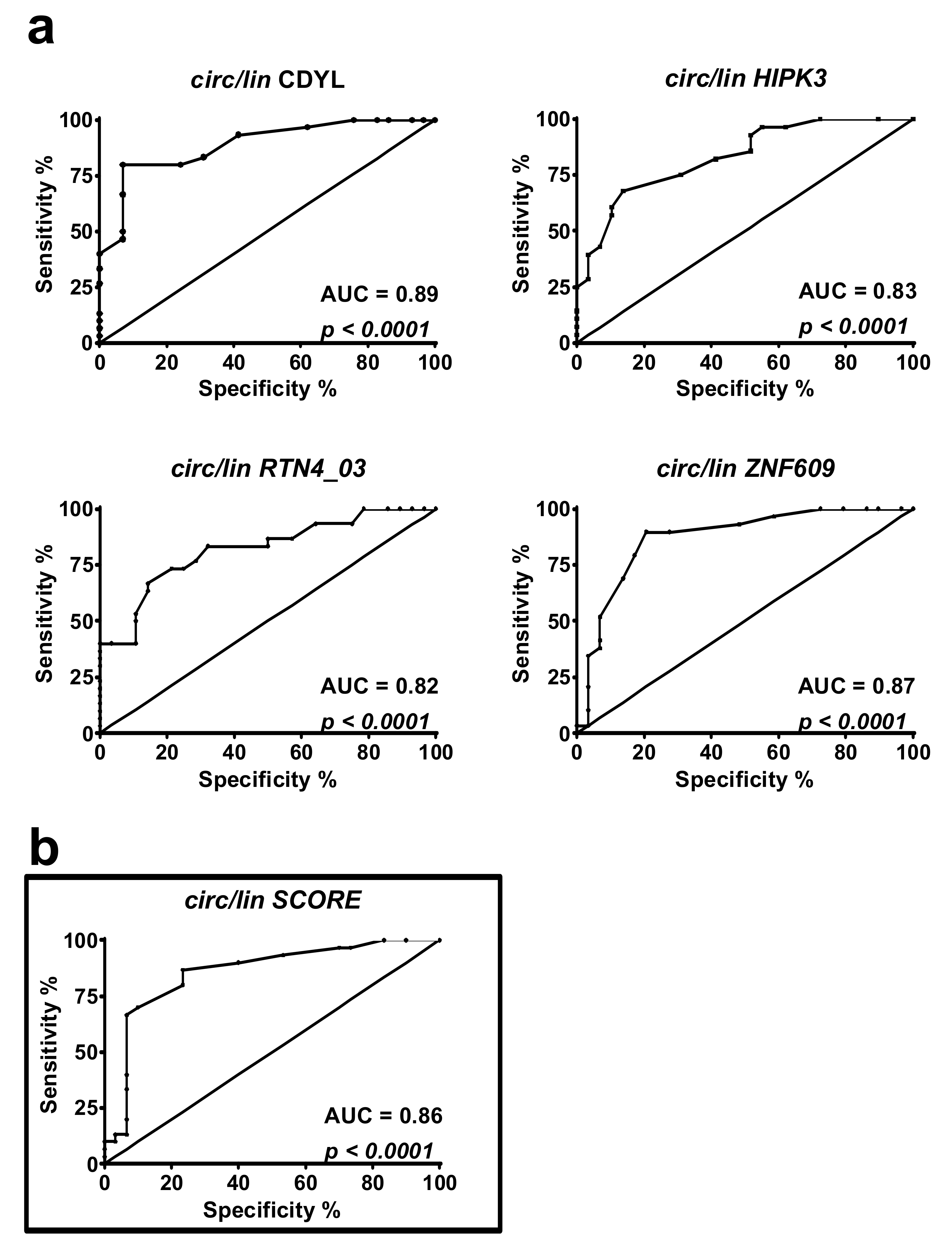
2.3. DM1-circRNAs Distinguish DM1 Patients from Controls
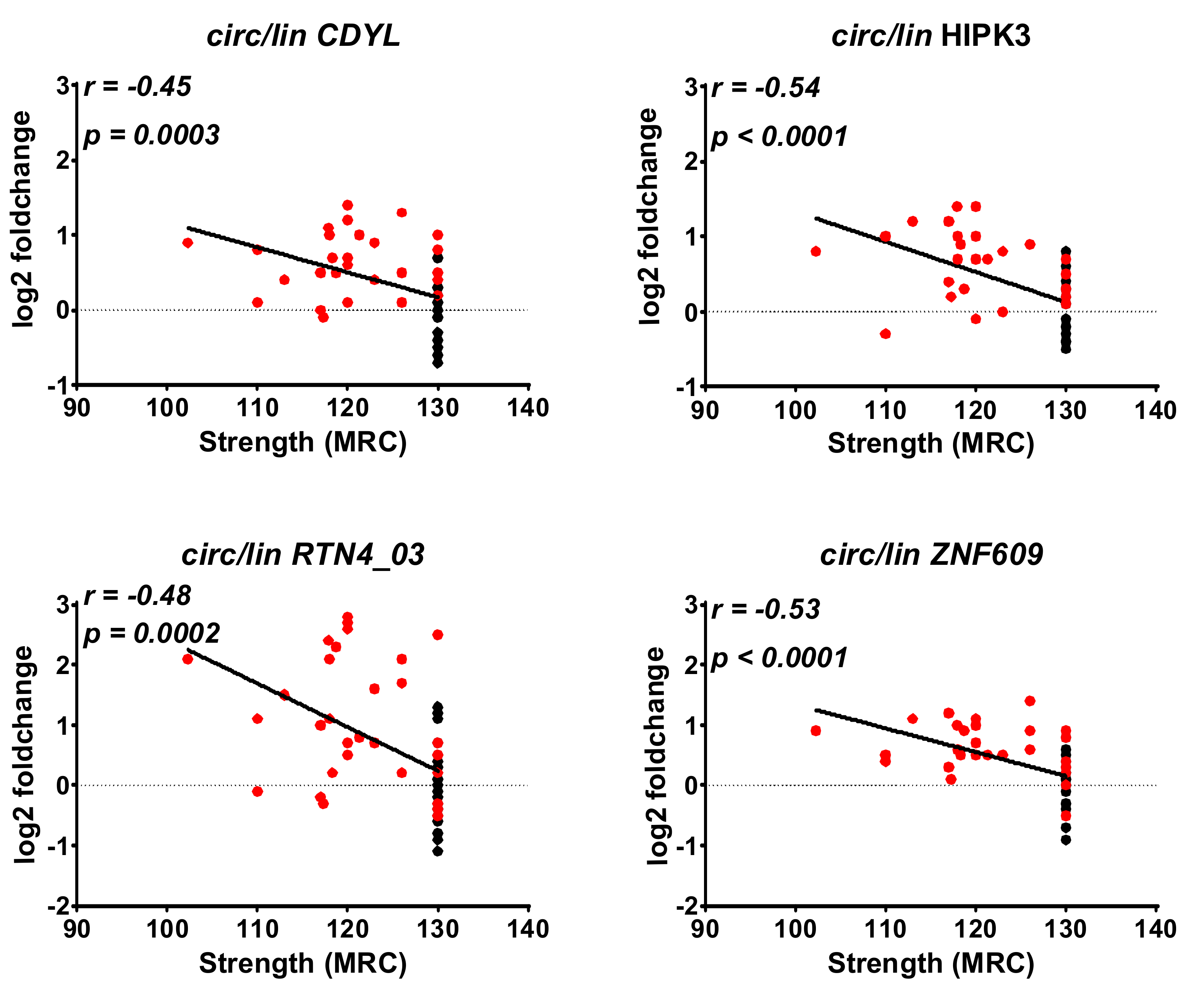
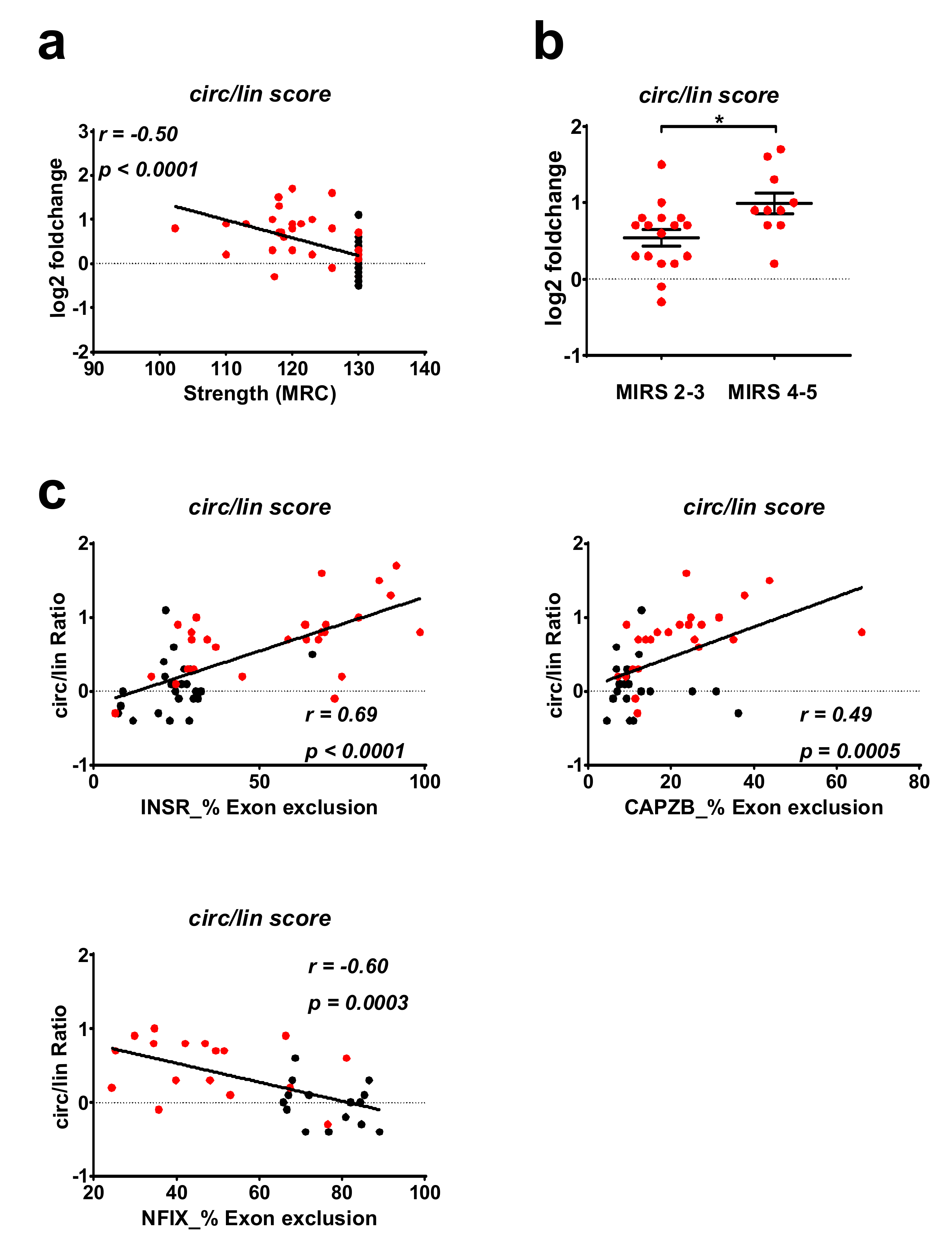
2.4. Correlation between DM1-circRNAs and Clinical Characteristics
2.5. Correlation of DM1-circRNA Levels and Alternative Splicing Events
2.6. DM1-circRNA levels in PBMCs and plasma of DM1 patients
2.7. DM1-circRNA Expression in DM1 Myogenic Cell Lines
3. Discussion
4. Materials and Methods
4.1. RNAseq and Bioinformatics Analysis
4.2. Patient Characteristics and Tissue Collection
4.3. Ethical Approval and Informed Consent
4.4. Histopathological Analysis
4.5. DM1 Myogenic Cell Lines
4.6. Isolation of Total RNA
4.7. Real-Time Reverse Transcriptase qPCR
4.8. Quantification of Alterntative Exon Usage
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meola, G.; Cardani, R. Myotonic Dystrophies: An Update on Clinical Aspects, Genetic, Pathology, and Molecular Pathomechanisms. Biochim. Biophys. Acta 2015, 1852, 594–606. [Google Scholar] [CrossRef] [PubMed]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G.; French Myotonic Dystrophy Clinical Network. Unravelling the Myotonic Dystrophy Type 1 Clinical Spectrum: A Systematic Registry-Based Study with Implications for Disease Classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Chouinard, M.C.; Laberge, L.; Veillette, S.; Begin, P.; Breton, R.; Jean, S.; Brisson, D.; Gaudet, D.; Mathieu, J. Health Supervision and Anticipatory Guidance in Adult Myotonic Dystrophy Type 1. Neuromuscul. Disord. 2010, 20, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, G.; Meola, G. Myotonic Dystrophies: State of the Art of New Therapeutic Developments for the CNS. Front. Cell. Neurosci. 2017, 11, 101. [Google Scholar] [CrossRef]
- Fu, Y.H.; Pizzuti, A.; Fenwick, R.G., Jr.; King, J.; Rajnarayan, S.; Dunne, P.W.; Dubel, J.; Nasser, G.A.; Ashizawa, T.; de Jong, P. An Unstable Triplet Repeat in a Gene Related to Myotonic Muscular Dystrophy. Science 1992, 255, 1256–1258. [Google Scholar]
- Bondy-Chorney, E.; Crawford Parks, T.E.; Ravel-Chapuis, A.; Klinck, R.; Rocheleau, L.; Pelchat, M.; Chabot, B.; Jasmin, B.J.; Cote, J. Staufen1 Regulates Multiple Alternative Splicing Events either Positively Or Negatively in DM1 Indicating its Role as a Disease Modifier. PLoS Genet. 2016, 12, e1005827. [Google Scholar]
- Lin, X.; Miller, J.W.; Mankodi, A.; Kanadia, R.N.; Yuan, Y.; Moxley, R.T.; Swanson, M.S.; Thornton, C.A. Failure of MBNL1-Dependent Post-Natal Splicing Transitions in Myotonic Dystrophy. Hum. Mol. Genet. 2006, 15, 2087–2097. [Google Scholar] [CrossRef]
- Laurent, F.X.; Sureau, A.; Klein, A.F.; Trouslard, F.; Gasnier, E.; Furling, D.; Marie, J. New Function for the RNA Helicase p68/DDX5 as a Modifier of MBNL1 Activity on Expanded CUG Repeats. Nucleic Acids Res. 2012, 40, 3159–3171. [Google Scholar] [CrossRef]
- Dansithong, W.; Paul, S.; Comai, L.; Reddy, S. MBNL1 is the Primary Determinant of Focus Formation and Aberrant Insulin Receptor Splicing in DM1. J. Biol. Chem. 2005, 280, 5773–5780. [Google Scholar] [CrossRef]
- Miller, J.W.; Urbinati, C.R.; Teng-Umnuay, P.; Stenberg, M.G.; Byrne, B.J.; Thornton, C.A.; Swanson, M.S. Recruitment of Human Muscleblind Proteins to (CUG)(N) Expansions Associated with Myotonic Dystrophy. EMBO J. 2000, 19, 4439–4448. [Google Scholar]
- Philips, A.V.; Timchenko, L.T.; Cooper, T.A. Disruption of Splicing Regulated by a CUG-Binding Protein in Myotonic Dystrophy. Science 1998, 280, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, L.T.; Miller, J.W.; Timchenko, N.A.; DeVore, D.R.; Datar, K.V.; Lin, L.; Roberts, R.; Caskey, C.T.; Swanson, M.S. Identification of a (CUG)N Triplet Repeat RNA-Binding Protein and its Expression in Myotonic Dystrophy. Nucleic Acids Res. 1996, 24, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Pan, H.; Lin, M.J.; Li, Y.Y.; Wang, L.C.; Wu, Y.C.; Hsiao, K.M. Length-Dependent Toxicity of Untranslated CUG Repeats on Caenorhabditis Elegans. Biochem. Biophys. Res. Commun. 2007, 352, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lopez, A.; Monferrer, L.; Garcia-Alcover, I.; Vicente-Crespo, M.; Alvarez-Abril, M.C.; Artero, R.D. Genetic and Chemical Modifiers of a CUG Toxicity Model in Drosophila. PLoS ONE 2008, 3, e1595. [Google Scholar] [CrossRef] [PubMed]
- Mankodi, A.; Logigian, E.; Callahan, L.; McClain, C.; White, R.; Henderson, D.; Krym, M.; Thornton, C.A. Myotonic Dystrophy in Transgenic Mice Expressing an Expanded CUG Repeat. Science 2000, 289, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Cline, M.S.; Osborne, R.J.; Tuttle, D.L.; Clark, T.A.; Donohue, J.P.; Hall, M.P.; Shiue, L.; Swanson, M.S.; Thornton, C.A. Aberrant Alternative Splicing and Extracellular Matrix Gene Expression in Mouse Models of Myotonic Dystrophy. Nat. Struct. Mol. Biol. 2010, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kalsotra, A.; Xiao, X.; Ward, A.J.; Castle, J.C.; Johnson, J.M.; Burge, C.B.; Cooper, T.A. A Postnatal Switch of CELF and MBNL Proteins Reprograms Alternative Splicing in the Developing Heart. Proc. Natl. Acad. Sci. USA 2008, 105, 20333–20338. [Google Scholar] [CrossRef] [PubMed]
- Kanadia, R.N.; Johnstone, K.A.; Mankodi, A.; Lungu, C.; Thornton, C.A.; Esson, D.; Timmers, A.M.; Hauswirth, W.W.; Swanson, M.S. A Muscleblind Knockout Model for Myotonic Dystrophy. Science 2003, 302, 1978–1980. [Google Scholar] [CrossRef]
- Batra, R.; Charizanis, K.; Manchanda, M.; Mohan, A.; Li, M.; Finn, D.J.; Goodwin, M.; Zhang, C.; Sobczak, K.; Thornton, C.A. Loss of MBNL Leads to Disruption of Developmentally Regulated Alternative Polyadenylation in RNA-Mediated Disease. Mol. Cell 2014, 56, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic Functions of Long Noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef] [PubMed]
- Holdt, L.M.; Kohlmaier, A.; Teupser, D. Molecular Roles and Function of Circular RNAs in Eukaryotic Cells. Cell Mol. Life Sci. 2018, 75, 1071–1098. [Google Scholar] [CrossRef] [PubMed]
- Du, W.W.; Zhang, C.; Yang, W.; Yong, T.; Awan, F.M.; Yang, B.B. Identifying and Characterizing circRNA-Protein Interaction. Theranostics 2017, 7, 4183–4191. [Google Scholar] [CrossRef]
- Carrara, M.; Fuschi, P.; Ivan, C.; Martelli, F. Circular RNAs: Methodological Challenges and Perspectives in Cardiovascular Diseases. J. Cell. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Cardinali, B.; Falcone, G.; Martelli, F. Circular RNAs in Muscle Function and Disease. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are Abundant, Conserved, and Associated with ALU Repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M. Circular RNAs are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M. Circ-ZNF609 is a Circular RNA that can be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37. [Google Scholar] [CrossRef]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glazar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R. Circular RNAs in the Mammalian Brain are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Wei, X.; Li, H.; Yang, J.; Hao, D.; Dong, D.; Huang, Y.; Lan, X.; Plath, M.; Lei, C.; Lin, F.; et al. Circular RNA Profiling Reveals an Abundant circLMO7 that Regulates Myoblasts Differentiation and Survival by Sponging miR-378a-3p. Cell. Death Dis. 2017, 8, e3153. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA Circles Function as Efficient microRNA Sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Piwecka, M.; Glazar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P. Loss of a Mammalian Circular RNA Locus Causes miRNA Deregulation and Affects Brain Function. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA Biogenesis Competes with Pre-mRNA Splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, L.; Han, L.; Qu, X.; Yang, Y.; Zhang, Y.; He, Z.; Wang, Y.; Li, J. Circular RNAs: Regulators of Cancer-Related Signaling Pathways and Potential Diagnostic Biomarkers for Human Cancers. Theranostics 2017, 7, 3106–3117. [Google Scholar] [CrossRef]
- Wagner, S.D.; Struck, A.J.; Gupta, R.; Farnsworth, D.R.; Mahady, A.E.; Eichinger, K.; Thornton, C.A.; Wang, E.T.; Berglund, J.A. Dose-Dependent Regulation of Alternative Splicing by MBNL Proteins Reveals Biomarkers for Myotonic Dystrophy. PLoS Genet. 2016, 12, e1006316. [Google Scholar] [CrossRef]
- Wang, E.T.; Treacy, D.; Eichinger, K.; Struck, A.; Estabrook, J.; Olafson, H.; Wang, T.T.; Bhatt, K.; Westbrook, T.; Sedehizadeh, S. Transcriptome Alterations in Myotonic Dystrophy Skeletal Muscle and Heart. Hum. Mol. Genet. 2019, 28, 1312–1321. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhao, F. CIRI: An Efficient and Unbiased Algorithm for De Novo Circular RNA Identification. Genome Biol. 2015, 16, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, J.; Zhao, F. Circular RNA Identification Based on Multiple Seed Matching. Brief Bioinform. 2017. [Google Scholar] [CrossRef]
- Udd, B.; Meola, G.; Krahe, R.; Thornton, C.; Ranum, L.P.; Bassez, G.; Kress, W.; Schoser, B.; Moxley, R. 140th ENMC International Workshop: Myotonic Dystrophy DM2/PROMM and Other Myotonic Dystrophies with Guidelines on Management. Neuromuscul. Disord. 2006, 16, 403–413. [Google Scholar] [CrossRef]
- Nakamori, M.; Sobczak, K.; Puwanant, A.; Welle, S.; Eichinger, K.; Pandya, S.; Dekdebrun, J.; Heatwole, C.R.; McDermott, M.P.; Chen, T. Splicing Biomarkers of Disease Severity in Myotonic Dystrophy. Ann. Neurol. 2013, 74, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Arandel, L.; Polay Espinoza, M.; Matloka, M.; Bazinet, A.; De Dea Diniz, D.; Naouar, N.; Rau, F.; Jollet, A.; Edom-Vovard, F.; Mamchaoui, K. Immortalized Human Myotonic Dystrophy Muscle Cell Lines to Assess Therapeutic Compounds. Dis. Model. Mech. 2017, 10, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakamori, M.; Lueck, J.D.; Pouliquin, P.; Aoike, F.; Fujimura, H.; Dirksen, R.T.; Takahashi, M.P.; Dulhunty, A.F.; Sakoda, S. Altered mRNA Splicing of the Skeletal Muscle Ryanodine Receptor and Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase in Myotonic Dystrophy Type 1. Hum. Mol. Genet. 2005, 14, 2189–2200. [Google Scholar] [CrossRef]
- Savkur, R.S.; Philips, A.V.; Cooper, T.A. Aberrant Regulation of Insulin Receptor Alternative Splicing is Associated with Insulin Resistance in Myotonic Dystrophy. Nat. Genet. 2001, 29, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Ward, A.J.; Cherone, J.M.; Giudice, J.; Wang, T.T.; Treacy, D.J.; Lambert, N.J.; Freese, P.; Saxena, T.; Cooper, T.A. Antagonistic Regulation of mRNA Expression and Splicing by CELF and MBNL Proteins. Genome Res. 2015, 25, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, K.K.; Kjems, J.; Hansen, T.B. Circular RNAs: Identification, Biogenesis and Function. Biochim. Biophys. Acta 2016, 1859, 163–168. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G. Circular RNA Profiling Reveals an Abundant circHIPK3 that Regulates Cell Growth by Sponging Multiple miRNAs. Nat. Commun. 2016, 7, 11215. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Zhou, M.; Fan, F.; Yu, M.; Gao, C.; Lu, Y.; Luo, Y. Circular RNA HIPK3 Regulates Human Lens Epithelial Cells Proliferation and Apoptosis by Targeting the miR-193a/CRYAA Axis. Biochem. Biophys. Res. Commun. 2018, 503, 2277–2285. [Google Scholar] [CrossRef]
- Perbellini, R.; Greco, S.; Sarra-Ferraris, G.; Cardani, R.; Capogrossi, M.C.; Meola, G.; Martelli, F. Dysregulation and Cellular Mislocalization of Specific miRNAs in Myotonic Dystrophy Type 1. Neuromuscul. Disord. 2011, 21, 81–88. [Google Scholar] [CrossRef]
- Greco, S.; Perfetti, A.; Fasanaro, P.; Cardani, R.; Capogrossi, M.C.; Meola, G.; Martelli, F. Deregulated microRNAs in Myotonic Dystrophy Type 2. PLoS ONE 2012, 7, e39732. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yu, J.; Guo, L.; Byers, MS.; Wang, Z.; Chen, X.; Xu, H.; Nie, Q. Circular RNA circHIPK3 Promotes the Proliferation and Differentiation of Chicken Myoblast Cells by Sponging miR-30a-3p. Cells 2019, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Liu, C.; Liu, B.H.; Chen, X.; Dong, R.; Liu, X.; Zhang, Y.Y.; Liu, B.; Zhang, S.J.; Wang, J.J. Circular Noncoding RNA HIPK3 Mediates Retinal Vascular Dysfunction in Diabetes Mellitus. Circulation 2017, 136, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Renna, L.V.; Bose, F.; Iachettini, S.; Fossati, B.; Saraceno, L.; Milani, V.; Colombo, R.; Meola, G.; Cardani, R. Receptor and Post-Receptor Abnormalities Contribute to Insulin Resistance in Myotonic Dystrophy Type 1 and Type 2 Skeletal Muscle. PLoS ONE 2017, 12, e0184987. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wang, Y.; Liu, J.; Zhang, M.; Fang, X.; Chen, H.; Zhang, C. A Zfp609 Circular RNA Regulates Myoblast Differentiation by Sponging miR-194-5p. Int. J. Biol. Macromol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, M.D.; Li, C.P.; Shan, K.; Yang, H.; Wang, J.J.; Liu, B.; Li, X.M.; Yao, J.; Jiang, Q. Silencing of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 2017, 7, 2863–2877. [Google Scholar] [CrossRef]
- Maatz, H.; Jens, M.; Liss, M.; Schafer, S.; Heinig, M.; Kirchner, M.; Adami, E.; Rintisch, C.; Dauksaite, V.; Radke, M.H. RNA-Binding Protein RBM20 Represses Splicing to Orchestrate Cardiac Pre-mRNA Processing. J. Clin. Invest. 2014, 124, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Maimaiti, R.; Zhu, C.; Cai, H.; Stern, A.; Mozdziak, P.; Ge, Y.; Ford, S.P.; Nathanielsz, P.W.; Guo, W. Z-Band and M-Band Titin Splicing and Regulation by RNA Binding Motif 20 in Striated Muscles. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Stepniak-Konieczna, E.; Sobczak, K. MBNL Proteins and their Target RNAs, Interaction and Splicing Regulation. Nucleic Acids Res. 2014, 42, 10873–10887. [Google Scholar] [CrossRef]
- Konieczny, P.; Stepniak-Konieczna, E.; Taylor, K.; Sznajder, L.J.; Sobczak, K. Autoregulation of MBNL1 Function by Exon 1 Exclusion from MBNL1 Transcript. Nucleic Acids Res. 2017, 45, 1760–1775. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Hansen, T.B.; Veno, M.T.; Kjems, J. Circular RNAs in Cancer: Opportunities and Challenges in the Field. Oncogene 2018, 37, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.M.; Swanson, M.S. RNA Mis-Splicing in Disease. Nat. Rev. Genet. 2016, 17, 19–32. [Google Scholar] [CrossRef]
- Antoury, L.; Hu, N.; Balaj, L.; Das, S.; Georghiou, S.; Darras, B.; Clark, T.; Breakefield, X.O.; Wheeler, T.M. Analysis of Extracellular mRNA in Human Urine Reveals Splice Variant Biomarkers of Muscular Dystrophies. Nat. Commun. 2018, 9, 3906. [Google Scholar] [CrossRef]
- Valaperta, R.; Sansone, V.; Lombardi, F.; Verdelli, C.; Colombo, A.; Valisi, M.; Brigonzi, E.; Costa, E.; Meola, G. Identification and Characterization of DM1 Patients by a New Diagnostic Certified Assay: Neuromuscular and Cardiac Assessments. Biomed. Res. Int. 2013, 2013, 958510. [Google Scholar] [CrossRef]
- Mathieu, J.; Boivin, H.; Meunier, D.; Gaudreault, M.; Begin, P. Assessment of a Disease-Specific Muscular Impairment Rating Scale in Myotonic Dystrophy. Neurology 2001, 56, 336–340. [Google Scholar] [CrossRef]
- Voellenkle, C.; van Rooij, J.; Cappuzzello, C.; Greco, S.; Arcelli, D.; Di Vito, L.; Melillo, G.; Rigolini, R.; Costa, E.; Crea, F. MicroRNA Signatures in Peripheral Blood Mononuclear Cells of Chronic Heart Failure Patients. Physiol. Genomics 2010, 42, 420–426. [Google Scholar] [CrossRef]
- Perfetti, A.; Greco, S.; Bugiardini, E.; Cardani, R.; Gaia, P.; Gaetano, C.; Meola, G.; Martelli, F. Plasma microRNAs as Biomarkers for Myotonic Dystrophy Type 1. Neuromuscul. Disord. 2014, 24, 509–515. [Google Scholar] [CrossRef]
- Perfetti, A.; Greco, S.; Cardani, R.; Fossati, B.; Cuomo, G.; Valaperta, R.; Ambrogi, F.; Cortese, A.; Botta, A.; Mignarri, A. Validation of Plasma microRNAs as Biomarkers for Myotonic Dystrophy Type 1. Sci. Rep. 2016, 6, 38174. [Google Scholar] [CrossRef] [PubMed]
- Dubowitz, V. A practical Approach. In Muscle Biopsy: A Practical Approach; Dubowitz, V., Ed.; Bailliere Tindall: London, UK, 1985; pp. 19–40. [Google Scholar]
- Greco, S.; De Simone, M.; Colussi, C.; Zaccagnini, G.; Fasanaro, P.; Pescatori, M.; Cardani, R.; Perbellini, R.; Isaia, E.; Sale, P. Common Micro-RNA Signature in Skeletal Muscle Damage and Regeneration Induced by Duchenne Muscular Dystrophy and Acute Ischemia. FASEB J. 2009, 23, 3335–3346. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | DM1 (n = 30) | CTRL (n = 29) |
|---|---|---|
| Age at sampling (average ± S.E.) | 42.2 ± 2.5 | 41.1 ± 1.1 |
| Sex (male/female) | 15/15 | 20/9 |
| MRC megascore (average ± S.E.) | 121.1 ± 1.3 | 130 ± 0.0 |
| Myotonia (% of patients) | 80 | 0 |
| Glucose (normal values: 70–110 mg/dL) | 88.4 ± 4.3 | 89.0 ± 3.8 |
| Cholesterol (normal values: <200 mg/dL) | 212.1 ± 10.4 | 230.8 ± 24.3 |
| CK (normal values: male <190 mg/dL; female <125 mg/dL) | Male: 266.8 ± 36.4 | N.A. |
| Female: 221.9 ± 40.2 | N.A. | |
| Arrhythmia (% of patients) | 17.6 | 0 |
| Cataract (% of patients) | 17.6 | 0 |
| ECG-QRS duration (normal values: 60–110 ms) | 105.2 ± 6.7 | N.A. |
| Number of CTG repeats (range) | 494.1 ± 43.9 (90–1100) | N.A. |
| Stage of disease (range 1–5) (% of patients at each stage) | Stage 1:0 | N.R. |
| Stage 2:25.9 | ||
| Stage 3:37.0 | ||
| Stage 4:33.3 | ||
| Stage 5:3.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voellenkle, C.; Perfetti, A.; Carrara, M.; Fuschi, P.; Renna, L.V.; Longo, M.; Sain, S.B.; Cardani, R.; Valaperta, R.; Silvestri, G.; et al. Dysregulation of Circular RNAs in Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2019, 20, 1938. https://doi.org/10.3390/ijms20081938
Voellenkle C, Perfetti A, Carrara M, Fuschi P, Renna LV, Longo M, Sain SB, Cardani R, Valaperta R, Silvestri G, et al. Dysregulation of Circular RNAs in Myotonic Dystrophy Type 1. International Journal of Molecular Sciences. 2019; 20(8):1938. https://doi.org/10.3390/ijms20081938
Chicago/Turabian StyleVoellenkle, Christine, Alessandra Perfetti, Matteo Carrara, Paola Fuschi, Laura Valentina Renna, Marialucia Longo, Simona Baghai Sain, Rosanna Cardani, Rea Valaperta, Gabriella Silvestri, and et al. 2019. "Dysregulation of Circular RNAs in Myotonic Dystrophy Type 1" International Journal of Molecular Sciences 20, no. 8: 1938. https://doi.org/10.3390/ijms20081938