UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca2+ Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries
Abstract
:1. Introduction
2. Results
2.1. Effects of OMC on Arterial Contractility
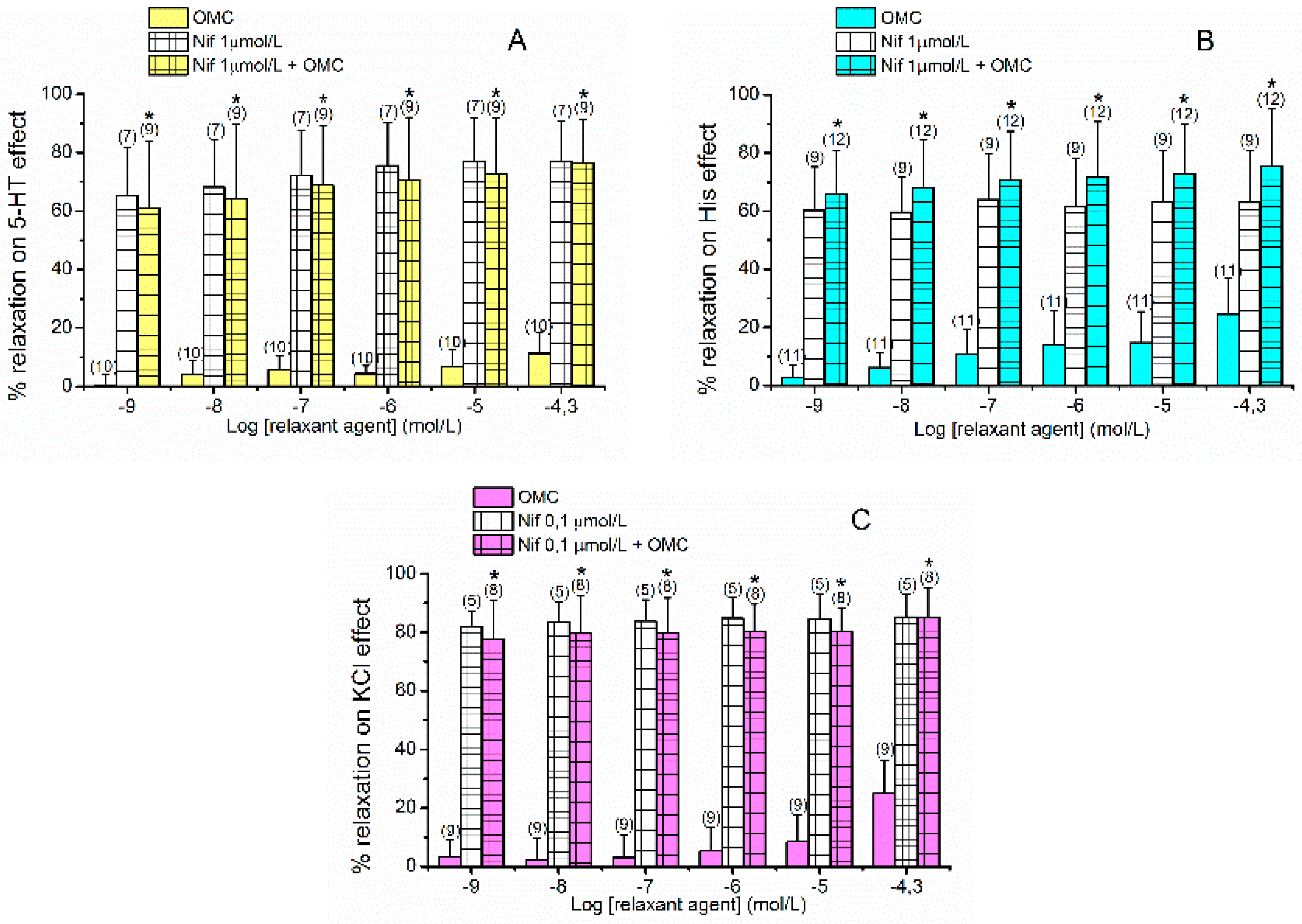
2.2. Effects of L-Type VOCC on OMC-Induced Vasorelaxation
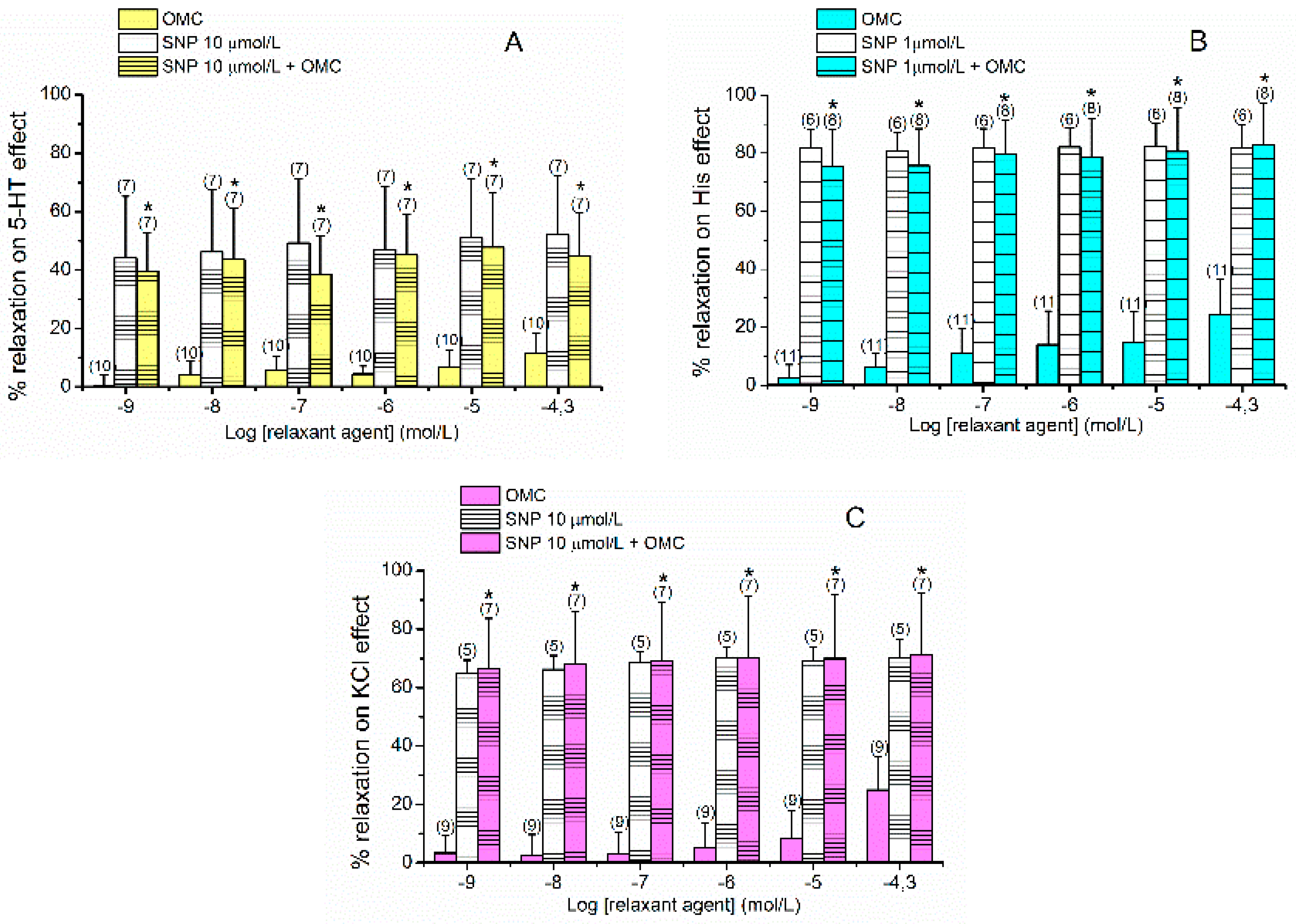
2.3. Effects of cGMP on OMC-Induced Vasorelaxation
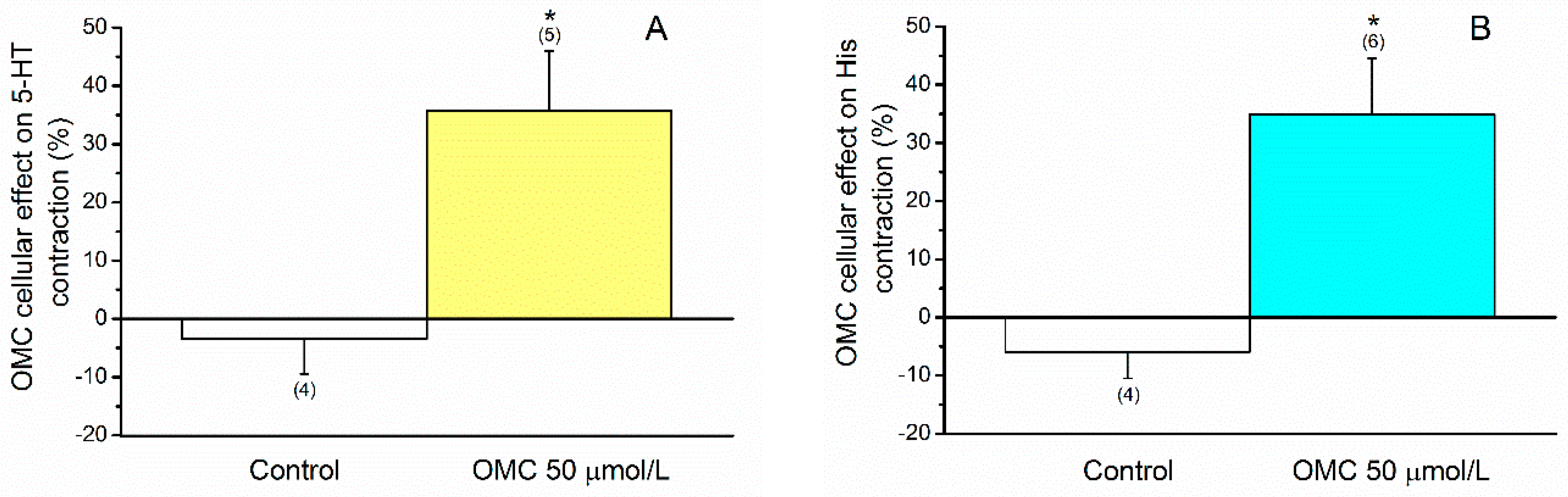
2.4. Effects of OMC on Cellular Contractility
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Arterial Contractility Experiments
4.3. Cell Dissociation and Culture
4.4. Cellular Contractility Experiments
4.5. Drugs and Chemicals
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| cAMP | Cyclic Adenosine Monophosphate |
| CDV | Cardiovascular Diseases |
| cGMP | cyclic Guanosine Monophosphate |
| CHUCB | “Centro Hospitalar Universitário da Cova da Beira E.P.E.” |
| CICS-UBI | Health Sciences Research Centre, University of Beira Interior |
| EDCs | Endocrine Disruptor Compounds |
| His | Histamine |
| HUA | Human Umbilical Artery |
| HUASMC | Human Umbilical Artery Smooth Muscle Cells |
| KCl | Potassium Chloride |
| L-Type VOCC | L-Type voltage-operated Ca2+ channels |
| MOA | Mode of Action |
| Nif | Nifedipine |
| OMC | Octylmethoxycinnamate |
| PCPs | Personal Care Products |
| PCSA | Planar Cell Surface Area |
| PKG | Protein Kinase G |
| PSS | Sterile Physiological Saline Solution |
| sGC | soluble Guanylyl Cyclase |
| SMC | Smooth Muscle Cells |
| SNP | Sodium Nitroprusside |
| UC | Umbilical Cord |
| US | United State |
| UV | Ultraviolet |
References
- Maipas, S.; Nicolopoulou-Stamati, P. Sun lotion chemicals as endocrine disruptors. Hormones 2015, 14, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, L.; Wu, S.; Lu, L.; Xu, Y.; Zhu, Y.; Guo, M.; Zhuang, S. Recent Advances on Endocrine Disrupting Effects of UV Filters. Int. J. Environ. Res. Public Health 2016, 13, 782. [Google Scholar] [CrossRef]
- Lee, D.H. Evidence of the Possible Harm of Endocrine-Disrupting Chemicals in Humans: Ongoing Debates and Key Issues. Endocrinol. Metab. 2018, 33, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, M.; Sciascia, F.; Cifelli, R.; Malatesta, L.; Bruni, P.; Croce, F. Analytical methods for the endocrine disruptor compounds determination in environmental water samples. J. Chromatogr. A 2016, 1434, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Vela-Soria, F.; Gallardo-Torres, M.E.; Ballesteros, O.; Diaz, C.; Perez, J.; Navalon, A.; Fernandez, M.F.; Olea, N. Assessment of parabens and ultraviolet filters in human placenta tissue by ultrasound-assisted extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Feiteiro, J.; Cairrao, E. How is the human umbilical artery regulated? J. Obstet. Gynaecol. Res. 2018, 44, 1193–1201. [Google Scholar] [CrossRef]
- Cairrao, E.; Santos-Silva, A.J.; Alvarez, E.; Correia, I.; Verde, I. Isolation and culture of human umbilical artery smooth muscle cells expressing functional calcium channels. In Vitro Cell. Dev. Biol. Anim. 2009, 45, 175–184. [Google Scholar] [CrossRef]
- Tapia-Orozco, N.; Santiago-Toledo, G.; Barron, V.; Espinosa-Garcia, A.M.; Garcia-Garcia, J.A.; Garcia-Arrazola, R. Environmental epigenomics: Current approaches to assess epigenetic effects of endocrine disrupting compounds (EDC’s) on human health. Environ. Toxicol. Pharmacol. 2017, 51, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Sharma, A.; Banyiova, K.; Babica, P.; El Yamani, N.; Collins, A.R.; Cupr, P. Different DNA damage response of cis and trans isomers of commonly used UV filter after the exposure on adult human liver stem cells and human lymphoblastoid cells. Sci. Total Environ. 2017, 593–594, 18–26. [Google Scholar] [CrossRef]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef]
- Lorigo, M.; Mariana, M.; Cairrao, E. Photoprotection of ultraviolet-B filters: Updated review of endocrine disrupting properties. Steroids 2018, 131, 46–58. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Jarry, H.; Christoffel, J.; Rimoldi, G.; Wuttke, W. Comparison of effects of estradiol (E2) with those of octylmethoxycinnamate (OMC) and 4-methylbenzylidene camphor (4MBC)—2 filters of UV light—On several uterine, vaginal and bone parameters. Toxicol. Appl. Pharmacol. 2006, 210, 246–254. [Google Scholar] [CrossRef]
- Klammer, H.; Schlecht, C.; Wuttke, W.; Jarry, H. Multi-organic risk assessment of estrogenic properties of octyl-methoxycinnamate in vivo A 5-day sub-acute pharmacodynamic study with ovariectomized rats. Toxicology 2005, 215, 90–96. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Christoffel, J.; Rimoldi, G.; Jarry, H.; Wuttke, W. Comparison of effects of estradiol with those of octylmethoxycinnamate and 4-methylbenzylidene camphor on fat tissue, lipids and pituitary hormones. Toxicol. Appl. Pharmacol. 2006, 214, 1–7. [Google Scholar] [CrossRef]
- Inui, M.; Adachi, T.; Takenaka, S.; Inui, H.; Nakazawa, M.; Ueda, M.; Watanabe, H.; Mori, C.; Iguchi, T.; Miyatake, K. Effect of UV screens and preservatives on vitellogenin and choriogenin production in male medaka (Oryzias latipes). Toxicology 2003, 194, 43–50. [Google Scholar] [CrossRef]
- Schreurs, R.; Lanser, P.; Seinen, W.; van der Burg, B. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch. Toxicol. 2002, 76, 257–261. [Google Scholar] [CrossRef]
- Gomez, E.; Pillon, A.; Fenet, H.; Rosain, D.; Duchesne, M.J.; Nicolas, J.C.; Balaguer, P.; Casellas, C. Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV screens, and musks. J. Toxicol. Environ. Health. Part A 2005, 68, 239–251. [Google Scholar] [CrossRef]
- Heneweer, M.; Muusse, M.; van den Berg, M.; Sanderson, J.T. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol. Appl. Pharmacol. 2005, 208, 170–177. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Fent, K. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat. Toxicol. 2006, 79, 305–324. [Google Scholar] [CrossRef] [PubMed]
- Axelstad, M.; Boberg, J.; Hougaard, K.S.; Christiansen, S.; Jacobsen, P.R.; Mandrup, K.R.; Nellemann, C.; Lund, S.P.; Hass, U. Effects of pre- and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol. 2011, 250, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Isling, L.K.; Boberg, J.; Jacobsen, P.R.; Mandrup, K.R.; Axelstad, M.; Christiansen, S.; Vinggaard, A.M.; Taxvig, C.; Kortenkamp, A.; Hass, U. Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters. Reproduction 2014, 147, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Schmutzler, C.; Hamann, I.; Hofmann, P.J.; Kovacs, G.; Stemmler, L.; Mentrup, B.; Schomburg, L.; Ambrugger, P.; Gruters, A.; Seidlova-Wuttke, D.; et al. Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology 2004, 205, 95–102. [Google Scholar] [CrossRef]
- Schmutzler, C.; Gotthardt, I.; Hofmann, P.J.; Radovic, B.; Kovacs, G.; Stemmler, L.; Nobis, I.; Bacinski, A.; Mentrup, B.; Ambrugger, P.; et al. Endocrine disruptors and the thyroid gland—A combined in vitro and in vivo analysis of potential new biomarkers. Environ. Health Perspect. 2007, 115 (Suppl. 1), 77–83. [Google Scholar] [CrossRef]
- Klammer, H.; Schlecht, C.; Wuttke, W.; Schmutzler, C.; Gotthardt, I.; Kohrle, J.; Jarry, H. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary-thyroid function in rats. Toxicology 2007, 238, 192–199. [Google Scholar] [CrossRef]
- Quintaneiro, C.; Teixeira, B.; Benede, J.L.; Chisvert, A.; Soares, A.; Monteiro, M.S. Toxicity effects of the organic UV-filter 4-Methylbenzylidene camphor in zebrafish embryos. Chemosphere 2019, 218, 273–281. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Shen, J.; Bao, X. Parental transfer of ethylhexyl methoxy cinnamate and induced biochemical responses in zebra fi sh. Aquat. Toxicol. 2019, 206, 24–32. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide (ZnO) and titanium dioxide (TiO2). Photodermatol. Photoimmunol. Photomed. 2018. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ma, C.Y.; Yiu, S.K.F.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ. Pollut. 2018, 245, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, S.; Oggier, D.M.; Fent, K. Global gene expression profile induced by the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) in zebrafish (Danio rerio). Environ. Pollut. 2011, 159, 3086–3096. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.W.; Quan, A.; Lao, T.T.; Man, R.Y. Efficacy of different vasodilators on human umbilical arterial smooth muscle under normal and reduced oxygen conditions. Early Hum. Dev. 2006, 82, 457–462. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Santos-Silva, A.J.; Verde, I. Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn-Schmiedebergs Arch. Pharmacol. 2008, 376, 375–383. [Google Scholar] [CrossRef]
- Tufan, H.; Ayan-Polat, B.; Tecder-Unal, M.; Polat, G.; Kayhan, Z.; Ogus, E. Contractile responses of the human umbilical artery to KCl and serotonin in Ca-free medium and the effects of levcromakalim. Life Sci. 2003, 72, 1321–1329. [Google Scholar] [CrossRef]
- Quan, A.; Leung, S.W.; Lao, T.T.; Man, R.Y. 5-hydroxytryptamine and thromboxane A2 as physiologic mediators of human umbilical artery closure. J. Soc. Gynecol. Investig. 2003, 10, 490–495. [Google Scholar] [CrossRef]
- Lovren, F.; Li, X.F.; Lytton, J.; Triggle, C. Functional characterization and m-RNA expression of 5-HT receptors mediating contraction in human umbilical artery. Br. J. Pharm. 1999, 127, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Santos-Silva, A.J.; Cairrao, E.; Morgado, M.; Alvarez, E.; Verde, I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur. J. Pharmacol. 2008, 582, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Hawley, J.; Rubin, P.C.; Hill, S.J. Distribution of receptors mediating phosphoinositide hydrolysis in cultured human umbilical artery smooth muscle and endothelial cells. Biochem. Pharmacol. 1995, 49, 1005–1011. [Google Scholar] [CrossRef]
- Santos-Silva, A.J.; Cairrao, E.; Marques, B.; Verde, I. Regulation of human umbilical artery contractility by different serotonin and histamine receptors. Reprod. Sci. 2009, 16, 1175–1185. [Google Scholar] [CrossRef]
- Schneider, A.; Riess, P.; Elbers, A.; Neugebauer, E.; Schaefer, U. Polyclonal anti-histamine H2 receptor antibodies detect differential expression of H2 receptor protein in primary vascular cell types. Inflamm. Res. 2004, 53, 223–229. [Google Scholar] [CrossRef]
- Alvarez, E.; Cairrao, E.; Morgado, M.; Morais, C.; Verde, I. Testosterone and cholesterol vasodilation of rat aorta involves L-type calcium channel inhibition. Adv. Pharmacol. Sci. 2010, 2010, 534184. [Google Scholar] [CrossRef]
- Saldanha, P.A.; Cairrao, E.; Maia, C.J.; Verde, I. Long- and short-term effects of androgens in human umbilical artery smooth muscle. Clin. Exp. Pharmacol. Physiol. 2013, 40, 181–189. [Google Scholar] [CrossRef]
- Smiley, D.A.; Khalil, R.A. Estrogenic Compounds, Estrogen Receptors and Vascular Cell Signaling in the Aging Blood Vessels. Curr. Med. Chem. 2009, 16, 1863–1887. [Google Scholar] [CrossRef]
- Miller, V.M. Sex-based differences in vascular function. Womens Health 2010, 6, 737–752. [Google Scholar] [CrossRef]
- Crews, J.K.; Khalil, R.A. Antagonistic effects of 17β-Estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1034–1040. [Google Scholar] [CrossRef]
- Cairrao, E.; Alvarez, E.; Carvas, J.M.; Santos-Silva, A.J.; Verde, I. Non-genomic vasorelaxant effects of 17beta-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacol. Sin. 2012, 33, 615–624. [Google Scholar] [CrossRef]
- Okabe, K.; Inoue, Y.; Soeda, H. Estradiol inhibits Ca2+ and K+ channels in smooth muscle cells from pregnant rat myometrium. Eur. J. Pharmacol. 1999, 376, 101–108. [Google Scholar] [CrossRef]
- Fausett, M.B.; Belfort, M.A.; Nanda, R.; Saade, G.R.; Vedernikov, Y. The effects of sex steroids on human umbilical artery and vein. J. Soc. Gynecol. Investig. 1999, 6, 27–31. [Google Scholar] [CrossRef]
- Silva de Sá, M.F.; Meirelles, R.S. Vasodilating Effect of Estrogen on the Human Umbilical Artery. Gynecol. Obstet. Investig. 1977, 8, 307–313. [Google Scholar] [CrossRef]
- Orshal, J.M.; Khalil, R.A. Gender, sex hormones, and vascular tone. American journal of physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R233–R249. [Google Scholar] [CrossRef]
- Hill, B.J.; Gebre, S.; Schlicker, B.; Jordan, R.; Necessary, S. Nongenomic inhibition of coronary constriction by 17ss-estradiol, 2-hydroxyestradiol, and 2-methoxyestradiol. Can. J. Physiol. Pharmacol. 2010, 88, 147–152. [Google Scholar] [CrossRef]
- Nakajima, T.; Kitazawa, T.; Hamada, E.; Hazama, H.; Omata, M.; Kurachi, Y. 17beta-Estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur. J. Pharmacol. 1995, 294, 625–635. [Google Scholar] [CrossRef]
- Zhang, M.; Benishin, C.G.; Pang, P.K. Rapid inhibition of the contraction of rat tail artery by progesterone is mediated by inhibition of calcium currents. J. Pharm. Pharmacol. 2002, 54, 1667–1674. [Google Scholar] [CrossRef]
- Ruehlmann, D.O.; Steinert, J.R.; Valverde, M.A.; Jacob, R.; Mann, G.E. Environmental estrogenic pollutants induce acute vascular relaxation by inhibiting L-type Ca2+ channels in smooth muscle cells. FASEB J. 1998, 12, 613–619. [Google Scholar] [CrossRef]
- Lovren, F.; Triggle, C. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br. J. Pharm. 2000, 131, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Mugge, A.; Riedel, M.; Barton, M.; Kuhn, M.; Lichtlen, P.R. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc. Res. 1993, 27, 1939–1942. [Google Scholar] [CrossRef]
- Cairrao, E.; Santos-Silva, A.J.; Verde, I. PKG is involved in testosterone-induced vasorelaxation of human umbilical artery. Eur. J. Pharmacol. 2010, 640, 94–101. [Google Scholar] [CrossRef]
- Martin de Llano, J.J.; Fuertes, G.; Garcia-Vicent, C.; Torro, I.; Fayos, J.L.; Lurbe, E. Procedure to consistently obtain endothelial and smooth muscle cell cultures from umbilical cord vessels. Transl. Res. 2007, 149, 1–9. [Google Scholar] [CrossRef]
- Mariana, M.; Feiteiro, J.; Cairrao, E.; Verde, I. Mifepristone is a Vasodilator Due to the Inhibition of Smooth Muscle Cells L-Type Ca2+ Channels. Reprod. Sci. 2016, 23, 723–730. [Google Scholar] [CrossRef]


 /Red arrows—inhibition;
/Red arrows—inhibition;  /Green arrows—Stimulation; Ca2+—calcium; cGMP—cyclic guanosine monophosphate; L-Type VOCC—L-type voltage-operated Ca2+ channels; OMC–octylmethoxycinnamate; PKG—protein kinase G.
/Green arrows—Stimulation; Ca2+—calcium; cGMP—cyclic guanosine monophosphate; L-Type VOCC—L-type voltage-operated Ca2+ channels; OMC–octylmethoxycinnamate; PKG—protein kinase G.
 /Red arrows—inhibition;
/Red arrows—inhibition;  /Green arrows—Stimulation; Ca2+—calcium; cGMP—cyclic guanosine monophosphate; L-Type VOCC—L-type voltage-operated Ca2+ channels; OMC–octylmethoxycinnamate; PKG—protein kinase G.
/Green arrows—Stimulation; Ca2+—calcium; cGMP—cyclic guanosine monophosphate; L-Type VOCC—L-type voltage-operated Ca2+ channels; OMC–octylmethoxycinnamate; PKG—protein kinase G.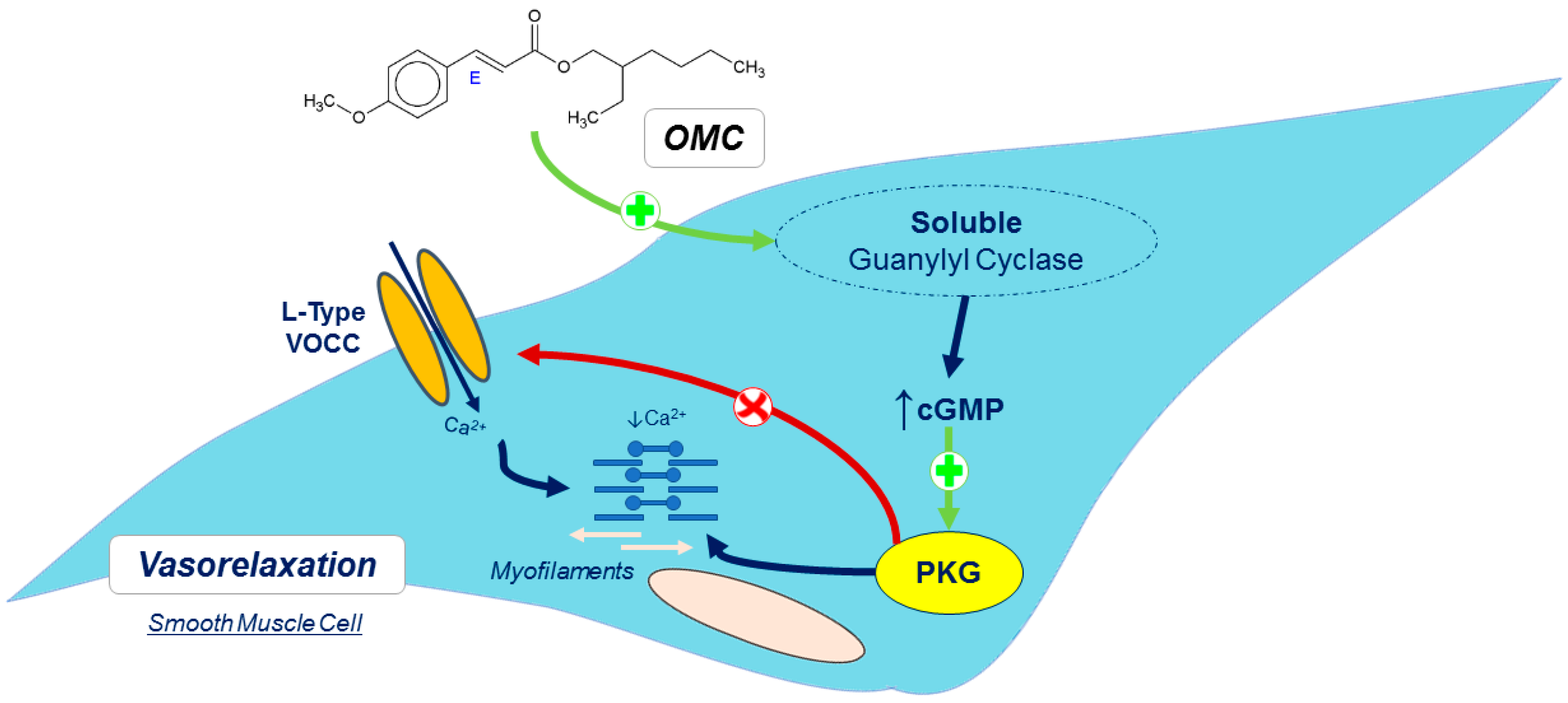
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorigo, M.; Quintaneiro, C.; Lemos, M.C.; Martinez-de-Oliveira, J.; Breitenfeld, L.; Cairrao, E. UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca2+ Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries. Int. J. Mol. Sci. 2019, 20, 1376. https://doi.org/10.3390/ijms20061376
Lorigo M, Quintaneiro C, Lemos MC, Martinez-de-Oliveira J, Breitenfeld L, Cairrao E. UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca2+ Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries. International Journal of Molecular Sciences. 2019; 20(6):1376. https://doi.org/10.3390/ijms20061376
Chicago/Turabian StyleLorigo, Margarida, Carla Quintaneiro, Manuel C. Lemos, José Martinez-de-Oliveira, Luiza Breitenfeld, and Elisa Cairrao. 2019. "UV-B Filter Octylmethoxycinnamate Induces Vasorelaxation by Ca2+ Channel Inhibition and Guanylyl Cyclase Activation in Human Umbilical Arteries" International Journal of Molecular Sciences 20, no. 6: 1376. https://doi.org/10.3390/ijms20061376






