Nitric Oxide Reverses the Position of the Heart during Embryonic Development
Abstract
:1. Introduction
2. Results
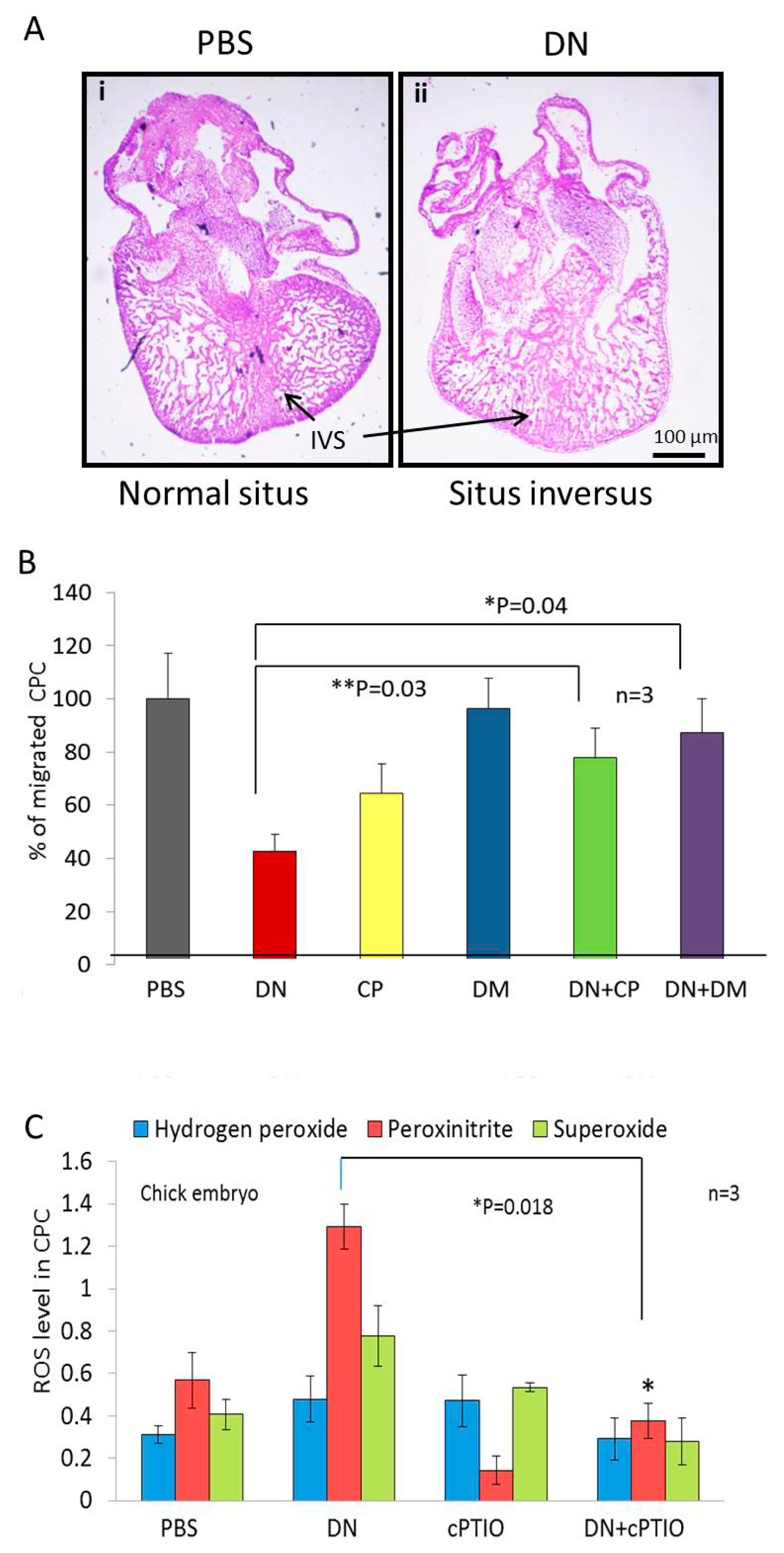
2.1. Situs Inversus Occurs with Nitric Oxide Overexpression in Chick Embryos
2.2. NO Causes Situs Inversus by Altering Cardiac Progenitor Cells Migration from the Blood Islands
2.3. Arrhythmia and Abnormal Vascular Patterning and Function Occurs in Embryos with Situs Inversus
2.4. NO Quencher cPTIO and BMP4 Inhibitor DM Restores Cell Migration
2.5. BMP4 Inhibitors Rescue Zebrafish Embryos from Situs Inversus
2.6. NO Causes Situs Inversus through BMP4-SMAD1 Mediated Pathway
3. Discussion
4. Materials and Methods
4.1. Chicken Embryos and Drug Treatment
4.2. Zebrafish (Danio Rerio) Embryos and Drug Treatment
4.3. Real Time Imaging of Heart Looping
4.4. Free Radical Measurement in Cardiac Progenitor Cells
4.5. Semi-Quantitative Reverse Transcriptase PCR
4.6. Immunohistochemistry
4.7. Western Blot Analyses
4.8. Whole Mount Antibody Staining
4.9. Cell Migration Assay Using Boyden Chamber
4.10. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Lacza, Z.; Pankotai, E.; Csordás, A.; Gero, D.; Kiss, L.; Horváth, E.M.; Kollai, M.; Busija, D.W.; Szabó, C.; Szabó, C. Mitochondrial NO and reactive nitrogen species production: Does mtNOS exist? Nitric Oxide 2006, 14, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A.; Furchgott, R. International Union of Pharmacology Nomenclature in Nitric Oxide Research. Pharmacol. Rev. 1997, 49, 137–142. [Google Scholar] [PubMed]
- Nicotera, P.; Bernassola, F.; Melino, G. Nitric oxide (NO), a signaling molecule with a killer soul. Cell Death Differ. 1999, 6, 931–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoport, R.M.; Murad, F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ. Res. 1983, 52, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 2, 1057–1058. [Google Scholar] [CrossRef]
- Garg, U.C.; Hassid, A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J. Clin. Investig. 1989, 83, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, S.; Arnold, W.; Mittal, C.; Murad, F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J. Cycl. Nucleotide Res. 1977, 3, 23–35. [Google Scholar]
- Carey, W.A.; Weaver, A.L.; Mara, K.C.; Clark, R.H. Inhaled Nitric Oxide in Extremely Premature Neonates with Respiratory Distress Syndrome. Pediatrics 2018, 141, e2017310. [Google Scholar] [CrossRef] [PubMed]
- Humpl, T.; Reyes, J.T.; Erickson, S.; Armano, R.; Holtby, H.; Adatia, I. Sildenafil therapy for neonatal and childhood pulmonary hypertensive vascular disease. Cardiol. Young 2011, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, J.; Rudolph, F.; Cseresnyes, Z.; Priller, F.; Otten, C.; Renz, M.; Schaefer, L.; Abdelilah-Seyfried, S. Unilateral Dampening of Bmp Activity by Nodal Generates Cardiac Left-Right Asymmetry. Dev. Cell 2013, 24, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, W.; Sukowati, E.W.; Sheng, G. On Hemangioblasts in Chicken. PLoS ONE 2007, 2, e1228. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; El-Remessy, A.; Gu, X.; Brooks, S.S.; Hamed, M.S.; Huang, P.; Caldwell, R.B. Normal vascular development in mice deficient in endothelial NO synthase: Possible role of neuronal NO synthase. Mol. Vis. 2003, 9, 549–558. [Google Scholar] [PubMed]
- Lancaster, J.R., Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl. Acad. Sci. USA 1994, 91, 8137–8141. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.; Burggren, W. Hypoxic incubation creates differential morphological effects during specific developmental critical windows in the embryo of the chicken (Gallus gallus). Respir. Physiol. Neurobiol. 2005, 145, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wanstall, J.C.; Jeffery, T.K.; Gambino, A.; Lovren, F.; Triggle, C.R. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide andnitroxyl ion. Br. J. Pharmacol. 2001, 134, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Pipili-Synetos, E.; Kritikou, S.; Papadimitriou, E.; Athanassiadou, A.; Flordellis, C.; Maragoudakis, M.E. Nitric oxide synthase expression, enzyme activity and NO production during angiogenesis in the chick chorioallantoic membrane. Br. J. Pharmacol. 2000, 129, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, M.; Pagan, S.; Roberts, D.J.; Cooke, J.; Kuehn, M.R.; Tabin, C.J. Left/Right Patterning Signals and the Independent Regulation of Different Aspects ofSitusin the Chick Embryo. Dev. Biol. 1997, 189, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.N.; Van Eeden, F.J.; Warren, K.S.; Chin, A.; Nusslein-Volhard, C.; Haffter, P.; Fishman, M.C. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development 1997, 124, 4373–4382. [Google Scholar] [PubMed]
- Schilling, T.F.; Concordet, J.-P.; Ingham, P.W. Regulation of Left–Right Asymmetries in the Zebrafish by Shh and BMP4. Dev. Biol. 1999, 210, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsoro-Burq, A.; le Douarin, N. Left-right asymmetry in BMP4 signalling pathway during chick gastrulation. Mech. Dev. 2000, 97, 105–108. [Google Scholar] [CrossRef]
- Brueckner, M. Heterotaxia, Congenital Heart Disease and Primary Ciliary Dyskinesia. Circulation 2007, 115, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Katritsis, D.G.; Papagiannis, J. Anatomically left-sided septal slow pathway ablation in dextrocardia and situs inversus totalis. Europace 2008, 10, 1004–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, I.; Schlueter, J.; Abu-Issa, R.; Brand, T.; Männer, J. Morphological and molecular left–right asymmetries in the development of the proepicardium: A comparative analysis on mouse and chick embryos. Dev. Dyn. 2007, 236, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Lee, G.T.; Lee, J.-H.; Kim, W.J.; Kim, I.Y. Bone morphogenetic protein-6 induces the expression of inducible nitric oxide synthase in macrophages. Immunology 2009, 128, e758–e765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimalawansa, S.J. Rationale for Using Nitric Oxide Donor Therapy for Prevention of Bone Loss and Treatment of Osteoporosis in Humans. Ann. N. Y. Acad. Sci. 2007, 1117, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, N.A.; Tabin, C.J. Wnt signaling during development of the gastrointestinal tract. Dev. Biol. 2003, 259, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Haffen, K.; Kedinger, M.; Simon-Assmann, P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ros, M.A.; Dahn, R.D.; Fernandez-Teran, M.; Rashka, K.; Caruccio, N.C.; Hasso, S.M.; Bitgood, J.J.; Lancman, J.J.; Fallon, J.F. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development 2003, 130, 527–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gros, J.; Feistel, K.; Viebahn, C.; Blum, M.; Tabin, C.J. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science 2009, 324, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.; Brown, N.A.; Wolpert, L. Development of left/right handedness in the chick heart. Development 1992, 115, 1071–1078. [Google Scholar] [PubMed]
- Zimmerman, L.B.; de Jesús-Escobar, J.M.; Harland, R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 1996, 86, 599–606. [Google Scholar] [CrossRef]
- Ueno, S.; Weidinger, G.; Osugi, T.; Kohn, A.D.; Golob, J.L.; Pabon, L.; Reinecke, H.; Moon, R.T.; Murry, C.E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 9685–9690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Kasiviswanathan, D.; Sundaresan, L.; Kathirvel, P.; Veeriah, V.; Dutta, P.; Sankaranarayanan, K.; Gupta, R.; Chatterjee, S. Harvesting clues from genome wide transcriptome analysis for exploring thalidomide mediated anomalies in eye development of chick embryo: Nitric oxide rectifies the thalidomide mediated anomalies by swinging back the system to normal transcriptome pattern. Biochimie 2016, 121, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, A.; Bader, D. In vitro analysis of cardiac progenitor cell differentiation. Dev. Biol. 1990, 139, 197–209. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Veeriah, V.; Priya, M.K.; Rajendran, S.; Saran, U.; Sinha, S.; Nagarajan, S.; Pradeep, T.; Chatterjee, S. Nitric oxide rescues thalidomide mediated teratogenicity. Sci. Rep. 2012, 2, 679. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, H.A.; Sundaresan, L.; Ghosh, A.; Kathirvel, P.; Thilak, A.; Katakia, Y.T.; Sankaranarayanan, K.; Chatterjee, S. Thalidomide remodels developing heart in chick embryo: Discovery of a thalidomide mediated hematoma in heart muscle. Naunyn Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Psychoyos, D.; Stern, C.D. Fates and migratory routes of primitive streak cells in the chick embryo. Development 1996, 122, 1523–1534. [Google Scholar] [PubMed]
- Park, Y.J.; Lee, T.; Ha, J.; Jung, I.M.; Chung, J.K.; Kim, S.J. Effect of nicotine on human umbilical vein endothelial cells (HUVECs) migration and angiogenesis. Vasc. Pharmacol. 2008, 49, 32–36. [Google Scholar] [CrossRef] [PubMed]




| 4th Day Avg. bpm | 5th Day Avg. bpm | 6th Day Avg. bpm | |
|---|---|---|---|
| PBS | 222 | 236 | 261 |
| Normal | 244 | 262 | 274 |
| Situs inversus | 265 | 280 | 297 |
| Internal Organs | Heart Left (L), Right (R) | Liver | Lungs | Gut | ||||
|---|---|---|---|---|---|---|---|---|
| PBS | YES | NO | NO | YES | NO | YES | NO | YES |
| DEAN | 144/200 embryos | 56/200 embryos | NO | YES | NO | YES | NO | YES |
| PBS | YES | NO | NA | NA | ND | ND | ND | ND |
| DEAN | 90/100 fishes | 10/100 fishes | NA | NA | ND | ND | ND | ND |
| Heart Contraction Area (Pixels) | Heart Relaxation Area (Pixels) | Vascular Bed Area (Pixels) | |
|---|---|---|---|
| PBS | 542.6 | 422.3 | 21.93 |
| SNP | 312 | 255 | 36.5 |
| DN100 | 655 | 456 | 17.7 |
| DN500 | 577 | 321 | 35.19 |
| DN1000 | 677 | 492 | 15.88 |
| Gene of Interest | Product Size | Annealing Temperature | Primer Sequence |
|---|---|---|---|
| BMP4 | 911 bp | 56.2 °C | Sense-5′ CTACTATGCCAAGTCCTGCT 3′ Anti-Sense-5′ TCGCTGAAATCCACATAGA 3′ |
| Shh | 508 bp | 56.4 °C | Sense- 5′ GTAATTGGATTCAATGGTCG 3′ Anti-Sense-5′GGCCAGCATTCCGTACTT 3′ |
| Pitx2 | 762 bp | 61 °C | Sense-5′ATGAGTTGCATGAAGGACCC 3′ Anti-Sense-5′TGCTCACACGGGCCTGTCCA 3′ |
| Nogg | 672 bp | 59 °C | Sense-5′ATGGATCATTCCCAGTGCCTTGT3′ Anti-Sense-5′CTAGCAGGAGCACTTGCACTC3′ |
| Nodal | 180 bp | 60 °C | Sense-5′CTGGATCGTCTACCCCAAGA3′ Anti-Sense-5′ATGGAGAGGGGACTCATCCT3′ |
| Wnt3a | 485 bp | 60 °C | Sense-5′GTTCTGCAGCGAAGTGGTG3′ Anti-Sense-5′GAGTGTCACAGCCGCAGATG3′ |
| β-Actin | 165 bp | 60.5 °C | Sense- 5′ TCTGACTGACCGCGTTACTC 3′ Anti-Sense-5′ CCATCACACCCTGATGTCTG 3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siamwala, J.H.; Kumar, P.; Veeriah, V.; Muley, A.; Rajendran, S.; Konikkat, S.; Majumder, S.; Mani, K.P.; Chatterjee, S. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. Int. J. Mol. Sci. 2019, 20, 1157. https://doi.org/10.3390/ijms20051157
Siamwala JH, Kumar P, Veeriah V, Muley A, Rajendran S, Konikkat S, Majumder S, Mani KP, Chatterjee S. Nitric Oxide Reverses the Position of the Heart during Embryonic Development. International Journal of Molecular Sciences. 2019; 20(5):1157. https://doi.org/10.3390/ijms20051157
Chicago/Turabian StyleSiamwala, Jamila H, Pavitra Kumar, Vimal Veeriah, Ajit Muley, Saranya Rajendran, Salini Konikkat, Syamantak Majumder, Krishna Priya Mani, and Suvro Chatterjee. 2019. "Nitric Oxide Reverses the Position of the Heart during Embryonic Development" International Journal of Molecular Sciences 20, no. 5: 1157. https://doi.org/10.3390/ijms20051157





