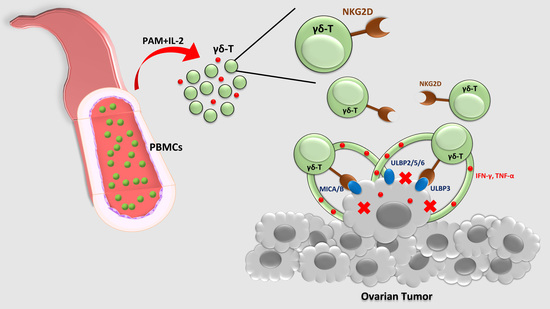
Ex Vivo Expanded Human Vγ9Vδ2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth
Abstract
:1. Introduction
2. Results
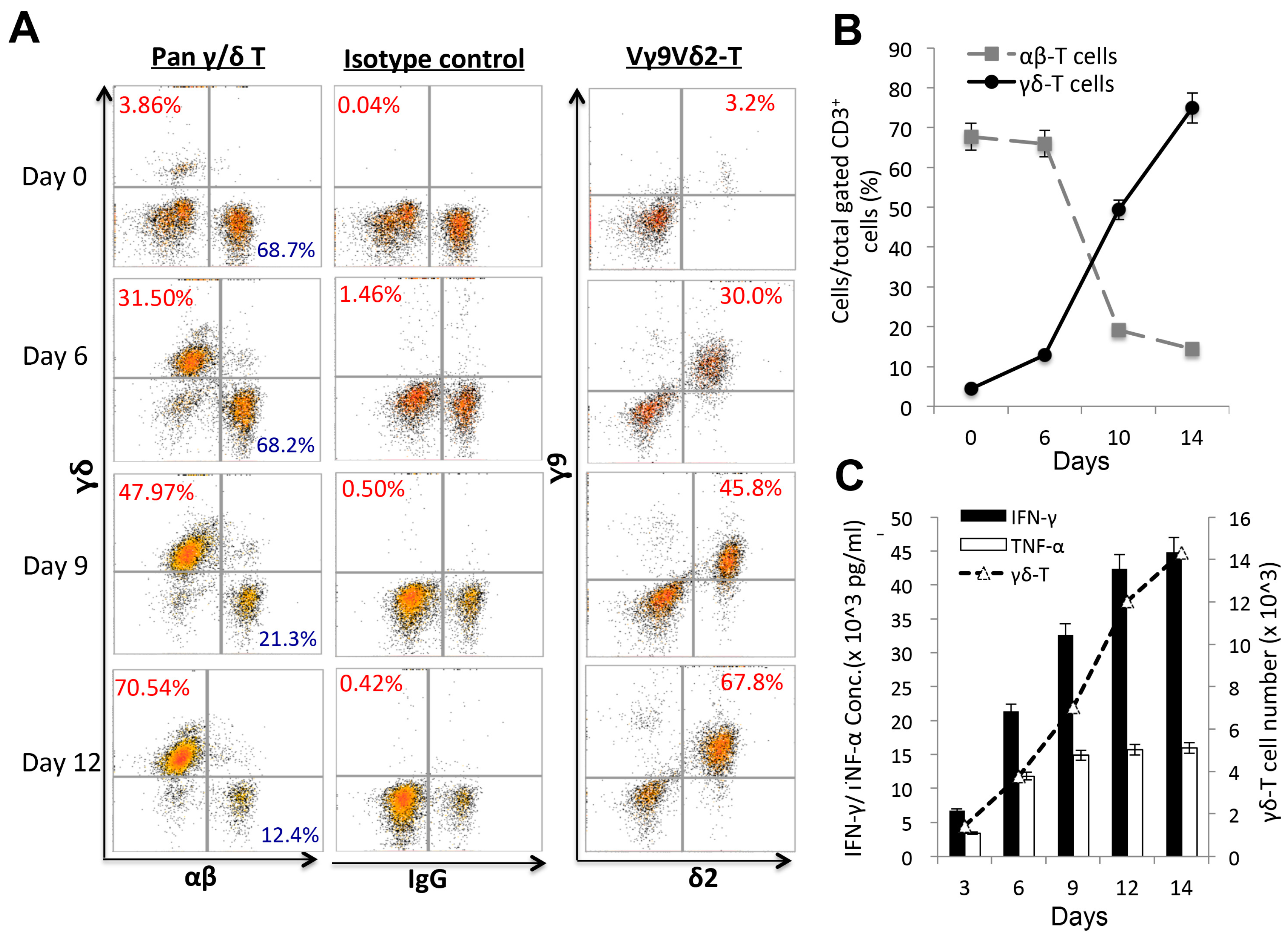
2.1. Ex vivo Expansion of Human Vγ9Vδ2 T-Cells from Healthy Donor PBMCs Depends on Co-Stimulation
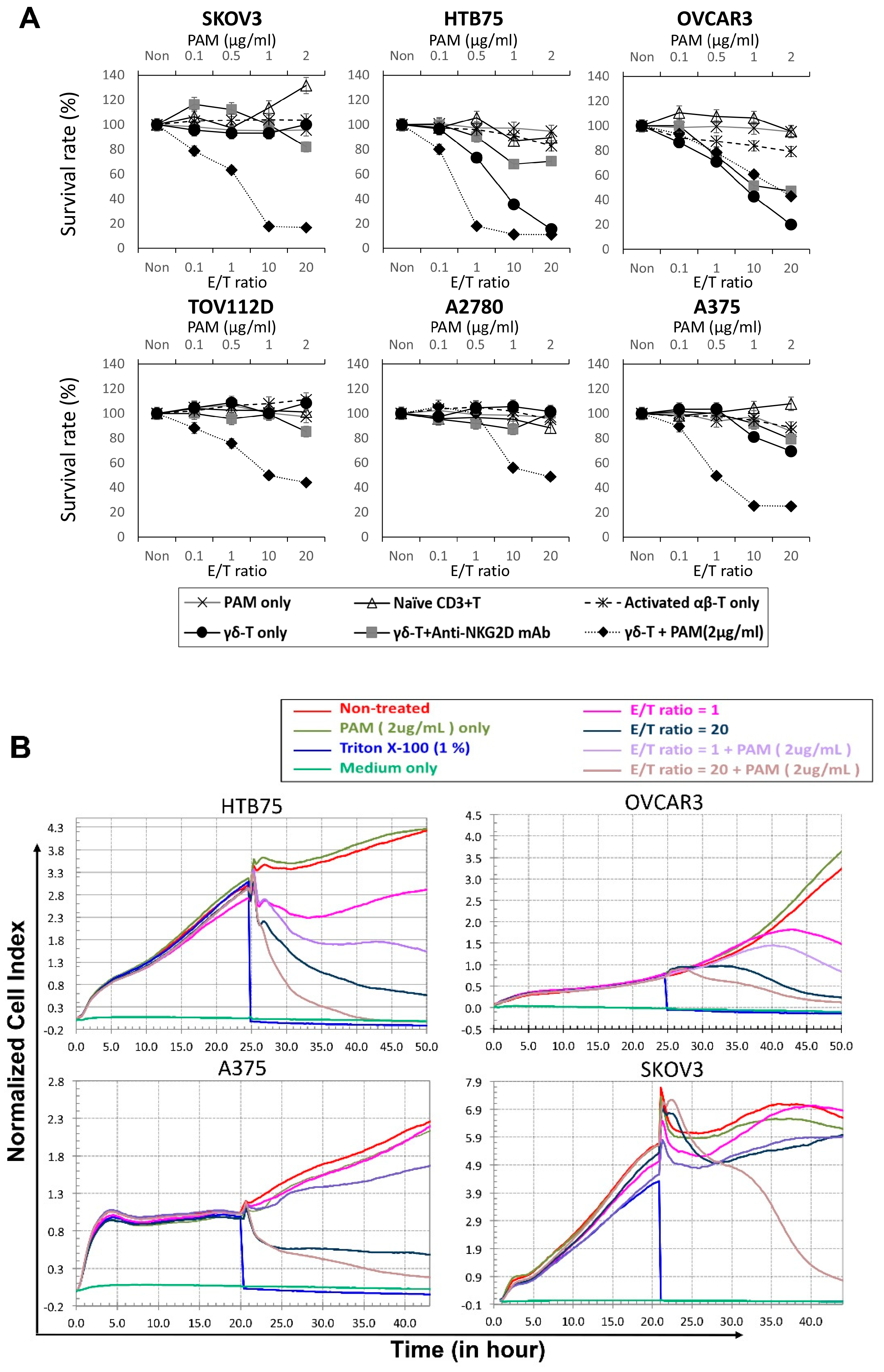
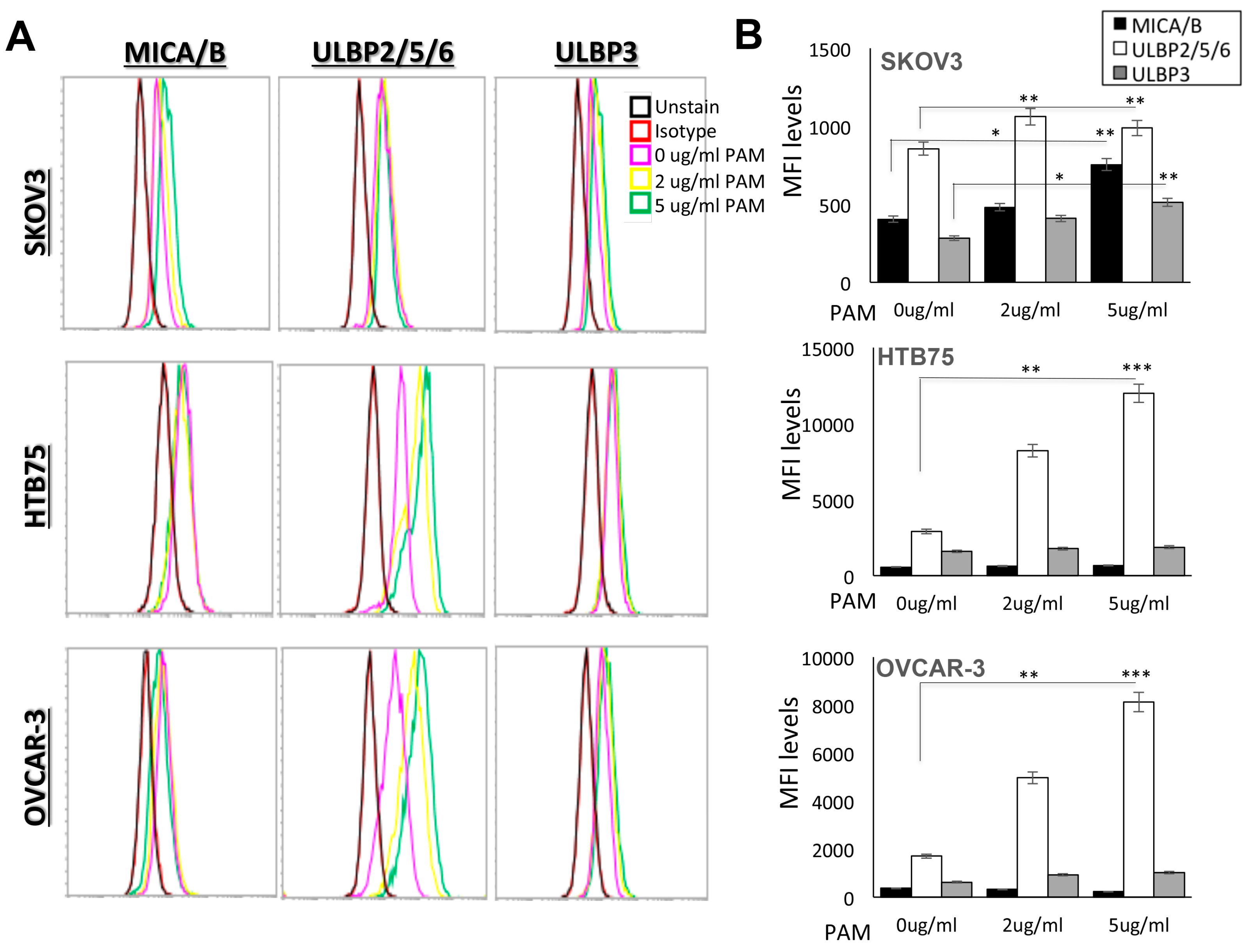
2.2. In vitro Cytotoxic Assays to Examine the Destruction of Epithelial Ovarian Tumors by Expanded Vγ9Vδ2 T-Cells Alone
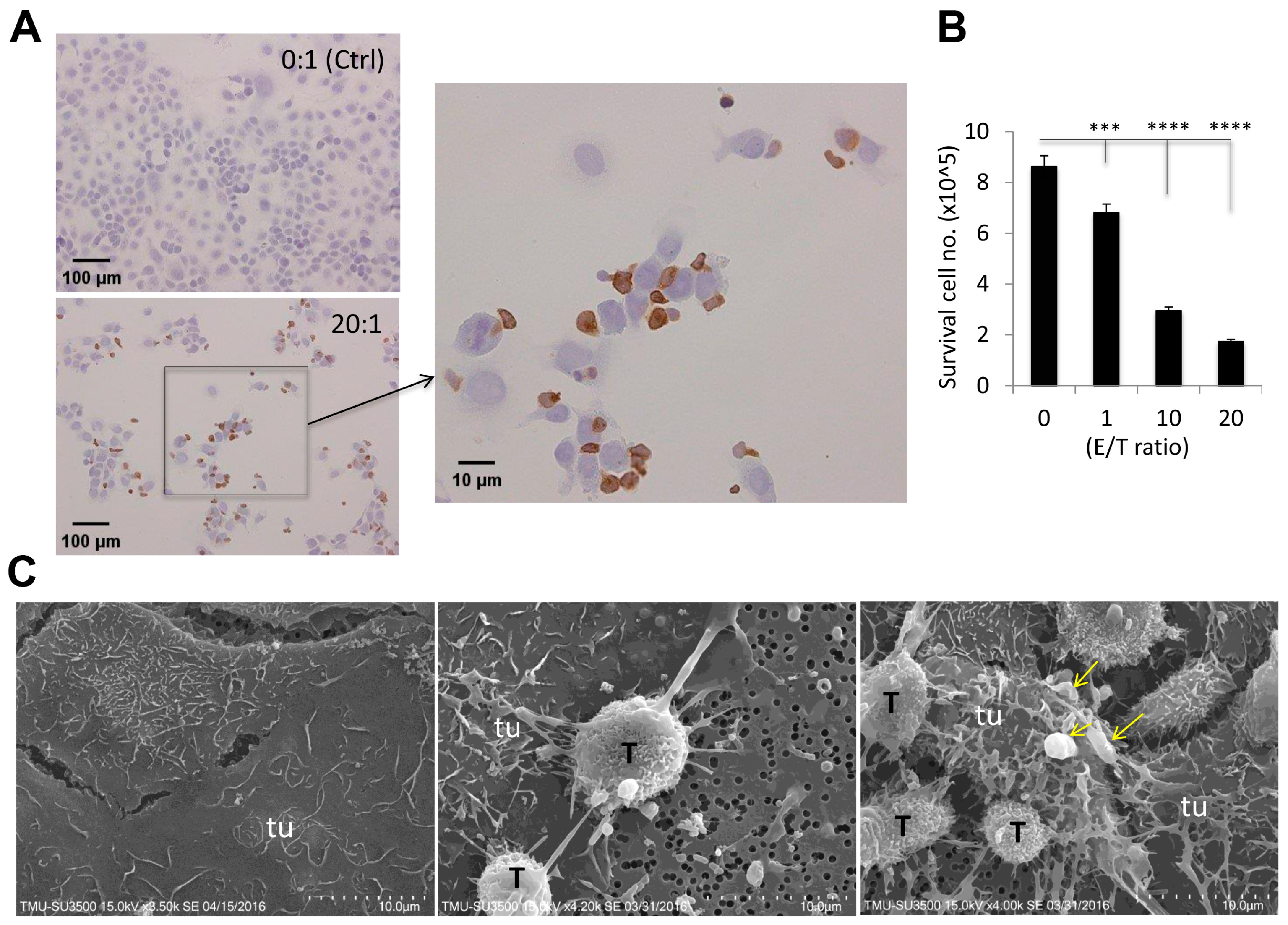
2.3. Visualization of γδ-T-Cell Contact Cytotoxicity Against Ovarian Cancer OVCAR3 Cells
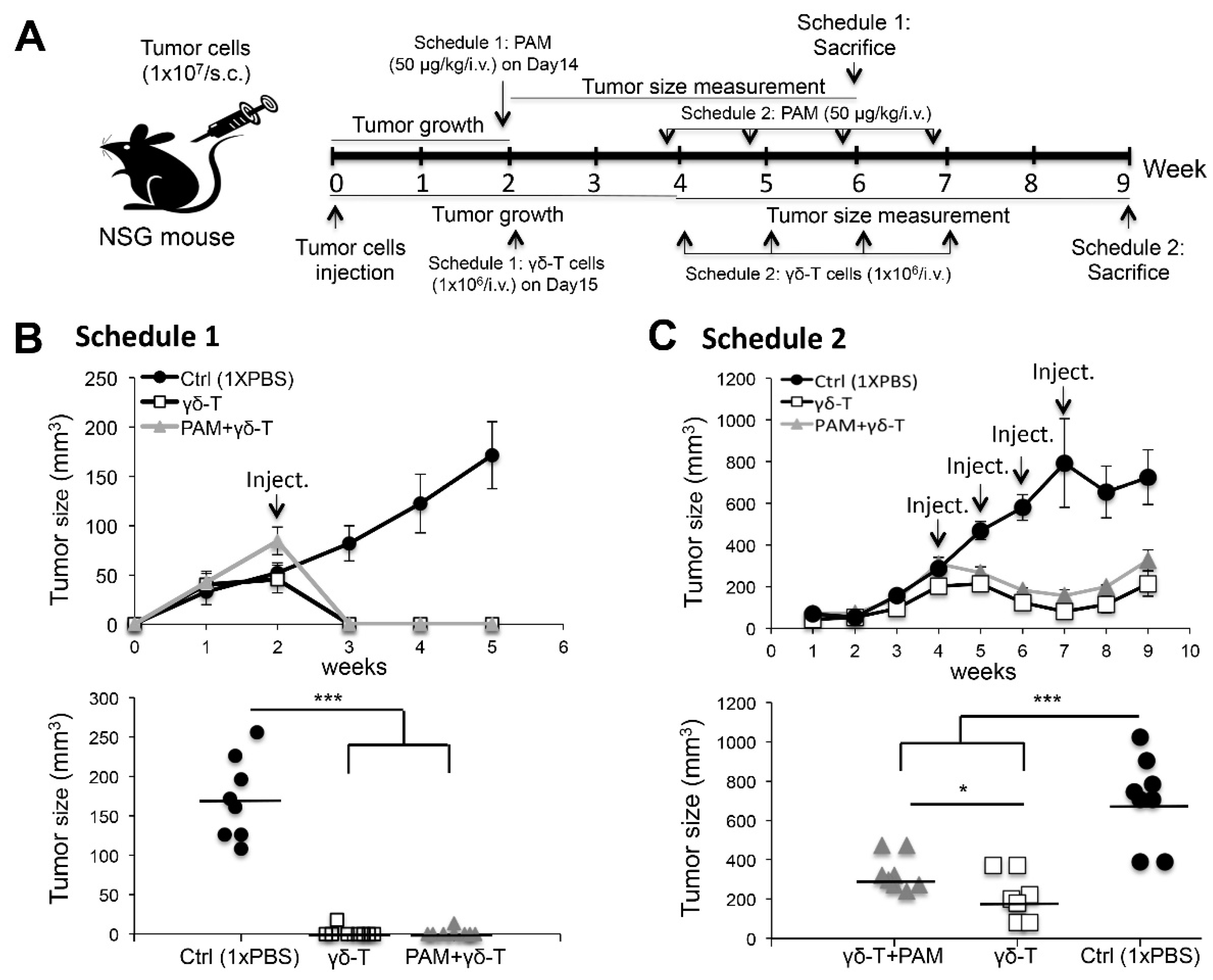
2.4. Adoptive Transfer of Expanded Vγ9Vδ2 T-Cells Completely Eliminated Smaller-Sized Tumors whereas Modulate the Growth of Large subcutaneous (s.c.) Xenograft Ovarian Tumors in NSG Mice
3. Discussion
4. Materials and Methods
4.1. Ethics, Consent, and Permission
4.2. Expansion of Human Vγ9Vδ2 T-Cells
4.3. Flow Cytometry
4.4. Tumor Cell Culture
4.5. Cytotoxicity Assays
4.6. Real-Time Cell Monitoring Using the xCELLigence System
4.7. In Vivo Studies
4.8. Visualization of Cell Attachment
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotsopoulos, I.C.; Papanikolaou, A.; Lambropoulos, A.F.; Papazisis, K.T.; Tsolakidis, D.; Touplikioti, P.; Tarlatzis, B.C. Serous ovarian cancer signaling pathways. Int. J. Gynecol. Cancer 2014, 24, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Girardi, M. Immunosurveillance and immunoregulation by gammadelta t cells. J. Investig. Dermatol. 2006, 126, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; De Libero, G. Human t cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Bauer, E.; Wilhelm, M. Gamma/delta t-cell stimulation by pamidronate. N. Engl. J. Med. 1999, 340, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.V.; d’Orey, F.; Cardoso, B.A.; Lanca, T.; Grosso, A.R.; deBarros, A.; Martins, L.R.; Barata, J.T.; Silva-Santos, B. Highly active microbial phosphoantigen induces rapid yet sustained mek/erk- and pi-3k/akt-mediated signal transduction in anti-tumor human gammadelta t-cells. PLoS ONE 2009, 4, e5657. [Google Scholar] [CrossRef] [PubMed]
- Wakita, D.; Sumida, K.; Iwakura, Y.; Nishikawa, H.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Nishimura, T. Tumor-infiltrating il-17-producing gammadelta t cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 2010, 40, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Anguille, S.; Van Tendeloo, V.F.; Lion, E. Empowering gamma delta t cells with antitumor immunity by dendritic cell-based immunotherapy. Oncoimmunology 2015, 4, e1021538. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of gammadelta t cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hannani, D.; Ma, Y.; Yamazaki, T.; Dechanet-Merville, J.; Kroemer, G.; Zitvogel, L. Harnessing gammadelta t cells in anticancer immunotherapy. Trends Immunol. 2012, 33, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, H.; Hu, H.; He, H.; Wang, Z.; Xu, Y.; Chen, H.; Cao, W.; Zhang, S.; Cui, L.; et al. Gammadelta t cells recognize tumor cells via cdr3delta region. Mol. Immunol. 2007, 44, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, D.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, D.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta t cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Deniger, D.C.; Maiti, S.N.; Mi, T.; Switzer, K.C.; Ramachandran, V.; Hurton, L.V.; Ang, S.; Olivares, S.; Rabinovich, B.A.; Huls, M.H.; et al. Activating and propagating polyclonal gamma delta t cells with broad specificity for malignancies. Clin. Cancer Res. 2014, 20, 5708–5719. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Goodwin, N.; Ishikawa, F.; Hosur, V.; Lyons, B.L.; Greiner, D.L. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb. Protocols 2014, 2014, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Brehm, M.A.; Garcia-Martinez, J.V.; Greiner, D.L. Humanized mice for immune system investigation: Progress, promise and challenges. Nat. Rev. Immunol. 2012, 12, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Osaka, Y.; Nakazawa, H.; Uchiyama, T.; Minato, N.; Toma, H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta t cells against advanced renal cell carcinoma: A pilot study. Cancer Immunol. Immunother. 2007, 56, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; Scotet, E. Human vgamma9vdelta2 t cells: Promising new leads for immunotherapy of infections and tumors. Curr. Opin. Immunol. 2006, 18, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, V.; Smetak, M.; Kimmel, B.; Weigang-Koehler, K.; Goebeler, M.; Birkmann, J.; Becker, J.; Schmidt-Wolf, I.G.; Einsele, H.; Wilhelm, M. Tumor-promoting versus tumor-antagonizing roles of gammadelta t cells in cancer immunotherapy: Results from a prospective phase i/ii trial. J. Immunother. 2012, 35, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Anglesio, M.S.; Wiegand, K.C.; Melnyk, N.; Chow, C.; Salamanca, C.; Prentice, L.M.; Senz, J.; Yang, W.; Spillman, M.A.; Cochrane, D.R.; et al. Type-specific cell line models for type-specific ovarian cancer research. PLoS ONE 2013, 8, e72162. [Google Scholar] [CrossRef]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xcelligence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Cao, X.; Shores, E.W.; Hu-Li, J.; Anver, M.R.; Kelsall, B.L.; Russell, S.M.; Drago, J.; Noguchi, M.; Grinberg, A.; Bloom, E.T.; et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995, 2, 223–238. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. Nod/scid/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Kekre, N.; Antin, J.H. Hematopoietic stem cell transplantation donor sources in the 21st century: Choosing the ideal donor when a perfect match does not exist. Blood 2014, 124, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human vdelta2(+) t-cell compartment comprises distinct innate-like vgamma9(+) and adaptive vgamma9(-) subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Cosman, D.; Mullberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. Ulbps, novel mhc class i-related molecules, bind to cmv glycoprotein ul16 and stimulate nk cytotoxicity through the nkg2d receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Nakajima, N.I.; Niimi, A.; Isono, M.; Oike, T.; Sato, H.; Nakano, T.; Shibata, A. Inhibition of the hdac/suv39/g9a pathway restores the expression of DNA damage-dependent major histocompatibility complex class i-related chain a and b in cancer cells. Oncol. Rep. 2017, 38, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Dansako, H.; Imai, H.; Ueda, Y.; Satoh, S.; Wakita, T.; Kato, N. Ulbp1 is induced by hepatitis c virus infection and is the target of the nk cell-mediated innate immune response in human hepatocytes. FEBS Open Bio. 2018, 8, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Neri, P.; Mesuraca, M.; Fulciniti, M.T.; Otsuki, T.; Pende, D.; Groh, V.; Spies, T.; Pollio, G.; Cosman, D.; et al. Hla class i, nkg2d, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood 2005, 105, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Tomogane, M.; Miyashita, M.; Ukimura, O.; Ashihara, E. Low dose gemcitabine increases the cytotoxicity of human vgamma9vdelta2 t cells in bladder cancer cells in vitro and in an orthotopic xenograft model. Oncoimmunology 2018, 7, e1424671. [Google Scholar] [CrossRef] [PubMed]
- Salih, H.R.; Rammensee, H.G.; Steinle, A. Cutting edge: Down-regulation of mica on human tumors by proteolytic shedding. J. Immunol. 2002, 169, 4098–4102. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011, 18, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Toyoda, F.; Imai, S.; Tanigawa, H.; Kumagai, K.; Matsuura, H.; Matsusue, Y. Lidocaine induces rock-dependent membrane blebbing and subsequent cell death in rabbit articular chondrocytes. J. Orthop. Res. 2016, 34, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Froelich, C.J.; Orth, K.; Turbov, J.; Seth, P.; Gottlieb, R.; Babior, B.; Shah, G.M.; Bleackley, R.C.; Dixit, V.M.; Hanna, W. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme b, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J. Biol. Chem. 1996, 271, 29073–29079. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.; Shi, L.; Feske, S.; Massol, R.; Navarro, F.; Kirchhausen, T.; Lieberman, J. Perforin triggers a plasma membrane-repair response that facilitates ctl induction of apoptosis. Immunity 2005, 23, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Sakuta, K.; Noguchi, A.; Ariyoshi, N.; Sato, K.; Sato, S.; Sato, K.; Hosoi, A.; Nakajima, J.; Yoshida, Y.; et al. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta t cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008, 10, 842–856. [Google Scholar] [CrossRef] [PubMed]
- Schilbach, K.E.; Geiselhart, A.; Wessels, J.T.; Niethammer, D.; Handgretinger, R. Human gammadelta t lymphocytes exert natural and il-2-induced cytotoxicity to neuroblastoma cells. J. Immunother. 2000, 23, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Lamb, L.S., Jr.; Musk, P.; Ye, Z.; van Rhee, F.; Geier, S.S.; Tong, J.J.; King, K.M.; Henslee-Downey, P.J. Human gammadelta(+) t lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transplant. 2001, 27, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Minato, N.; Tanabe, K. Phase i/ii study of adoptive transfer of gammadelta t cells in combination with zoledronic acid and il-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, T.; Robard, M.; Leger, A.; Catros, V.; Bonneville, M.; Scotet, E. Repeated systemic administrations of both aminobisphosphonates and human vgamma9vdelta2 t cells efficiently control tumor development in vivo. J. Immunol. 2013, 191, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Benzaid, I.; Monkkonen, H.; Stresing, V.; Bonnelye, E.; Green, J.; Monkkonen, J.; Touraine, J.L.; Clezardin, P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote vgamma9vdelta2 t-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011, 71, 4562–4572. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.F.; Su, Y.Z.; Tseng, Y.H.; Wang, S.Y.; Wang, H.M.; Chueh, P.J. Flavokawain b, a novel chalcone from alpinia pricei hayata with potent apoptotic activity: Involvement of ros and gadd153 upstream of mitochondria-dependent apoptosis in hct116 cells. Free Radic. Biol. Med. 2010, 49, 214–226. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.L.; Miao, C.H.; Liao, Y.J.; Chen, Y.J.; Yeh, C.Y.; Liu, C.L. Ex Vivo Expanded Human Vγ9Vδ2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2019, 20, 1139. https://doi.org/10.3390/ijms20051139
Mao TL, Miao CH, Liao YJ, Chen YJ, Yeh CY, Liu CL. Ex Vivo Expanded Human Vγ9Vδ2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth. International Journal of Molecular Sciences. 2019; 20(5):1139. https://doi.org/10.3390/ijms20051139
Chicago/Turabian StyleMao, Tsui Lien, Carol H. Miao, Yi Jen Liao, Ying Jen Chen, Chia Yu Yeh, and Chao Lien Liu. 2019. "Ex Vivo Expanded Human Vγ9Vδ2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth" International Journal of Molecular Sciences 20, no. 5: 1139. https://doi.org/10.3390/ijms20051139
APA StyleMao, T. L., Miao, C. H., Liao, Y. J., Chen, Y. J., Yeh, C. Y., & Liu, C. L. (2019). Ex Vivo Expanded Human Vγ9Vδ2 T-Cells Can Suppress Epithelial Ovarian Cancer Cell Growth. International Journal of Molecular Sciences, 20(5), 1139. https://doi.org/10.3390/ijms20051139