Osseointegration of Alkali-Modified NANOZR Implants: An In Vivo Study
Abstract
:1. Introduction
2. Results
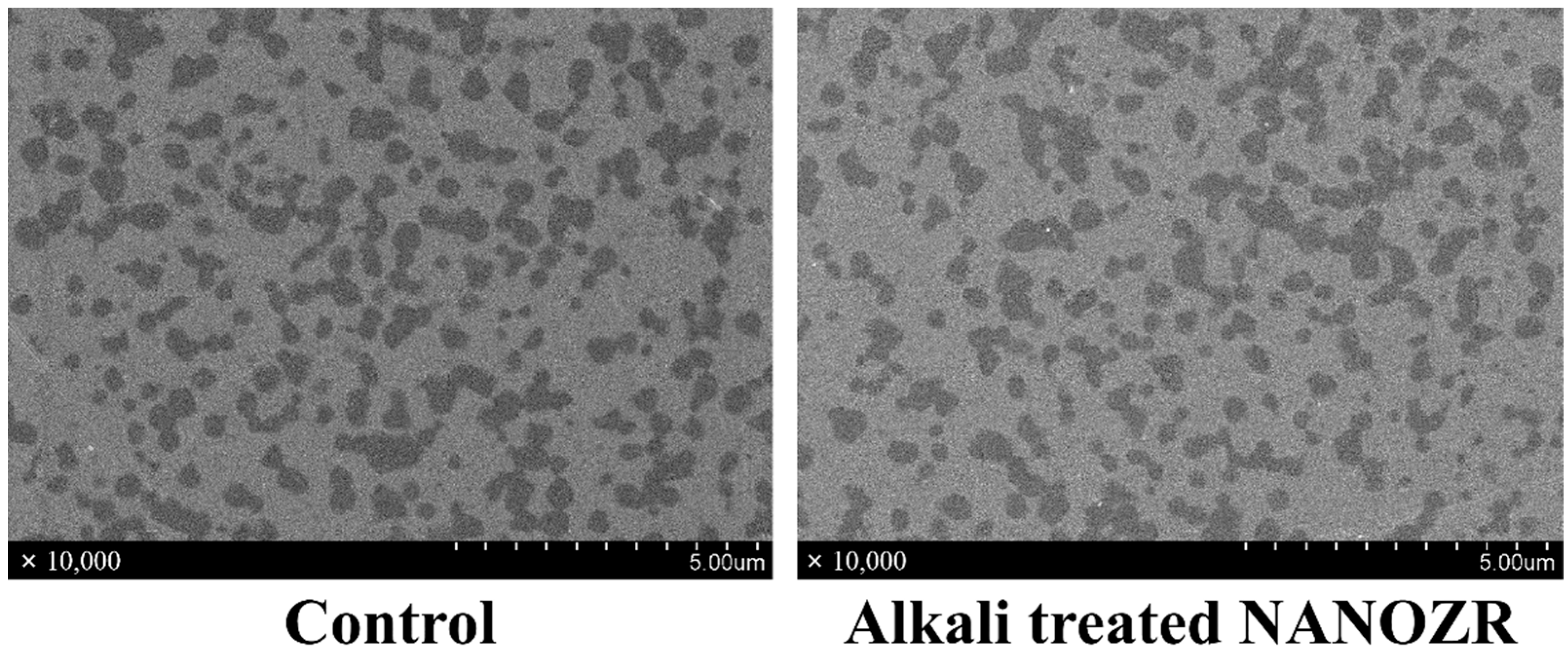
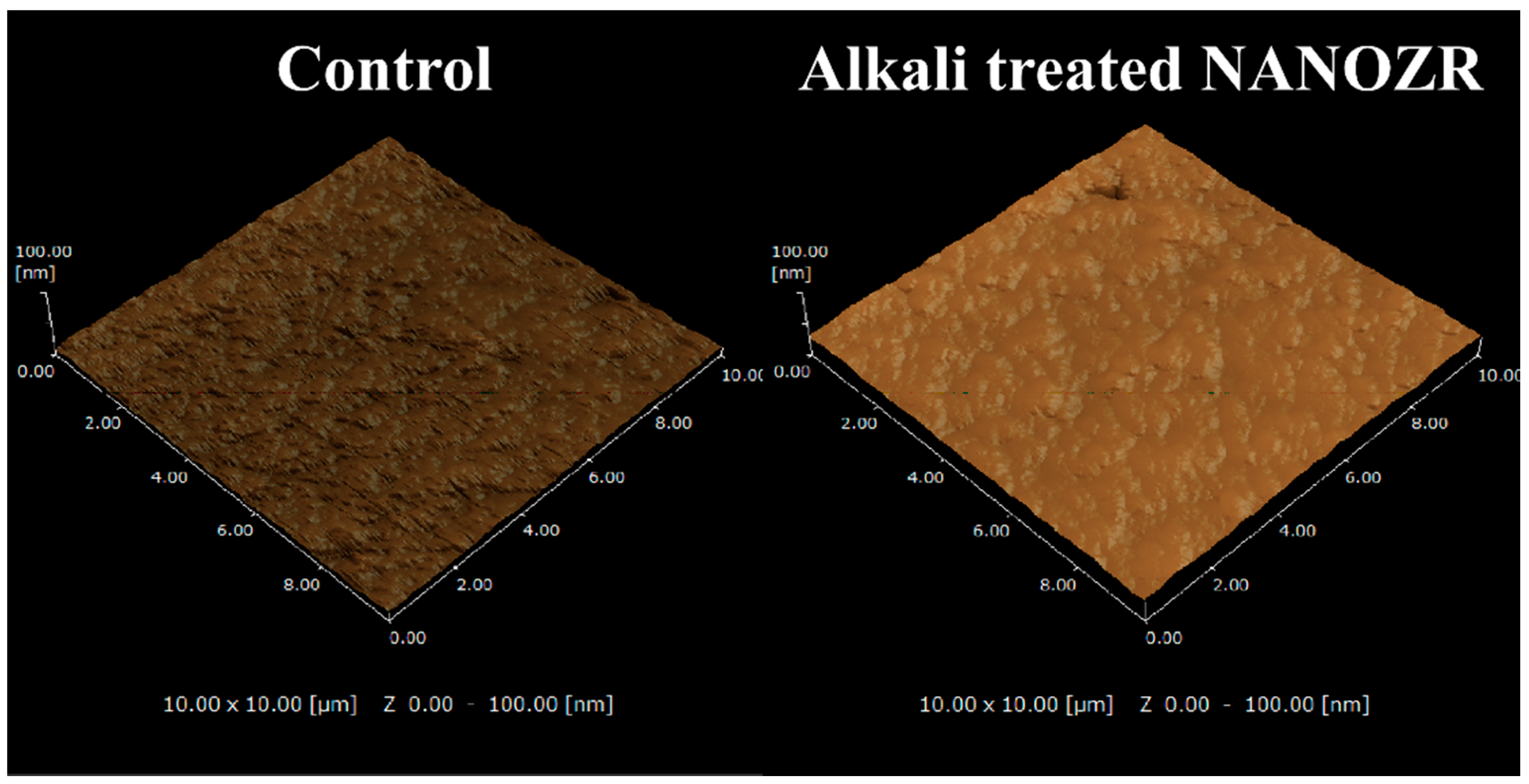
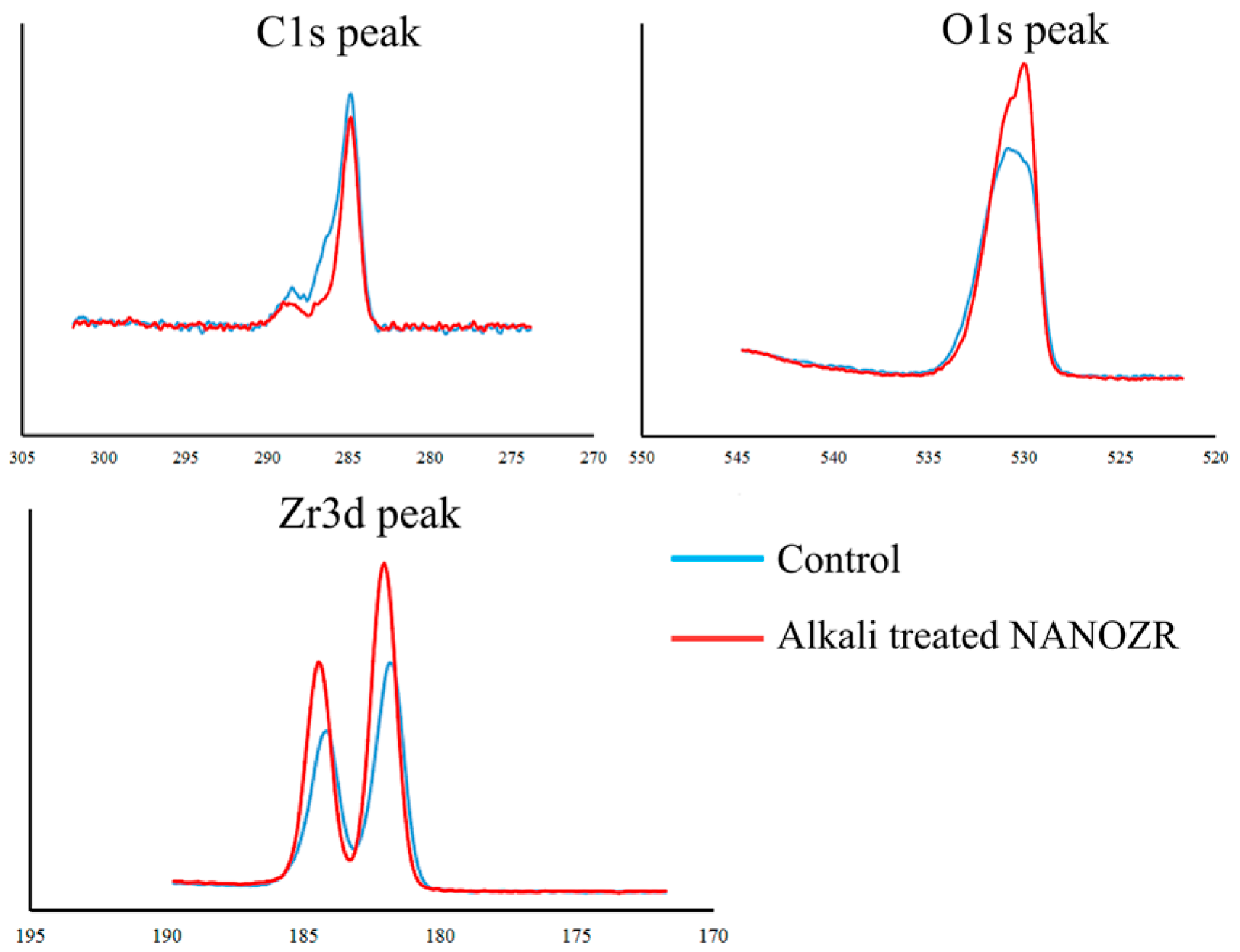
2.1. Surface Characterization
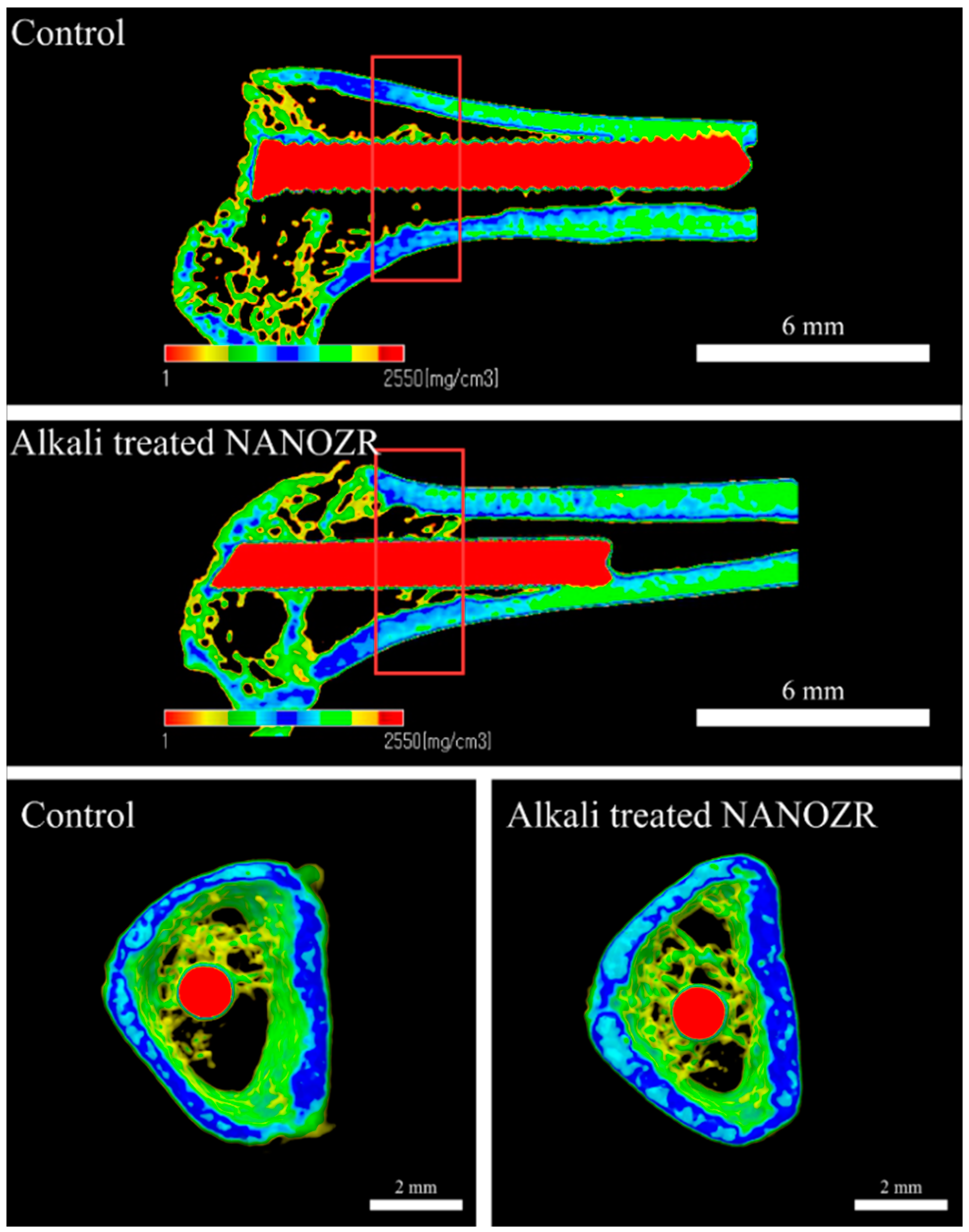
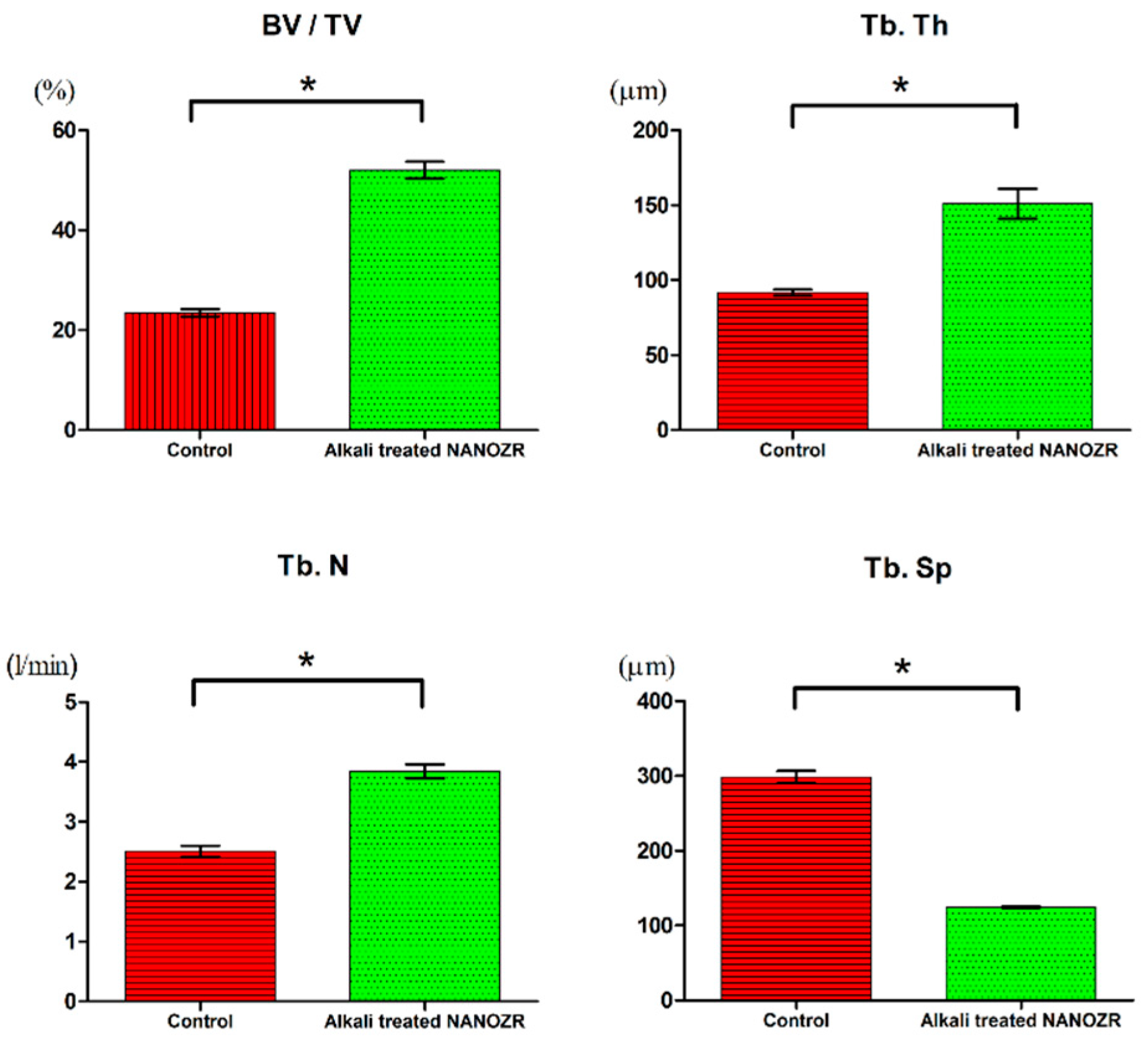
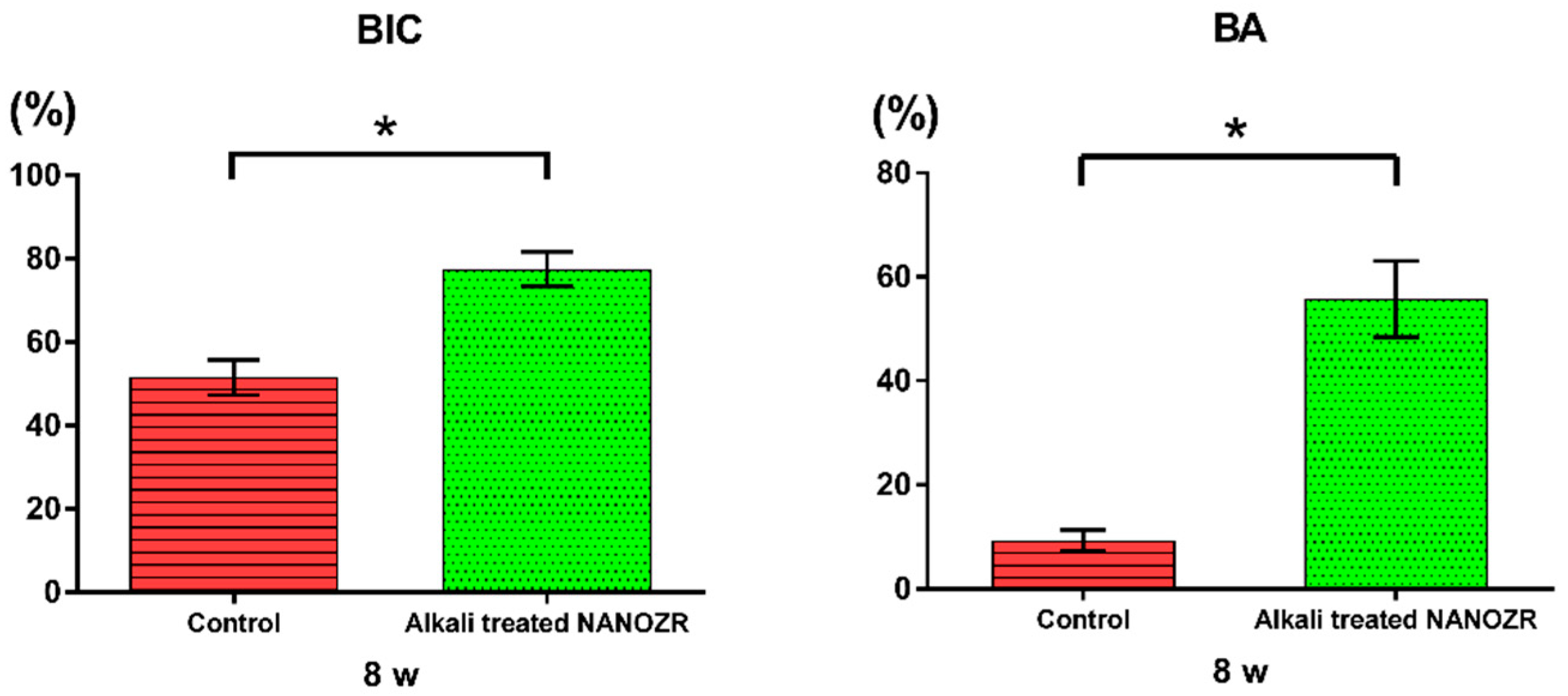
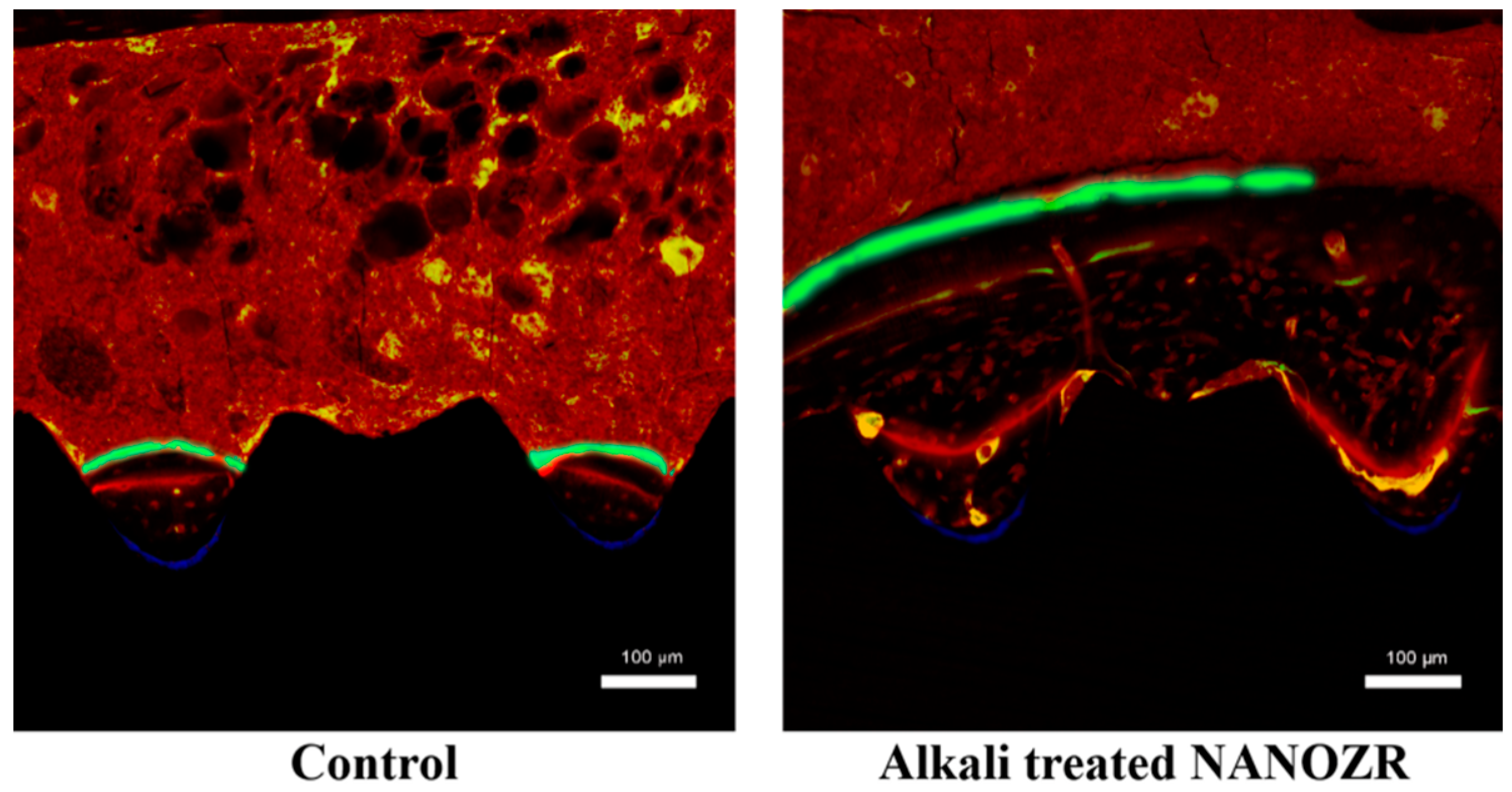
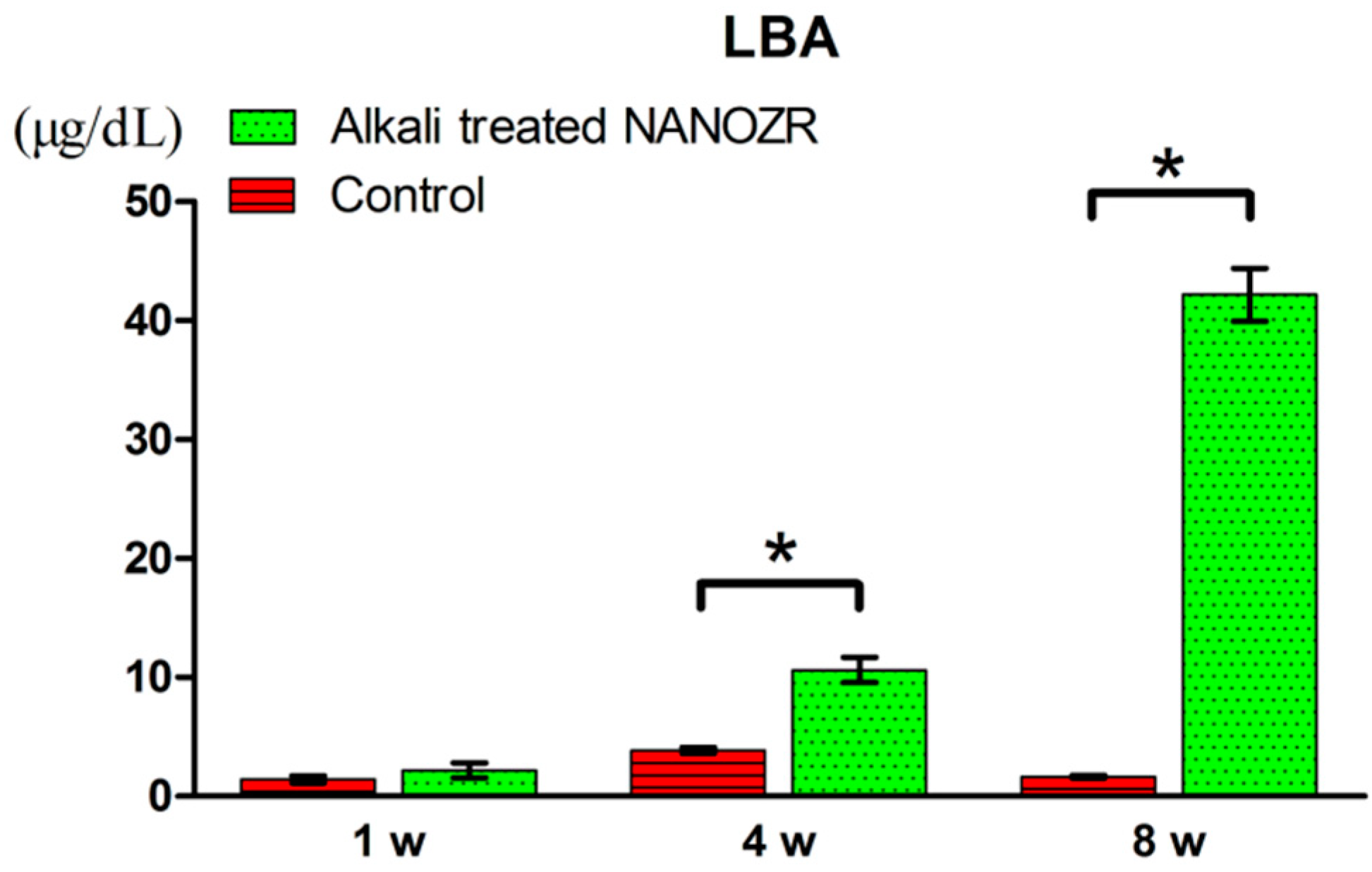
2.2. Alkali Treatment-Induced Bone Differentiation on the NANOZR Surface In Vivo
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Surface Characterization
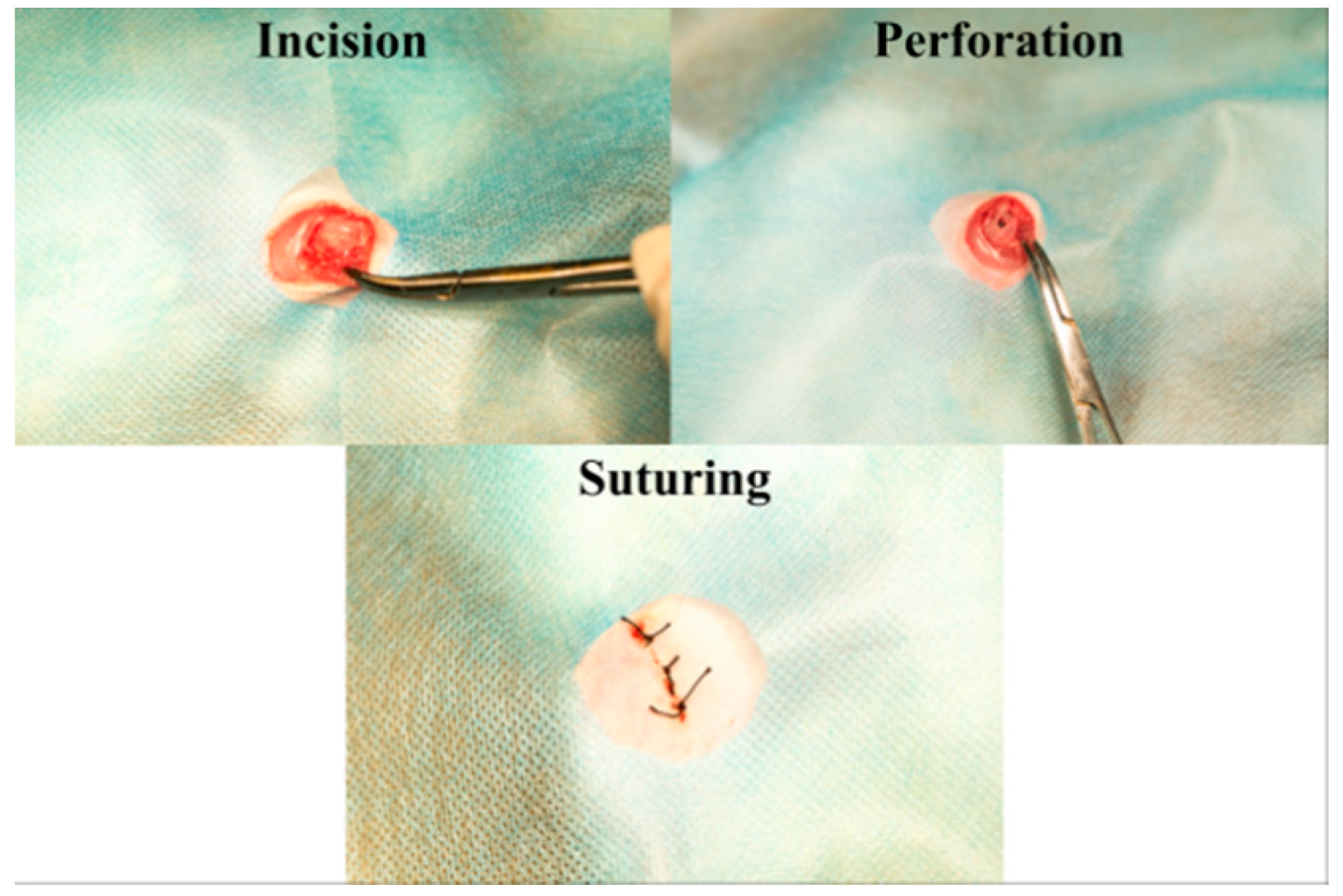
4.3. Animal Model and Surgical Procedures
4.4. Sequential Fluorescent Labeling
4.5. Alkali Treatment-Induced Bone Differentiation on NANOZR Surface In Vivo
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adell, R.; Eriksson, B.; Lekholm, U. A long-term follow up study of osseointegrated implants in the treatment of totally edentulous jaws. Int. J. Oral Maxillofac. Implants 1990, 5, 347–359. [Google Scholar] [PubMed]
- Jemt, T. Fixed implant-supported protheses in the edentulous maxilla. A five-years follow-up report. Clin. Oral Imlpants Res. 1994, 5, 142–147. [Google Scholar] [CrossRef]
- Van Steenberge, D. A retrospective multicenter evaluation of the survival rate of osseointegrated fixture supporting fixed partial prostheses in the treatment of partial edentulism. J. Prosthet. Dent. 1989, 61, 217–223. [Google Scholar] [CrossRef]
- Henry, P.J.; Laney, W.R.; Jemt, T. Osseointegrated implants for single-tooth replacement: A prospective 5-year multicenter study. Int. J. Oral Maxillofac. Implants 1996, 11, 450–455. [Google Scholar] [PubMed]
- Akagawa, Y.; Hashimoto, M.; Kondo, N.; Yamasaki, A.; Tsuru, H. Tissue reaction to implanted biomaterials. J. Prosthet. Dent. 1985, 53, 681–686. [Google Scholar] [CrossRef]
- Nagai, N.; Takashita, N.; Hayashi, J. Biological reaction of zirconia ceramic as a new implant material in the dental field. J. Oral. Biol. 1982, 24, 759–762. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahl, E.; Enbom, L. Osseointegrated oral implants. A Swedish multi-center study of 8139 consecutively inserted Nobelpharma implants. J. Periodontal. 1988, 59, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tripett, R.G.; Froberg, U.; Sykaras, N.; Woody, R.D. Implant materials, design, and surface topographies: Their influence on osseointegration of dental implants. J. Long Term Eff. Med. Implants 2003, 13, 485–501. [Google Scholar]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Buser, D.L.; Bruggenate, C.M.; Weingart, D.; Taylor, T.M.; Bernard, J.P.; Peters, F.; Simpson, J.P. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI SLA implants. Clin. Oral. Implants Res. 2002, 13, 144–153. [Google Scholar] [CrossRef]
- Svanborg, L.M.; Andersson, M.; Wennerberg, A. Surface characterization of commercial oral implants on the nanometer level. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Komasa, S.; Taguchi, Y.; Nishida, H.; Tanaka, M.; Kawazoe, T. Bioactivity of nanostructure on titanium surface modified by chemical processing at room temperature. J. Periodontics 2012, 56, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surface with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Taguchi, Y.; Komasa, S.; Sekino, T.; Tanaka, M. Cell differentiation on nanoscale features of a titanium surface: Effects of deposition time in NaOH solution. J. Hard Tissue Biol. 2014, 23, 63–70. [Google Scholar] [CrossRef]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Rat endothelial cell attachment, behavior and gene expression on NaOH-treated titanium surfaces. J. Oral Tissue Eng. 2013, 11, 189–200. [Google Scholar]
- Hara, Y.; Komasa, S.; Yoshimine, S.; Nishizaki, H.; Okazaki, J. Effect of nano modified titanium surface on adsorption of rat periodontal ligament cells. J. Osaka Dent. Univ. 2018, 52, 37–44. [Google Scholar]
- Jungm, R.E.; Sailer, I.; Hammerle, C.H.; Attin, T.; Schmidlin, P. In vitro color changes of soft tissue caused by restorative materials. Int. J. Periodontics Restor. Dent. 2007, 27, 251–257. [Google Scholar]
- Traini, T.; Pettinicchio, M.; Murmura, G.; Varvara, G.; Di Lullo, N.; Sinjari, B.; Caputi, S. Esthetic outcome of an immediately place maxillary anterior single-tooth implant restored with a custom-made zirconia-ceramic abutment and crown: A staged treatment. Quintessence Int. 2011, 42, 103–108. [Google Scholar] [PubMed]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. Tough and strong Ce-TZP/alumina nanocomposites doped with titania. Ceramic Int. 1998, 24, 497–506. [Google Scholar] [CrossRef]
- Nawa, M.; Bamba, N.; Sekino, T.; Niihara, K. The effect of TiO2 addition on strengthening and toughening in intragranular type of 12Ce-TZP/Al2O3 nanocomposites. J. Eur. Ceram. Soc. 1998, 18, 209–219. [Google Scholar] [CrossRef]
- Ban, S. Reliability and properties of core materials for all-ceramic dental restorations. Jpn. Dent. Sci Rev. 2008, 44, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tamura, J.; Kawanabe, K. Ce-TZP/Al2O3 nanocomposite as a bearing materials in total joint replacement. J. Biomed. Mater. Res. 2002, 63, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tasaka, A.; Yoshinari, M.; Sakurai, K. Fatigue strength of Ce-TZP/ Al2O3 nanocomposite with different surfaces. J. Dent. Res. 2012, 91, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; de Groot, K. The role of hydrated silica, titania, and alumina in inducing apatite on implants. J. Biomed. Mater. Res. A 1992, 28, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J., Jr.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells(MG63). J. Biomed. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Ko, H.C.; Han, J.S.; Bachle, M.; Jang, J.H.; Shin, S.W.; Kim, D.J. Initial osteoblast-like cell response to pure titanium and zirconia/alumina ceramics. Dent. Mater. 2007, 23, 1349–1355. [Google Scholar] [CrossRef]
- Depprich, R.; Ommerborn, M.; Zipprich, H.; Naujok, C.; Handschel, J.; Wiesmann, H.P.; Kubler, N.R.; Meyer, U. Behavior of osteoblastic cells cultured on titanium and structured zirconia surfaces. Head Face Med. 2008, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Komasa, S.; Kusumoto, T.; Taguchi, Y.; Nishizaki, H.; Sekino, T.; Umeda, M.; Okazaki, J.; Kawazoe, T. Effect of nanosheet surface structure of titanium alloys on cell differentiation. J. Nanomater. 2004, 2014, 642527. [Google Scholar] [CrossRef]
- Nishizaki, M.; Komasa, S.; Taguchi, Y.; Nishizaki, H.; Okazaki, J. Bioactivity of NANOZR Induced by Alkali Treatment. Int. J. Mol. Sci. 2017, 18, 780. [Google Scholar] [CrossRef] [PubMed]
- Komasa, S.; Nishizaki, M.; Kusumoto, T.; Terada, C.; Yin, D.; Kawamoto, A.; Yamamoto, S.; Yoshimine, S.; Nishizaki, H.; Shimizu, H.; et al. Osteogenesis-related gene expression on alkali-modified NANOZR and titanium surfaces with nanonetwork structures. J. Bio-Integr. 2017, 26, 355–360. [Google Scholar]
- Sul, Y.-T. The significance of the surface properties of oxidized titanium to the bone response: Special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials 2003, 24, 3893–3907. [Google Scholar] [CrossRef]
- Baier, R.E.; Meyer, A.E.; Natiella, J.R.; Natiella, R.R.; Carter, J.M. Surface properties determine bioadhesive outcomes: Methods and results. J. Biomed. Mater. Res. 1984, 18, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Bächle, M.; Kohal, R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin. Oral Implants Res. 2004, 15, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Setzer, B.; Bächle, M.; Metzger, M.C.; Kohal, R.J. The gene-expression and phenotypic response of hFOB 1.19 osteoblasts to surface-modified titanium and zirconia. Biomaterials 2009, 30, 979–990. [Google Scholar]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Apatite formation on zirconia metal treated with aqueous NaOH. Biomaterials 2002, 23, 313–317. [Google Scholar] [CrossRef]
- Miyake, N.; Miura, T.; Tanabe, K.; Hisanaga, R.; Yamashita, S.; Sato, T.; Yoshinari, M. Effect of phycochemical surface modifications on bovine serum albumin adsorption to tetragonal zirconia polycrystal in vitro through the change of the zeta potential. J. Oleo Sci. 2016, 65, 1003–1010. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; van den Dolder, J.; Bancroft, G.N.; Jansen, J.A.; Mikos, A.G. Influence of the in vitro culture period on the in vivo performance of cell/titanium bone tissue-engineered constructs using a rat cranial critical size defect model. J. Biomed. Mater. Res. 2003, 67, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.M.; Moodie, G.D.; Dimond, P.M.; Giorani, C.W.; Ehrlich, M.G.; Valentini, R.F. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar] [CrossRef]
- Depprich, R.; Zipprich, H.; Ommerborn, M.; Naujoks, C.; Wiesmann, H.P.; Kiattavorncharoen, S.; Lauer, H.C.; Meyer, U.; Kubler, N.R.; Handschel, J. Osseointegration of zirconia implants compared with titanium: An in vivo study. Head Face Med. 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Gudehus, T.; Eichhorm, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implants Res. 2007, 18, 662–668. [Google Scholar] [CrossRef]
- Koch, F.P.; Weng, D.; Kramer, S.; Biesterfeld, S.; Eimermacher, A.J.; Wagner, W. Osseointegration of one-piece zirconia implants compared with a titanium implant of identical design: A histomorphometric study in the dog. Clin. Oral Implants Res. 2010, 21, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Kohal, R.J.; Weng, D.; Bachle, M.; Strub, J.R. Loaded custom-made zirconia and titanium implants show similar osseointegration: An animal experiment. J. Peridontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komasa, S.; Nishizaki, M.; Zhang, H.; Takao, S.; Yin, D.; Terada, C.; Kobayashi, Y.; Kusumoto, T.; Yoshimine, S.; Nishizaki, H.; et al. Osseointegration of Alkali-Modified NANOZR Implants: An In Vivo Study. Int. J. Mol. Sci. 2019, 20, 842. https://doi.org/10.3390/ijms20040842
Komasa S, Nishizaki M, Zhang H, Takao S, Yin D, Terada C, Kobayashi Y, Kusumoto T, Yoshimine S, Nishizaki H, et al. Osseointegration of Alkali-Modified NANOZR Implants: An In Vivo Study. International Journal of Molecular Sciences. 2019; 20(4):842. https://doi.org/10.3390/ijms20040842
Chicago/Turabian StyleKomasa, Satoshi, Mariko Nishizaki, Honghao Zhang, Seiji Takao, Derong Yin, Chisato Terada, Yasuyuki Kobayashi, Tetsuji Kusumoto, Shigeki Yoshimine, Hiroshi Nishizaki, and et al. 2019. "Osseointegration of Alkali-Modified NANOZR Implants: An In Vivo Study" International Journal of Molecular Sciences 20, no. 4: 842. https://doi.org/10.3390/ijms20040842




