Radiobiological Characterization of Canine Malignant Melanoma Cell Lines with Different Types of Ionizing Radiation and Efficacy Evaluation with Cytotoxic Agents
Abstract
:1. Introduction
2. Results
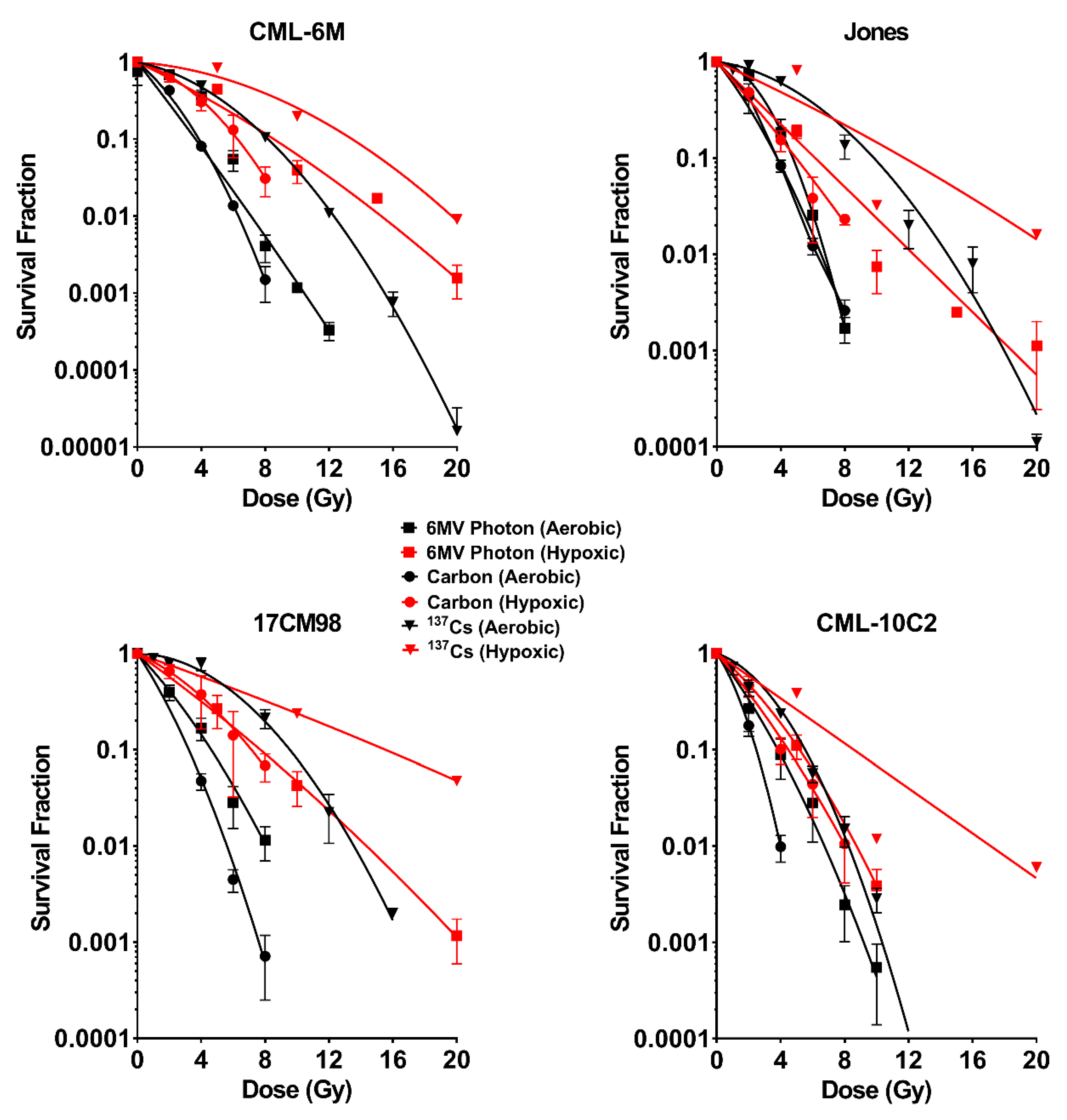
2.1. Survival Curves
2.2. α/β Ratio
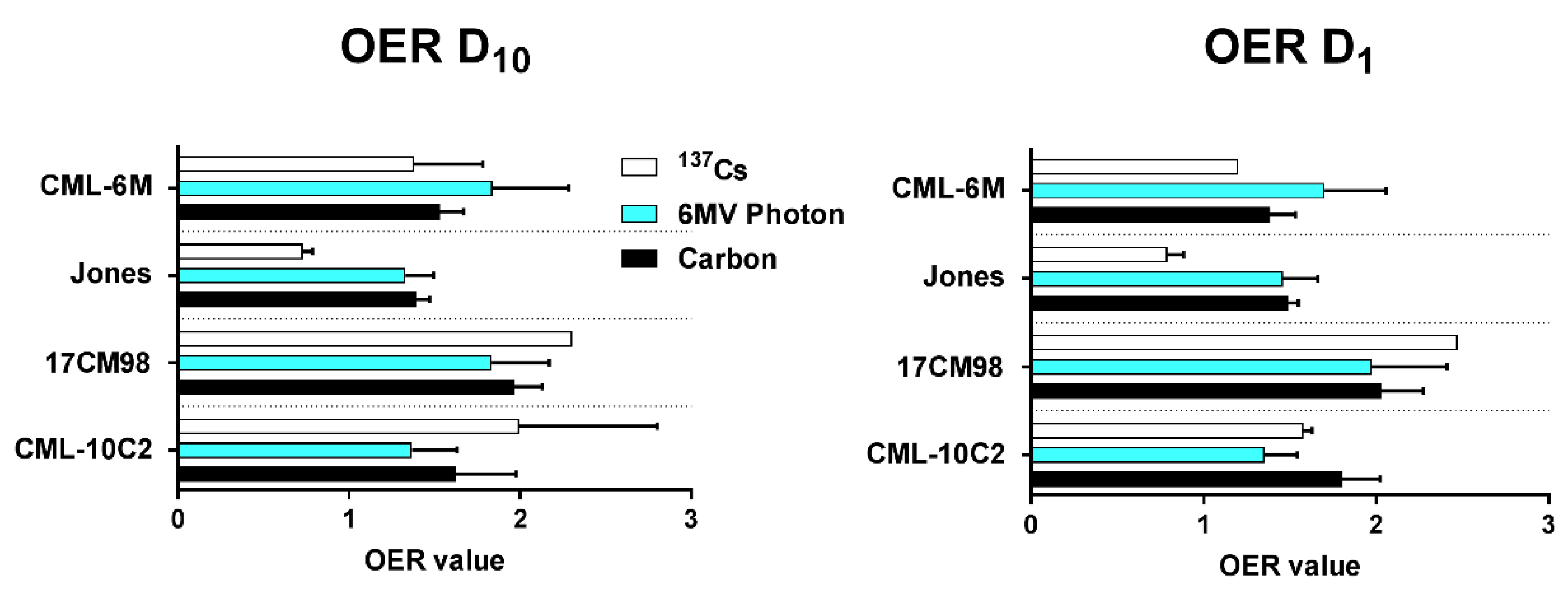
2.3. Oxygen Enhancement Ratio
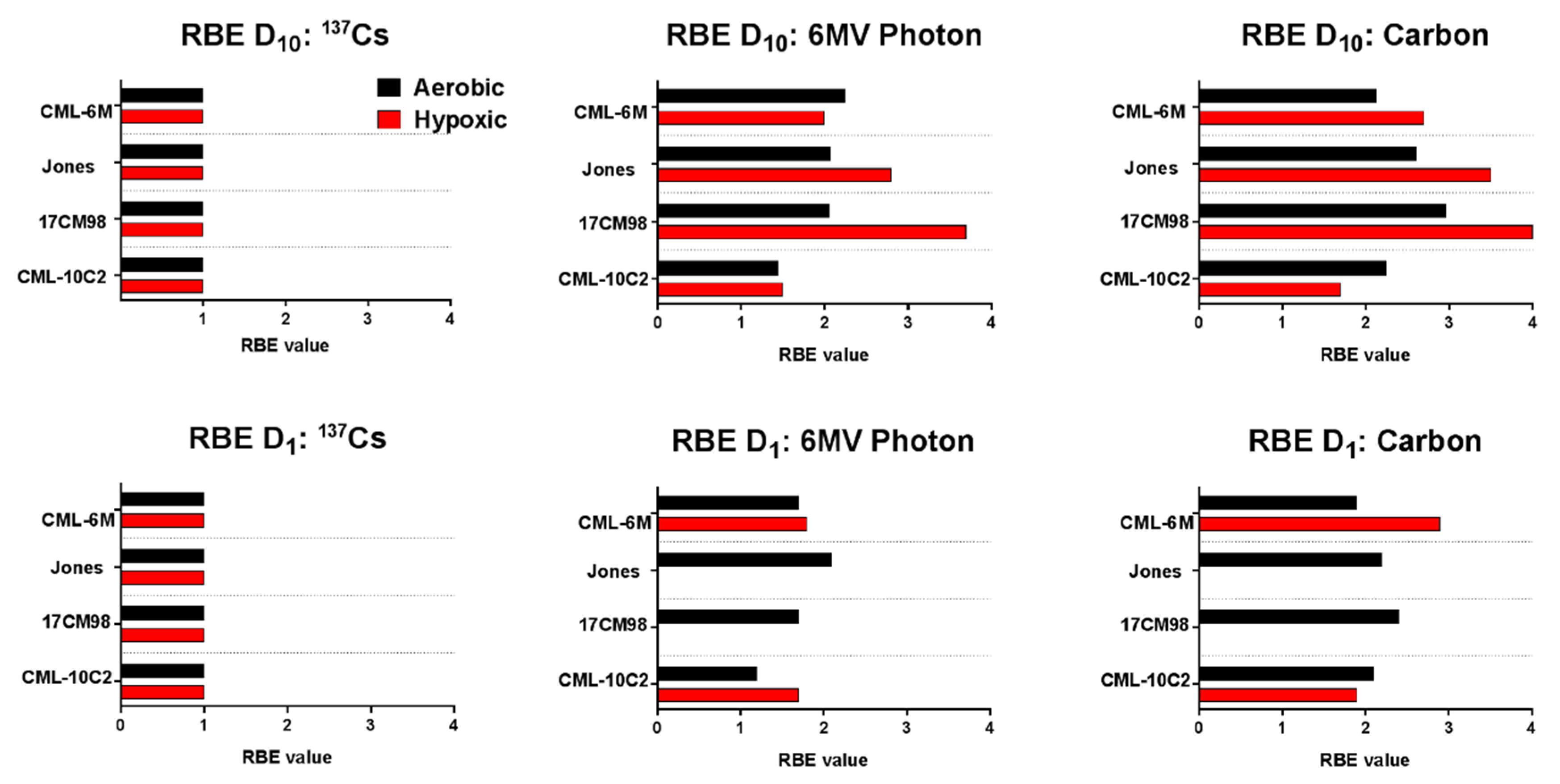
2.4. Relative Biological Effectiveness
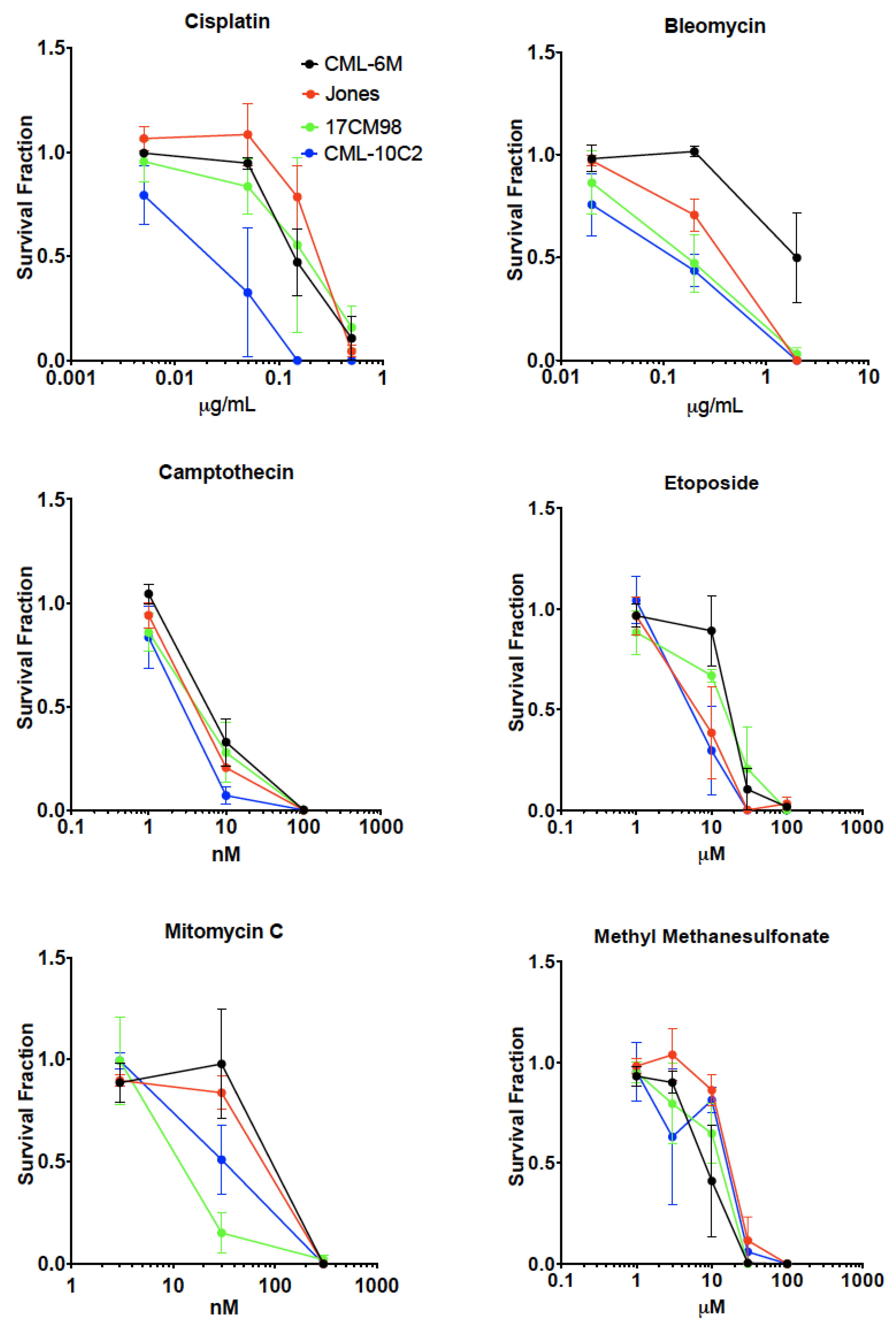
2.5. Drug Sensitivity Test
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Hypoxic Treatment
4.3. Irradiation
4.4. Colony Formation Assay
4.5. Drug Sensitivity Study
4.6. Data Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CMM | Canine malignant melanoma |
| OER | Oxygen enhancement ratio |
| RBE | Relative biological effectiveness |
| SF | Survival fraction |
| SOBP | Spread out Bragg peak |
| LET | Linear energy transfer |
References
- Hernandez, B.; Adissu, H.A.; Wei, B.R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.K. Multimodality therapy for head and neck cancer. Vet. Clin. North Am. Small Anim. Pract. 2003, 33, 615–628. [Google Scholar] [CrossRef]
- Theon, A.P.; Rodriguez, C.; Madewell, B.R. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J. Am. Vet. Med. Assoc. 1997, 210, 778–784. [Google Scholar] [PubMed]
- Tuohy, J.L.; Selmic, L.E.; Worley, D.R.; Ehrhart, N.P.; Withrow, S.J. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998–2011). J. Am. Vet. Med. Assoc. 2014, 245, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Bateman, K.E.; Catton, P.A.; Pennock, P.W.; Kruth, S.A. 0-7-21 radiation therapy for the treatment of canine oral melanoma. J. Vet. Intern. Med. 1994, 8, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, L.; Dobson, J.M. Radiotherapy of oral malignant melanomas in dogs. J. Am. Vet. Med. Assoc. 1996, 209, 98–102. [Google Scholar] [PubMed]
- Proulx, D.R.; Ruslander, D.M.; Dodge, R.K.; Hauck, M.L.; Williams, L.E.; Horn, B.; Price, G.S.; Thrall, D.E. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet. Radiol. Ultrasound. 2003, 44, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Hayes, A.M.; Blackwood, L.; Maglennon, G.; Pattinson, H.; Sparkes, A.H. Oral malignant melanoma - the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet. Comp. Oncol. 2005, 3, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Boston, S.E.; Lu, X.; Culp, W.T.; Montinaro, V.; Romanelli, G.; Dudley, R.M.; Liptak, J.M.; Mestrinho, L.A.; Buracco, P. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001–2012). J. Am. Vet. Med. Assoc. 2014, 245, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, S.; Rohrer Bley, C.; Aresu, L.; Dacasto, M.; Leone, V.F.; Pizzoni, S.; Gracis, M.; Marconato, L. Efficacy and side effects of radiation therapy in comparison with radiation therapy and temozolomide in the treatment of measurable canine malignant melanoma. Vet. Comp. Oncol. 2016, 14, e146–e157. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Giaccia, A. Linear energy transfer and relative biologic effectiveness. In Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018; pp. 101–110. [Google Scholar]
- Hayashi, K.; Koto, M.; Demizu, Y.; Saitoh, J.I.; Suefuji, H.; Okimoto, T.; Ohno, T.; Shioyama, Y.; Takagi, R.; Ikawa, H.; et al. A retrospective multicenter study of carbon-ion radiotherapy for external auditory canal and middle ear carcinomas. Cancer Med. 2018, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Demizu, Y.; Nagano, F.; Terashima, K.; Fujii, O.; Jin, D.; Mima, M.; Niwa, Y.; Katsui, K.; Suga, M.; et al. Treatment outcomes of proton or carbon ion therapy for skull base chordoma: a retrospective study. Radiat. Oncol. 2018, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Ohno, T.; Koto, M.; Demizu, Y.; Suefuji, H.; Tsuji, H.; Okimoto, T.; Shioyama, Y.; Saitoh, J.I.; Shirai, K.; et al. A multi-institutional retrospective study of carbon-ion radiotherapy for non-squamous cell malignant tumors of the nasopharynx: Subanalysis of Japan Carbon-Ion Radiation Oncology Study Group study 1402 HN. Cancer Med. 2018, 7, 6077–6083. [Google Scholar] [CrossRef] [PubMed]
- Boria, P.A.; Murry, D.J.; Bennett, P.F.; Glickman, N.W.; Snyder, P.W.; Merkel, B.L.; Schlittler, D.L.; Mutsaers, A.J.; Thomas, R.M.; Knapp, D.W. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 2004, 224, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rassnick, K.M.; Ruslander, D.M.; Cotter, S.M.; Al-Sarraf, R.; Bruyette, D.S.; Gamblin, R.M.; Meleo, K.A.; Moore, A.S. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989–2000). J. Am. Vet. Med. Assoc. 2001, 218, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Wouda, R.M.; Hocker, S.E.; Higginbotham, M.L. Safety evaluation of combination carboplatin and toceranib phosphate (Palladia) in tumour-bearing dogs: A phase I dose finding study. Vet. Comp. Oncol. 2018, 16, E52–E60. [Google Scholar] [CrossRef] [PubMed]
- Choisunirachon, N.; Jaroensong, T.; Yoshida, K.; Saeki, K.; Mochizuki, M.; Nishimura, R.; Sasaki, N.; Nakagawa, T. Effects of low-dose cyclophosphamide with piroxicam on tumour neovascularization in a canine oral malignant melanoma-xenografted mouse model. Vet. Comp. Oncol. 2015, 13, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.K.; Obradovich, J.E.; Elmslie, R.E.; Vail, D.M.; Moore, A.S.; Straw, R.C.; Dickinson, K.; Cooper, M.F.; Withrow, S.J. Efficacy of mitoxantrone against various neoplasms in dogs. J. Am. Vet. Med. Assoc. 1991, 198, 1618–1621. [Google Scholar] [PubMed]
- Cichorek, M. Camptothecin-induced death of amelanotic and melanotic melanoma cells in different phases of cell cycle. Neoplasma 2011, 58, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Zhou, S.T.; Li, X.Y.; Chen, X.C.; Zhao, X.; Qian, Z.Y.; Zhou, L.N.; Li, Z.Y.; Wang, Y.M.; Zhong, Q.; et al. Anti-tumor activity of N-trimethyl chitosan-encapsulated camptothecin in a mouse melanoma model. J. Exp. Clin. Cancer Res. 2010, 29, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loch-Neckel, G.; Nemen, D.; Puhl, A.C.; Fernandes, D.; Stimamiglio, M.A.; Alvarez Silva, M.; Hangai, M.; Santos Silva, M.C.; Lemos-Senna, E. Stealth and non-stealth nanocapsules containing camptothecin: in-vitro and in-vivo activity on B16-F10 melanoma. J. Pharm. Pharmacol. 2007, 59, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Dora, C.L.; Alvarez-Silva, M.; Trentin, A.G.; de Faria, T.J.; Fernandes, D.; da Costa, R.; Stimamiglio, M.; Lemos-Senna, E. Evaluation of antimetastatic activity and systemic toxicity of camptothecin-loaded microspheres in mice injected with B16-F10 melanoma cells. J. Pharm. Pharm. Sci. 2006, 9, 22–31. [Google Scholar] [PubMed]
- Gottlieb, J.A.; Luce, J.K. Treatment of malignant melanoma with camptothecin (NSC-100880). Cancer Chemother. Rep. 1972, 56, 103–105. [Google Scholar] [PubMed]
- Li, S.; Au, W.W.; Schmoyer, R.L., Jr.; Hsu, T.C. Baseline and mitomycin-C-induced sister chromatic exchanges ina melanoma and a colon tumor cell line. Cancer Genet. Cytogenet. 1982, 6, 243–248. [Google Scholar] [CrossRef]
- Pedersen, J.E.; Barron, G. Radiation and concurrent chemotherapy for the treatment of Lewis lung tumor and B16 melanoma tumor in C57/BL mice. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1479–1482. [Google Scholar] [CrossRef]
- Creagan, E.T.; Edmonson, J.H.; Ahmann, D.L.; Chang, M. Phase II study of mitomycin in disseminated malignant melanoma. Cancer Treat Rep. 1985, 69, 1451–1452. [Google Scholar] [PubMed]
- Nathanson, L.; Wittenberg, B.K. Pilot study of vinblastine and bleomycin combinations in the treatment of metastatic melanoma. Cancer Treat Rep. 1980, 64, 133–137. [Google Scholar] [PubMed]
- Nathanson, L.; Kaufman, S.D.; Carey, R.W. Vinblastine, infusion, bleomycin, and cis-dichlorodiammine-platinum chemotherapy in metastatic melanoma. Cancer 1981, 48, 1290–1294. [Google Scholar] [CrossRef] [Green Version]
- Bajetta, E.; Buzzoni, R.; Viviani, S.; Vaglini, M.; Nava, M.; Bonadonna, G. Prospective randomized trial in advanced malignant melanoma with cis-platinum, vindesine, and etoposide vs. cis-platinum, vindesine, and lomustine. Am. J. Clin. Oncol. 1985, 8, 401–405. [Google Scholar] [CrossRef]
- Freeman, K.P.; Hahn, K.A.; Harris, F.D.; King, G.K. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987–1997). J. Vet. Intern. Med. 2003, 17, 96–101. [Google Scholar]
- Milevoj, N.; Tratar, U.L.; Nemec, A.; Brozic, A.; Znidar, K.; Sersa, G.; Cemazar, M.; Tozon, N. A combination of electrochemotherapy, gene electrotransfer of plasmid encoding canine IL-12 and cytoreductive surgery in the treatment of canine oral malignant melanoma. Res. Vet. Sci. 2018, 122, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Batschinski, K.; Dervisis, N.; Kitchell, B.; Newman, R.; Erfourth, T. Combination of Bleomycin and Cytosine Arabinoside Chemotherapy for Relapsed Canine Lymphoma. J. Am. Anim. Hosp. Assoc. 2018, 54, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Kim, D.G.; Han, E.H.; Kim, Y.B.; Hwang, I.C.; Kim, C.Y. Toxicity study of a new camptothecin anti-cancer agent CKD-602 in dogs: 4-week continuous intravenous dose by infusion pump and 4-week repeated intravenous dose. Regul. Toxicol. Pharmacol. 2010, 58, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Boye, P.; Serres, F.; Marescaux, L.; Hordeaux, J.; Bouchaert, E.; Gomes, B.; Tierny, D. Dose escalation study to evaluate safety, tolerability and efficacy of intravenous etoposide phosphate administration in 27 dogs with multicentric lymphoma. PLoS ONE 2017, 12, e0177486. [Google Scholar] [CrossRef] [PubMed]
- Flory, A.B.; Rassnick, K.M.; Balkman, C.E.; Kiselow, M.A.; Autio, K.; Beaulieu, B.B.; Lewis, L.D. Oral bioavailability of etoposide after administration of a single dose to tumor-bearing dogs. Am. J. Vet. Res. 2008, 69, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Abbo, A.H.; Jones, D.R.; Masters, A.R.; Stewart, J.C.; Fourez, L.; Knapp, D.W. Phase I clinical trial and pharmacokinetics of intravesical mitomycin C in dogs with localized transitional cell carcinoma of the urinary bladder. J. Vet. Intern. Med. 2010, 24, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, W.; Kitchell, B.E.; Fossum, T.W.; Couto, C.G.; Theilen, G. Cisplatin for treatment of transitional cell and squamous cell carcinomas in dogs. J. Am. Vet. Med. Assoc. 1988, 193, 1530–1533. [Google Scholar]
- Shapiro, W.; Fossum, T.W.; Kitchell, B.E.; Couto, C.G.; Theilen, G.H. Use of cisplatin for treatment of appendicular osteosarcoma in dogs. J. Am. Vet. Med. Assoc. 1988, 192, 507–511. [Google Scholar]
- Knapp, D.W.; Richardson, R.C.; Bonney, P.L.; Hahn, K. Cisplatin therapy in 41 dogs with malignant tumors. J. Vet. Intern. Med. 1988, 2, 41–46. [Google Scholar] [CrossRef]
- Kitchell, B.E.; Brown, D.M.; Luck, E.E.; Woods, L.L.; Orenberg, E.K.; Bloch, D.A. Intralesional implant for treatment of primary oral malignant melanoma in dogs. J. Am. Vet. Med. Assoc. 1994, 204, 229–236. [Google Scholar]
- Maeda, J.; Froning, C.E.; Brents, C.A.; Rose, B.J.; Thamm, D.H.; Kato, T.A. Intrinsic Radiosensitivity and Cellular Characterization of 27 Canine Cancer Cell Lines. PLoS ONE 2016, 11, e0156689. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.G.; Oliver, J.L.; Smith, B.B.; Toivio-Kinnucan, M.A.; Powers, R.D.; Brawner, W.R.; Henderson, R.A.; Hankes, G.H. Biologic characterization of canine melanoma cell lines. Am. J. Vet. Res. 1987, 48, 1642–1648. [Google Scholar] [PubMed]
- Fowles, J.S.; Dailey, D.D.; Gustafson, D.L.; Thamm, D.H.; Duval, D.L. The Flint Animal Cancer Center (FACC) Canine Tumour Cell Line Panel: a resource for veterinary drug discovery, comparative oncology and translational medicine. Vet. Comp. Oncol. 2017, 15, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.M.; Su, C.; Haskins, J.S.; Salinas, V.A.; Sunada, S.; Yu, H.; Uesaka, M.; Hirakawa, H.; Chen, D.J.; Fujimori, A.; et al. DNA Repair Deficient Chinese Hamster Ovary Cells Exhibiting Differential Sensitivity to Charged Particle Radiation under Aerobic and Hypoxic Conditions. Int. J. Mol. Sci. 2018, 19, 2228. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Tsuji, H.; Yanagi, T.; Mizoe, J.E.; Miyamoto, T.; Kato, H.; Yamada, S.; Morita, S.; Yoshikawa, K.; et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J. Clin. Oncol. 2002, 20, 4466–4471. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, I.M.; Bell, J.J.; Maeda, J.; Genet, M.D.; Romero, A.; Fujii, Y.; Fujimori, A.; Kitamuta, H.; Kamada, T.; Chen, D.J.; et al. Effects of targeted phosphorylation site mutations in the DNA-PKcs phosphorylation domain on low and high LET radiation sensitivity. Oncol. Lett. 2015, 9, 1621–1627. [Google Scholar] [CrossRef] [Green Version]
- McMillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, Y.; et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef]

| SF2 and SF8 | ||||||
|---|---|---|---|---|---|---|
| CML-6M | Jones | 17CM98 | CML-10C2 | |||
| SF2 | 137Cs | Aerobic | 0.75 | 0.84 | 0.89 | 0.63 |
| Hypoxic | 0.85 | 0.77 | 0.85 | 0.55 | ||
| 6MV Photon | Aerobic | 0.29 | 0.65 | 0.41 | 0.33 | |
| Hypoxic | 0.62 | 0.47 | 0.57 | 0.48 | ||
| Carbon | Aerobic | 0.37 | 0.33 | 0.27 | 0.18 | |
| Hypoxic | 0.66 | 0.41 | 0.65 | 0.39 | ||
| SF8 | 137Cs | Aerobic | 0.11 | 0.20 | 0.20 | 0.01 |
| Hypoxic | 0.44 | 0.35 | 0.53 | 0.09 | ||
| 6MV Photon | Aerobic | 0.01 | 0.002 | 0.01 | 0.003 | |
| Hypoxic | 0.12 | 0.05 | 0.09 | 0.02 | ||
| Carbon | Aerobic | 0.001 | 0.002 | 0.001 | <0.001 | |
| Hypoxic | 0.03 | 0.02 | 0.07 | 0.01 | ||
| α/β ratio | |||||
|---|---|---|---|---|---|
| CML-6M | Jones | 17CM98 | CML-10C2 | ||
| 137Cs | Aerobic | 4.3 | 2.9 | 0.3 | 2.4 |
| Hypoxic | 22.7 | >100 | >100 | >100 | |
| 6MV Photon | Aerobic | >100 | 0.3 | 19.2 | 17.9 |
| Hypoxic | >100 | 47.8 | 83.3 | 14.1 | |
| Carbon | Aerobic | 7.4 | 13.8 | 13.1 | 3.9 |
| Hypoxic | 3.6 | 77.9 | 8.4 | 27.5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, H.; Sunada, S.; Hirakawa, H.; Fujimori, A.; Elmegerhi, S.; Leary, D.; Kato, T.A. Radiobiological Characterization of Canine Malignant Melanoma Cell Lines with Different Types of Ionizing Radiation and Efficacy Evaluation with Cytotoxic Agents. Int. J. Mol. Sci. 2019, 20, 841. https://doi.org/10.3390/ijms20040841
Yoshikawa H, Sunada S, Hirakawa H, Fujimori A, Elmegerhi S, Leary D, Kato TA. Radiobiological Characterization of Canine Malignant Melanoma Cell Lines with Different Types of Ionizing Radiation and Efficacy Evaluation with Cytotoxic Agents. International Journal of Molecular Sciences. 2019; 20(4):841. https://doi.org/10.3390/ijms20040841
Chicago/Turabian StyleYoshikawa, Hiroto, Shigeaki Sunada, Hirokazu Hirakawa, Akira Fujimori, Suad Elmegerhi, Del Leary, and Takamitsu A. Kato. 2019. "Radiobiological Characterization of Canine Malignant Melanoma Cell Lines with Different Types of Ionizing Radiation and Efficacy Evaluation with Cytotoxic Agents" International Journal of Molecular Sciences 20, no. 4: 841. https://doi.org/10.3390/ijms20040841





