Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease—A Next-Generation Sequencing-Guided Bioinformatic Approach
Abstract
:1. Introduction
2. Results
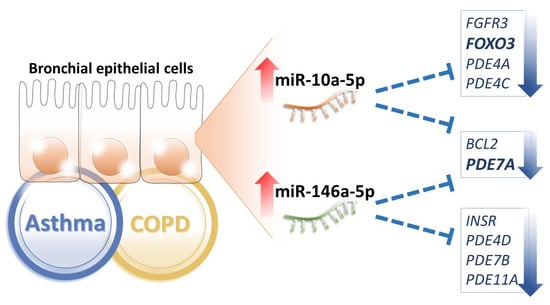
2.1. Common MicroRNA Changes in the Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Diseases (COPD)
2.2. Potential Targets of the Upregulated MicroRNAs in the Bronchial Epithelial Cells of Asthma and COPD
2.3. Serum MicroRNA Changes in Asthma and COPD Patients
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Next-Generation Sequencing (NGS) of Small RNA
4.3. The MicroRNA and mRNA Levels in Bronchial Epithelial Cells
4.4. Ingenuity Pathway Analysis (IPA)
4.5. MicroRNA Target Prediction
4.6. Gene Expression Omnibus (GEO) Database Analysis
4.7. Gene Ontology (GO) Analysis Using Database for Annotation, Visualization and Integrated Discovery (DAVID)
4.8. Analysis of Serum MicroRNA Levels
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Number of Databases Suggesting the microRNA-mRNA Relationship | Source | GSE67940 | GSE41861 | GSE41861 | GSE63142 | GSE43696 | GSE4302 | GSE38974 | GSE20257 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (Control) | 31 control | 30 control | 30 control | 27 control | 20 control | 28 control | 9 control | 53 control | ||||||||||
| n (Case) | 73 asthma | 26 asthma (moderate & severe) | 51 asthma | 128 asthma | 88 asthma (moderate & severe) | 42 asthma | 23 COPD | 23 COPD | ||||||||||
| miR-10a-5p | miR-146a-5p | Gene | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC | adj. p | log2FC |
| 8 | 3 | FOXO3 | 0.04 | −0.24 | 0.85 | −0.02 | 0.84 | −0.04 | 0.84 | 0.07 | 0.06 | −0.47 | 0.46 | 0.22 | ||||
| 8 | 8 | GRIN3A | 0.98 | −0.01 | 0.34 | 0.18 | 0.41 | 0.27 | 0.39 | −0.10 | 0.93 | −0.04 | 0.03 | 0.70 | ||||
| 7 | 3 | ADRB3 | 0.66 | 0.10 | 0.93 | 0.01 | 0.90 | 0.01 | 0.39 | 0.13 | 0.12 | 0.42 | 0.87 | −0.02 | 0.36 | −0.23 | 0.25 | 0.40 |
| 7 | 4 | BCL2 | 0.92 | −0.05 | 0.01 | −0.41 | 0.01 | 0.13 | 0.29 | −0.11 | 0.51 | −0.16 | 0.60 | −0.04 | 0.06 | −0.51 | 0.49 | −0.27 |
| 2 | 7 | CD8A | 0.96 | −0.06 | 0.88 | 0.05 | 0.84 | 0.06 | 0.85 | −0.07 | 0.83 | −0.13 | 0.98 | −0.01 | 0.67 | −0.18 | 0.04 | 0.78 |
| 7 | 7 | GRIN2A | 0.63 | −0.20 | 0.74 | 0.03 | 0.51 | 0.04 | 0.56 | −0.03 | 0.69 | 0.14 | 0.78 | −0.03 | 0.32 | −0.32 | 0.31 | 0.49 |
| 3 | 7 | HDAC2 | 0.41 | 0.08 | 0.01 | 0.17 | 0.02 | 0.13 | 0.67 | 0.04 | 0.97 | 0.01 | 0.88 | 0.03 | 0.43 | −0.27 | 0.06 | 0.54 |
| 7 | MPO | 0.62 | 0.22 | 0.58 | 0.04 | 0.30 | 0.05 | 0.97 | 0.00 | 0.91 | 0.05 | 0.57 | 0.03 | 0.16 | 0.55 | 0.63 | −0.25 | |
| 6 | CCL5 | 0.89 | −0.15 | 0.63 | 0.15 | 0.47 | 0.19 | 0.95 | −0.04 | 0.96 | −0.05 | 0.45 | 0.13 | 0.80 | −0.11 | 0.27 | 0.50 | |
| 5 | 6 | CYSLTR2 | 0.51 | 0.16 | 0.84 | −0.02 | 0.83 | −0.01 | 0.94 | 0.01 | 0.83 | 0.10 | 1.00 | 0.00 | 0.62 | −0.14 | 0.87 | 0.08 |
| 6 | GATA3 | 0.94 | −0.03 | 0.58 | −0.08 | 0.88 | 0.03 | 0.74 | 0.06 | 0.91 | −0.09 | 0.49 | −0.07 | 0.71 | −0.12 | 1.00 | 0.00 | |
| 6 | HMGCR | 0.86 | −0.05 | 0.28 | −0.13 | 0.03 | −0.21 | 0.93 | −0.02 | 0.48 | 0.20 | 0.11 | −0.22 | 0.78 | −0.08 | 0.69 | 0.14 | |
| 3 | 6 | PDE11A | 0.19 | −0.48 | 0.53 | 0.04 | 0.57 | 0.16 | 0.10 | −0.17 | 0.23 | −0.30 | 0.07 | 0.69 | ||||
| 6 | 6 | PDE3A | 0.86 | 0.02 | 0.98 | 0.00 | 0.87 | 0.01 | 0.96 | −0.01 | 0.95 | −0.03 | 0.69 | −0.03 | 0.43 | −0.19 | 0.76 | −0.18 |
| 6 | PDE4A | 0.01 | −0.23 | 0.57 | −0.05 | 0.40 | −0.06 | 0.73 | 0.03 | 0.08 | −0.24 | 0.45 | 0.07 | 0.05 | −0.28 | 0.46 | 0.23 | |
| 6 | PDE7B | 0.87 | 0.09 | 0.28 | −0.09 | 0.00 | −0.35 | 0.82 | −0.02 | 0.28 | −0.24 | 0.25 | 0.06 | 0.71 | −0.14 | 0.20 | −0.44 | |
| 2 | 6 | PGR | 0.52 | 0.13 | 0.66 | −0.03 | 0.59 | −0.16 | 0.57 | −0.04 | 0.05 | −0.40 | 0.80 | −0.14 | ||||
| 6 | 3 | VDR | 0.81 | −0.05 | 0.42 | 0.05 | 0.08 | 0.08 | 0.77 | 0.05 | 0.95 | −0.02 | 0.55 | 0.04 | 0.05 | 0.50 | 0.29 | 0.42 |
| 5 | 2 | CRP | 0.10 | −0.45 | 0.96 | 0.01 | 0.93 | 0.06 | 0.89 | 0.02 | 0.60 | 0.19 | 0.61 | −0.24 | ||||
| 5 | 5 | CTSS | 0.37 | 0.15 | 0.10 | 0.20 | 0.10 | 0.17 | 0.68 | −0.09 | 0.76 | −0.11 | 0.56 | −0.06 | 0.00 | 2.14 | 0.36 | 0.24 |
| 5 | FGFR3 | 0.89 | 0.05 | 0.00 | −0.49 | 0.01 | −0.37 | 0.48 | −0.16 | 0.85 | −0.10 | 0.80 | 0.02 | 0.06 | −0.48 | 0.40 | −0.26 | |
| 3 | 5 | GRIN2B | 0.72 | 0.09 | 0.73 | 0.03 | 0.52 | 0.05 | 0.98 | 0.00 | 0.63 | 0.07 | 0.44 | −0.06 | 0.74 | 0.11 | 0.03 | 1.04 |
| 5 | 5 | PDE7A | 0.70 | −0.15 | 0.02 | −0.23 | 0.00 | −0.38 | 0.03 | −0.23 | 0.57 | −0.12 | 0.17 | −0.08 | 0.23 | −0.38 | 0.09 | 0.66 |
| 5 | SELE | 0.66 | −0.22 | 0.88 | 0.01 | 0.98 | 0.01 | 0.68 | −0.03 | 0.15 | 1.34 | 0.40 | 0.44 | |||||
| 5 | TARP | 0.94 | 0.04 | 0.87 | −0.04 | 0.79 | −0.17 | 0.72 | 0.05 | 0.84 | −0.08 | 0.14 | −0.60 | |||||
| 5 | TBX21 | 0.98 | −0.02 | 0.40 | 0.08 | 0.37 | 0.07 | 0.90 | 0.02 | 0.97 | 0.03 | 0.45 | 0.08 | 0.50 | −0.31 | 1.00 | 0.00 | |
| 4 | CHRM1 | 0.98 | 0.01 | 0.27 | 0.10 | 0.30 | 0.29 | 0.55 | 0.04 | 0.35 | −0.21 | 0.13 | 0.66 | |||||
| 3 | 4 | CHRM2 | 0.72 | 0.12 | 0.82 | −0.02 | 0.73 | −0.12 | 0.79 | −0.02 | 0.00 | −1.22 | 0.90 | 0.07 | ||||
| 4 | CHRM3 | 0.67 | 0.15 | 0.29 | −0.09 | 0.28 | −0.06 | 0.41 | −0.04 | 0.46 | −0.37 | 0.17 | 0.09 | 0.00 | −1.32 | 0.35 | −0.46 | |
| 4 | GRIN1 | 0.72 | 0.16 | 0.27 | 0.07 | 0.26 | 0.10 | 0.04 | 0.35 | 0.07 | 0.40 | 0.53 | 0.05 | 0.73 | 0.08 | 0.44 | 0.32 | |
| 4 | GRIN2C | 0.83 | 0.07 | 0.41 | 0.05 | 0.85 | 0.02 | 0.51 | 0.08 | 0.63 | 0.21 | 0.94 | −0.01 | 0.92 | −0.04 | 0.32 | 0.44 | |
| 4 | HLA−DQA1 | 0.75 | −0.37 | 0.89 | 0.13 | 0.98 | 0.03 | 0.24 | −0.22 | 0.62 | 0.40 | 0.52 | −0.55 | 0.66 | 0.75 | 0.50 | −0.66 | |
| 2 | 4 | INSR | 0.87 | −0.04 | 0.00 | −0.48 | 0.00 | −0.33 | 0.01 | −0.27 | 0.04 | −0.38 | 0.22 | −0.32 | 0.17 | −0.56 | 0.26 | −0.34 |
| 4 | MYLK | 0.75 | 0.24 | 0.45 | 0.09 | 0.93 | −0.02 | 0.43 | −0.10 | 0.68 | −0.12 | 0.49 | 0.13 | 0.01 | −0.75 | 0.10 | 0.59 | |
| 4 | PDE4B | 0.26 | −0.47 | 0.54 | 0.06 | 0.44 | 0.06 | 0.57 | −0.14 | 0.58 | −0.11 | 0.22 | −0.11 | 0.35 | −0.22 | 0.04 | 0.91 | |
| 4 | PDE4C | 0.20 | −0.34 | 0.41 | −0.11 | 0.78 | −0.07 | 0.01 | −0.54 | |||||||||
| 2 | 4 | PDE4D | 0.67 | −0.32 | 0.76 | −0.02 | 0.57 | 0.05 | 0.59 | 0.05 | 0.17 | −0.31 | 0.42 | 0.08 | 0.12 | −0.34 | 0.26 | 0.33 |
| 3 | 2 | ADRA1A | 0.55 | −0.24 | 0.94 | 0.01 | 0.65 | 0.21 | 0.67 | 0.03 | 0.42 | 0.28 | 0.37 | 0.48 | ||||
| 3 | 2 | ADRB2 | 0.00 | 0.58 | 0.56 | −0.09 | 0.26 | −0.12 | 0.49 | 0.13 | 0.45 | 0.23 | 0.72 | −0.10 | 0.04 | −0.79 | 0.53 | 0.17 |
| 3 | CHRNA4 | 0.55 | 0.10 | 0.49 | 0.06 | 0.10 | 0.11 | 0.55 | 0.13 | 0.46 | 0.24 | 0.60 | 0.05 | 0.77 | −0.07 | 0.64 | 0.14 | |
| 3 | 2 | CHRNB2 | 0.81 | −0.11 | 0.52 | 0.06 | 0.31 | 0.07 | 0.96 | 0.02 | 0.61 | 0.20 | 0.45 | 0.07 | 0.12 | 0.32 | 0.46 | 0.28 |
| 3 | 2 | HMOX1 | 0.79 | 0.04 | 0.38 | −0.22 | 0.12 | −0.30 | 0.67 | −0.15 | 0.59 | −0.26 | 0.42 | −0.13 | 0.01 | 1.32 | 0.34 | 0.34 |
| 2 | 3 | HRH1 | 0.08 | −0.30 | 0.16 | 0.11 | 0.06 | 0.12 | 0.38 | 0.08 | 0.28 | 0.34 | 0.48 | 0.08 | 0.00 | 0.84 | 0.25 | 0.45 |
| 3 | IL13 | 0.69 | 0.24 | 0.49 | 0.06 | 0.11 | 0.11 | 0.37 | 0.07 | 0.76 | 0.17 | 0.17 | 0.10 | 0.55 | 0.49 | 0.07 | 0.71 | |
| 2 | 3 | IL6 | 0.87 | −0.10 | 0.24 | −0.21 | 0.04 | −0.25 | 0.01 | −0.36 | 0.05 | −0.94 | 0.87 | 0.02 | 0.01 | 2.37 | 0.61 | −0.26 |
| 2 | ADORA2A | 0.76 | −0.11 | 0.81 | 0.02 | 0.66 | 0.12 | 0.00 | 0.57 | |||||||||
| 2 | CHRNA7 | 0.92 | −0.03 | 0.50 | −0.07 | 0.84 | −0.08 | 0.32 | −0.27 | |||||||||
| 2 | IL10 | 0.57 | 0.21 | 0.96 | −0.01 | 0.79 | −0.12 | 0.84 | 0.01 | 0.05 | 0.65 | 0.21 | 0.54 | |||||
| 2 | IL1R1 | 0.77 | 0.10 | 0.17 | −0.20 | 0.73 | −0.06 | 0.73 | −0.05 | 0.76 | −0.10 | 0.56 | 0.06 | 0.40 | 0.22 | 0.80 | 0.12 | |
| 2 | 2 | IL2RA | 0.93 | 0.07 | NA | NA | NA | NA | 0.45 | 0.08 | 0.67 | 0.20 | 0.75 | −0.03 | 0.01 | 1.04 | 0.55 | 0.23 |
| 2 | NOS2 | 0.48 | 0.58 | 0.01 | 1.10 | 0.08 | 1.15 | 0.02 | 0.29 | 0.50 | 0.31 | 0.65 | 0.24 | |||||
| 2 | PDE3B | 0.71 | −0.10 | 0.70 | 0.03 | 0.56 | 0.04 | 0.52 | 0.07 | 0.87 | 0.08 | 0.76 | 0.02 | 0.13 | 0.29 | 0.02 | 0.99 | |
| 2 | PDE5A | 0.78 | −0.09 | 0.25 | 0.08 | 0.17 | 0.08 | 0.93 | 0.01 | 0.50 | −0.25 | 0.46 | −0.05 | 0.00 | −0.79 | 0.38 | 0.43 | |
| 2 | TNF | 0.55 | 0.15 | 0.92 | −0.02 | 0.46 | −0.08 | 0.76 | 0.03 | 0.87 | 0.10 | 0.90 | 0.02 | 0.27 | −0.24 | 0.27 | 0.43 | |
| microRNA | Primer Sequence |
|---|---|
| hsa-miR-10a-3p | 5′ CAAATTCGTATCTAGGGGAATA 3′ |
| hsa-miR-10a-5p | 5′ TACCCTGTAGATCCGAATTTGTG 3′ |
| hsa-miR-146a-5p | 5′ TGAGAACTGAATTCCATGGGTT 3′ |
| hsa-miR-203a-3p | 5′ GTGAAATGTTTAGGACCACTAG 3′ |
| hsa-miR-3130-5p | 5′ TACCCAGTCTCCGGTGCAGCC 3′ |
| hsa-miR-365a-5p | 5′ AGGGACTTTTGGGGGCAGATGTG 3′ |
| hsa-miR-548d-5p | 5′ AAAAGTAATTGTGGTTTTTGCC 3′ |
| hsa-miR-487a-3p | 5′ AATCATACAGGGACATCCAGTT 3′ |
| hsa-miR-873-5p | 5′ GCAGGAACTTGTGAGTCTCCT 3′ |
| cel-miR-39-3p | 5′ TCACCGGGTGTAAATCAGCTTG 3′ |
| FOXO3 forward | 5′ GGACAAACGGCTCACTCTGT 3′ |
| FOXO3 reverse | 5′ GCTCTTGCCAGTTCCCTCAT 3′ |
| BCL2 forward | 5′ GTGGTGGAGGAGCTCTTCAG 3′ |
| BCL2 reverse | 5′ GCCGGTTCAGGTACTCAGTC 3′ |
| FGFR3_H_F2 | 5′ CCAATGTCTCCGAGCTCGAG 3′ |
| FGFR3_H_R2 | 5′ TCCTTGTCAGTGGCATCGTC 3′ |
| PDE11A forward | 5′ CCACAACTGGAGACATGCCT 3′ |
| PDE11A reverse | 5′ TTGTTGGTTCCCCTGTGGTC 3′ |
| INSR forward | 5′ TGGCAACATCACCCACTACC 3′ |
| INSR reverse | 5′ CCGGCCGAATCCTCATACTC 3′ |
| PDE7A forward | 5′ TTGCAGCTGCCACTCATGAT 3′ |
| PDE7A reverse | 5′ GTCTCCATTTGTTGCCTGCT 3′ |
| PDE4D forward | 5′ ACCGGCCCTTGACTGTTATC 3′ |
| PDE4D reverse | 5′ ACTGGACAACATCTGCAGCA 3′ |
| PDE4A forward | 5′ CGATTTGGGGTGAAGACCGA 3′ |
| PDE4A reverse | 5′ TGTCACCATCGTGTCCACAG 3′ |
| PDE4C forward | 5′ CTGACCAGGAGGAGCAACTG 3′ |
| PDE4C reverse | 5′ TGACCTTCCAGCATCAGCAG 3′ |
| PDE7B forward | 5′ TGTGTTTGCATGCTGGGAGA 3′ |
| PDE7B reverse | 5′ TTCCACGAAGCAGCCTTGAT 3′ |
References
- Sheu, C.C.; Tsai, M.J.; Chen, F.W.; Chang, K.F.; Chang, W.A.; Chong, I.W.; Kuo, P.L.; Hsu, Y.L. Identification of novel genetic regulations associated with airway epithelial homeostasis using next-generation sequencing data and bioinformatics approaches. Oncotarget 2017, 8, 82674–82688. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.A.; Tsai, M.J.; Jian, S.F.; Sheu, C.C.; Kuo, P.L. Systematic analysis of transcriptomic profiles of COPD airway epithelium using next-generation sequencing and bioinformatics. Int. J. Chronic Obstr. Pulm. Dis. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Chang, W.A.; Jian, S.F.; Chang, K.F.; Sheu, C.C.; Kuo, P.L. Possible mechanisms mediating apoptosis of bronchial epithelial cells in chronic obstructive pulmonary disease—A next-generation sequencing approach. Pathol. Res. Pract. 2018, 214, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- GINA. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma (GINA): Fontana, WI, USA, 2018. [Google Scholar]
- GOLD. Global Strategy for Diagnosis, Management, and Prevention of COPD; Global Initiative for Obstructive Lung Disease (GOLD), 2018. Available online: https://goldcopd.org/ (accessed on 28 January 2019).
- Tsai, M.J.; Hsu, Y.L.; Wang, T.N.; Wu, L.Y.; Lien, C.T.; Hung, C.H.; Kuo, P.L.; Huang, M.S. Aryl hydrocarbon receptor (AhR) agonists increase airway epithelial matrix metalloproteinase activity. J. Mol. Med. 2014, 92, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Wang, T.N.; Lin, Y.S.; Kuo, P.L.; Hsu, Y.L.; Huang, M.S. Aryl hydrocarbon receptor agonists upregulate VEGF secretion from bronchial epithelial cells. J. Mol. Med. 2015, 93, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.M.; Wang, C.H.; Lee, M.J.; He, J.R.; Huang, H.Y.; Chao, M.W.; Chung, K.F.; Kuo, H.P. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy 2018, 73, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.Y.; Chiang, S.R.; Tsai, M.J.; Tsai, Y.M.; Chong, I.W.; Shieh, J.M.; Hsu, Y.L. LIGHT is a crucial mediator of airway remodeling. J. Cell. Physiol. 2015, 230, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.L.; Hsu, Y.L.; Tsai, M.J.; Huang, M.S. Pterostilbene suppresses benzo[a]pyrene-induced airway remodeling. J. Agric. Food Chem. 2011, 59, 8028–8035. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, L.R.; Harris, A. The role of microRNAs in chronic respiratory disease: Recent insights. Biol. Chem. 2018, 399, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Svitich, O.A.; Sobolev, V.V.; Gankovskaya, L.V.; Zhigalkina, P.V.; Zverev, V.V. The role of regulatory RNAs (miRNAs) in asthma. Allergol. Immunopathol. 2018, 46, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Baskoro, H.; Rennard, S.I.; Seyama, K.; Takahashi, K. MicroRNAs as Therapeutic Targets in Lung Disease: Prospects and Challenges. Chronic Obstr. Pulm. Dis. 2015, 3, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Yatagai, Y.; Yamada, H.; Iijima, H.; Masuko, H.; Sakamoto, T.; Hizawa, N. The search for common pathways underlying asthma and COPD. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 65–78. [Google Scholar]
- Jardim, M.J.; Dailey, L.; Silbajoris, R.; Diaz-Sanchez, D. Distinct microRNA expression in human airway cells of asthmatic donors identifies a novel asthma-associated gene. Am. J. Respir. Cell Mol. Biol. 2012, 47, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Ezzie, M.E.; Crawford, M.; Cho, J.H.; Orellana, R.; Zhang, S.; Gelinas, R.; Batte, K.; Yu, L.; Nuovo, G.; Galas, D.; et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax 2012, 67, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Pan, W.; Fedulov, A.V.; Jester, W.; Jones, M.R.; Weiss, S.T.; Panettieri, R.A., Jr.; Tantisira, K.; Lu, Q. MicroRNA-10a controls airway smooth muscle cell proliferation via direct targeting of the PI3 kinase pathway. FASEB J. 2014, 28, 2347–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.Y.; Tang, X.Y.; Li, N.; Zhao, L.M.; Guo, Y.L.; Li, X.S.; Tian, C.J.; Cheng, D.J.; Chen, Z.C.; Zhang, L.X. GAS5 promotes airway smooth muscle cell proliferation in asthma via controlling miR-10a/BDNF signaling pathway. Life Sci. 2018, 212, 93–101. [Google Scholar] [CrossRef]
- Xie, T.; Liang, J.; Guo, R.; Liu, N.; Noble, P.W.; Jiang, D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol. Genomics 2011, 43, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Liu, L.; Li, J.; Yan, M.; Lin, H.; Liu, Y.; Chu, D.; Tu, H.; Gu, A.; Yao, M. MiRNA-10a is upregulated in NSCLC and may promote cancer by targeting PTEN. Oncotarget 2015, 6, 30239–30250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinegaglia, N.C.; Andrade, S.C.; Tokar, T.; Pinheiro, M.; Severino, F.E.; Oliveira, R.A.; Hasimoto, E.N.; Cataneo, D.C.; Cataneo, A.J.; Defaveri, J.; et al. Integrative transcriptome analysis identifies deregulated microRNA-transcription factor networks in lung adenocarcinoma. Oncotarget 2016, 7, 28920–28934. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, J.; Lin, Z.; Cao, J.; Huang, H.; Jiang, Y.; He, H.; Yang, L.; Ren, N.; Liu, G. Role of deregulated microRNAs in non-small cell lung cancer progression using fresh-frozen and formalin-fixed, paraffin-embedded samples. Oncol. Lett. 2016, 11, 801–808. [Google Scholar] [CrossRef]
- Markou, A.; Sourvinou, I.; Vorkas, P.A.; Yousef, G.M.; Lianidou, E. Clinical evaluation of microRNA expression profiling in non small cell lung cancer. Lung Cancer 2013, 81, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ma, Y.; Chen, P.; Wang, D. MicroRNA-10a silencing reverses cisplatin resistance in the A549/cisplatin human lung cancer cell line via the transforming growth factor-beta/Smad2/STAT3/STAT5 pathway. Mol. Med. Rep. 2015, 11, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A. microRNA and Allergy. Adv. Exp. Med. Biol. 2015, 888, 331–352. [Google Scholar] [PubMed]
- Comer, B.S.; Camoretti-Mercado, B.; Kogut, P.C.; Halayko, A.J.; Solway, J.; Gerthoffer, W.T. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, L727–L734. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Morales, S.; Gamboa-Becerra, R.; Baca, V.; Del Rio-Navarro, B.E.; Lopez-Ley, D.Y.; Velazquez-Cruz, R.; Saldana-Alvarez, Y.; Salas-Martinez, G.; Orozco, L. MiR-146a polymorphism is associated with asthma but not with systemic lupus erythematosus and juvenile rheumatoid arthritis in Mexican patients. Tissue Antigens 2012, 80, 317–321. [Google Scholar]
- Hu, D.; Zhang, Z.; Ke, X.; Kang, H.; Hong, S. A functional variant of miRNA-149 confers risk for allergic rhinitis and comorbid asthma in Chinese children. Int. J. Immunogenet. 2017, 44, 62–70. [Google Scholar] [CrossRef]
- Trinh, H.K.T.; Pham, D.L.; Kim, S.C.; Kim, R.Y.; Park, H.S.; Kim, S.H. Association of the miR-196a2, miR-146a, and miR-499 Polymorphisms with Asthma Phenotypes in a Korean Population. Mol. Diagn. Ther. 2017, 21, 547–554. [Google Scholar] [CrossRef]
- Carreras-Badosa, G.; Runnel, T.; Plaas, M.; Karner, J.; Ruckert, B.; Lattekivi, F.; Koks, S.; Akdis, C.A.; Kingo, K.; Rebane, A. microRNA-146a is linked to the production of IgE in mice but not in atopic dermatitis patients. Allergy 2018. [Google Scholar] [CrossRef]
- Li, F.; Huang, Y.; Huang, Y.Y.; Kuang, Y.S.; Wei, Y.J.; Xiang, L.; Zhang, X.J.; Jia, Z.C.; Jiang, S.; Li, J.Y.; et al. MicroRNA-146a promotes IgE class switch in B cells via upregulating 14-3-3sigma expression. Mol. Immunol. 2017, 92, 180–189. [Google Scholar] [CrossRef]
- Hammad Mahmoud Hammad, R.; Hamed, D.; Eldosoky, M.; Ahmad, A.; Osman, H.M.; Abd Elgalil, H.M.; Mahmoud Hassan, M.M. Plasma microRNA-21, microRNA-146a and IL-13 expression in asthmatic children. Innate Immun. 2018, 24, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xue, Y.; Liu, Y.; Song, G.; Lv, G.; Wang, Y.; Wang, Y.; Li, X.; Yang, L. MicroRNA-146a expression inhibits the proliferation and promotes the apoptosis of bronchial smooth muscle cells in asthma by directly targeting the epidermal growth factor receptor. Exp. Ther. Med. 2016, 12, 854–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, K.A.; Roff, A.N.; Panganiban, R.P.; Douglas, S.; Ishmael, F.T. MicroRNA-146a is induced by inflammatory stimuli in airway epithelial cells and augments the anti-inflammatory effects of glucocorticoids. PLoS ONE 2018, 13, e0205434. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hong, H.; Tang, J.; Wu, X.; Lin, Z.; Ma, R.; Fan, Y.; Xu, G.; Liu, D.; Li, H. Increased Expression of miR-146a in Children with Allergic Rhinitis After Allergen-Specific Immunotherapy. Allergy Asthma Immunol. Res. 2016, 8, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Tsitsiou, E.; Williams, A.E.; Moschos, S.A.; Patel, K.; Rossios, C.; Jiang, X.; Adams, O.D.; Macedo, P.; Booton, R.; Gibeon, D.; et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 2012, 129, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Ma, C.; Bao, L.; Lv, L.; Huang, M. miR-146a Mimics Attenuate Allergic Airway Inflammation by Impacted Group 2 Innate Lymphoid Cells in an Ovalbumin-Induced Asthma Mouse Model. Int. Arch. Allergy Immunol. 2018, 177, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Faiz, A.; Weckmann, M.; Tasena, H.; Vermeulen, C.J.; Van den Berge, M.; Ten Hacken, N.H.T.; Halayko, A.J.; Ward, J.P.T.; Lee, T.H.; Tjin, G.; et al. Profiling of healthy and asthmatic airway smooth muscle cells following interleukin-1beta treatment: A novel role for CCL20 in chronic mucus hypersecretion. Eur. Respir. J. 2018, 52, 1800310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, Y.; Li, M.; Wu, P.; Sun, G.; Fei, G.; Xu, X.; Zhou, X.; Zhou, L.; Wang, R. Combined Effects of PVT1 and MiR-146a Single Nucleotide Polymorphism on the Lung Function of Smokers with Chronic Obstructive Pulmonary Disease. Int. J. Biol. Sci. 2018, 14, 1153–1162. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Zhou, S.; Zeng, D.; Xu, X.; Xu, R.; Sun, G. Effect of a single nucleotide polymorphism in miR-146a on COX-2 protein expression and lung function in smokers with chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 463–473. [Google Scholar]
- Chen, B.B.; Li, Z.H.; Gao, S. Circulating miR-146a/b correlates with inflammatory cytokines in COPD and could predict the risk of acute exacerbation COPD. Medicine 2018, 97, e9820. [Google Scholar] [CrossRef] [Green Version]
- Osei, E.T.; Florez-Sampedro, L.; Tasena, H.; Faiz, A.; Noordhoek, J.A.; Timens, W.; Postma, D.S.; Hackett, T.L.; Heijink, I.H.; Brandsma, C.A. miR-146a-5p plays an essential role in the aberrant epithelial-fibroblast cross-talk in COPD. Eur. Respir. J. 2017, 49, 1602538. [Google Scholar] [CrossRef]
- Sato, T.; Liu, X.; Nelson, A.; Nakanishi, M.; Kanaji, N.; Wang, X.; Kim, M.; Li, Y.; Sun, J.; Michalski, J.; et al. Reduced miR-146a increases prostaglandin E(2)in chronic obstructive pulmonary disease fibroblasts. Am. J. Respir. Crit. Care Med. 2010, 182, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Tasena, H.; Faiz, A.; Timens, W.; Noordhoek, J.; Hylkema, M.N.; Gosens, R.; Hiemstra, P.S.; Spira, A.; Postma, D.S.; Tew, G.W.; et al. microRNA-mRNA regulatory networks underlying chronic mucus hypersecretion in COPD. Eur. Respir. J. 2018, 52, 1701556. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Brookes-Fazakerley, S.; Donnelly, L.E.; Barnes, P.J.; Barnette, M.S.; Giembycz, M.A. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L279–L289. [Google Scholar] [CrossRef] [PubMed]
- Giembycz, M.A.; Smith, S.J. Phosphodiesterase 7A: A new therapeutic target for alleviating chronic inflammation? Curr. Pharm. Des. 2006, 12, 3207–3220. [Google Scholar] [CrossRef] [PubMed]
- Fan Chung, K. Phosphodiesterase inhibitors in airways disease. Eur. J. Pharmacol. 2006, 533, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Page, C.P. Phosphodiesterase inhibitors for the treatment of asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 2014, 165, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, E.; Lagente, V.; Dupont, M.; Fargeau, H.; Palazzi, X.; Richard, V.; Dassaud, M.; Fric, M.; Coupe, M.; Carre, C.; et al. Lack of involvement of type 7 phosphodiesterase in an experimental model of asthma. Eur. Respir. J. 2012, 39, 582–588. [Google Scholar] [CrossRef]
- Myatt, S.S.; Lam, E.W. The emerging roles of forkhead box (Fox) proteins in cancer. Nat. Rev. Cancer 2007, 7, 847–859. [Google Scholar] [CrossRef]
- Barkund, S.; Shah, T.; Ambatkar, N.; Gadgil, M.; Joshi, K. FOXO3a Gene Polymorphism Associated with Asthma in Indian Population. Mol. Biol. Int. 2015, 2015, 638515. [Google Scholar] [CrossRef]
- Amarin, J.Z.; Naffa, R.G.; Suradi, H.H.; Alsaket, Y.M.; Obeidat, N.M.; Mahafza, T.M.; Zihlif, M.A. An intronic single-nucleotide polymorphism (rs13217795) in FOXO3 is associated with asthma and allergic rhinitis: A case-case-control study. BMC Med. Genet. 2017, 18, 132. [Google Scholar] [CrossRef]
- Hwang, J.W.; Rajendrasozhan, S.; Yao, H.; Chung, S.; Sundar, I.K.; Huyck, H.L.; Pryhuber, G.S.; Kinnula, V.L.; Rahman, I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J. Immunol. 2011, 187, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, Y.; Xing, S.; Ma, P.; Lin, D. SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO3. Cell Stress Chaperones 2015, 20, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Vincenzo, S.; Heijink, I.H.; Noordhoek, J.A.; Cipollina, C.; Siena, L.; Bruno, A.; Ferraro, M.; Postma, D.S.; Gjomarkaj, M.; Pace, E. SIRT1/FoxO3 axis alteration leads to aberrant immune responses in bronchial epithelial cells. J. Cell. Mol. Med. 2018, 22, 2272–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Sundar, I.K.; Ahmad, T.; Lerner, C.; Gerloff, J.; Friedman, A.E.; Phipps, R.P.; Sime, P.J.; McBurney, M.W.; Guarente, L.; et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L816–L828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.H.; An, H.J.; Kim, K.A.; Ko, J.J.; Kim, J.H.; Kim, Y.R.; Ahn, E.H.; Rah, H.; Lee, W.S.; Kim, N.K. Single nucleotide polymorphisms at miR-146a/196a2 and their primary ovarian insufficiency-related target gene regulation in granulosa cells. PLoS ONE 2017, 12, e0183479. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Kuo, P.L.; Hung, W.W.; Wu, L.Y.; Wu, P.H.; Chang, W.A.; Kuo, M.C.; Hsu, Y.L. Angpt2 Induces Mesangial Cell Apoptosis through the MicroRNA-33-5p-SOCS5 Loop in Diabetic Nephropathy. Mol. Ther. Nucleic Acids 2018, 13, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hung, J.Y.; Chang, W.A.; Jian, S.F.; Lin, Y.S.; Pan, Y.C.; Wu, C.Y.; Kuo, P.L. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol. Ther. 2018, 26, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Kuo, P.L.; Kuo, M.C.; Hung, W.W.; Wu, L.Y.; Chang, W.A.; Wu, P.H.; Lee, S.C.; Chen, H.C.; Hsu, Y.L. The Interaction of miR-378i-Skp2 Regulates Cell Senescence in Diabetic Nephropathy. J. Clin. Med. 2018, 7, 468. [Google Scholar] [CrossRef]
- Yen, M.C.; Chou, S.K.; Kan, J.Y.; Kuo, P.L.; Hou, M.F.; Hsu, Y.L. Solute Carrier Family 27 Member 4 (SLC27A4) Enhances Cell Growth, Migration, and Invasion in Breast Cancer Cells. Int. J. Mol. Sci. 2018, 19, 3434. [Google Scholar] [CrossRef]
- Dweep, H.; Gretz, N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Li, X.; Hastie, A.T.; Hawkins, G.A.; Moore, W.C.; Ampleford, E.J.; Milosevic, J.; Li, H.; Busse, W.W.; Erzurum, S.C.; Kaminski, N.; et al. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy 2015, 70, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Modena, B.D.; Tedrow, J.R.; Milosevic, J.; Bleecker, E.R.; Meyers, D.A.; Wu, W.; Bar-Joseph, Z.; Erzurum, S.C.; Gaston, B.M.; Busse, W.W.; et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am. J. Respir. Crit. Care Med. 2014, 190, 1363–1372. [Google Scholar] [CrossRef]
- Voraphani, N.; Gladwin, M.T.; Contreras, A.U.; Kaminski, N.; Tedrow, J.R.; Milosevic, J.; Bleecker, E.R.; Meyers, D.A.; Ray, A.; Ray, P.; et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol. 2014, 7, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Boushey, H.A.; Dolganov, G.M.; Barker, C.S.; Yang, Y.H.; Donnelly, S.; Ellwanger, A.; Sidhu, S.S.; Dao-Pick, T.P.; Pantoja, C.; et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc. Natl. Acad. Sci. USA 2007, 104, 15858–15863. [Google Scholar] [CrossRef] [PubMed]
- Shaykhiev, R.; Otaki, F.; Bonsu, P.; Dang, D.T.; Teater, M.; Strulovici-Barel, Y.; Salit, J.; Harvey, B.G.; Crystal, R.G. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell. Mol. Life Sci. 2011, 68, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]







| microRNA | Expression Level (rpm) | Ratio (AHBE/NHBE) | Expression Level (rpm) | Ratio (CHBE/NHBE) | ||
|---|---|---|---|---|---|---|
| AHBE | NHBE * | CHBE | NHBE * | |||
| hsa-miR-10a-3p | 6.51 | 2.58 | 2.52 | 2.39 | 0.14 | 17.07 |
| hsa-miR-10a-5p | 6937.77 | 2251.48 | 3.08 | 52,697.51 | 24,821.91 | 2.12 |
| hsa-miR-146a-5p | 157.81 | 53.57 | 2.95 | 526.01 | 223.11 | 2.36 |
| hsa-miR-203a | 2852.52 | 1184.56 | 2.41 | 445.33 | 187.00 | 2.38 |
| hsa-miR-3130-5p | 1.92 | 0.65 | 2.95 | 19.10 | 1.67 | 11.44 |
| hsa-miR-365a-5p | 5.18 | 2.58 | 2.01 | 7.50 | 2.79 | 2.69 |
| hsa-miR-548d-5p | 5.47 | 2.42 | 2.26 | 5.12 | 2.37 | 2.16 |
| hsa-miR-487a | 1.04 | 2.74 | 0.38 | 2.39 | 5.86 | 0.41 |
| hsa-miR-873-5p | 1.63 | 9.84 | 0.17 | 1.88 | 8.65 | 0.22 |
| Normal Controls | Asthma Patients | COPD Patients | p Value * | |
|---|---|---|---|---|
| n | 22 | 86 | 16 | |
| Age | 58.67 ± 13.66 | 46.51 ± 16.27 | 65.07 ± 12.68 | <0.01 |
| Sex | <0.01 | |||
| Female | 16 (73%) | 45 (51%) | 2 (13%) | |
| Male | 6 (27%) | 43 (49%) | 14 (88%) | |
| Body mass index (BMI) (kg/m2) | 22.77 ± 2.79 | 25.6 ± 5.26 | 22.97 ± 4.01 | 0.01 |
| Obesity (BMI ≥ 27 kg/m2) | <0.01 | |||
| No | 21 (95%) | 53 (60%) | 14 (88%) | |
| Yes | 1 (5%) | 35 (40%) | 2 (13%) | |
| Allergic rhinitis | <0.01 | |||
| No | 19 (86%) | 27 (31%) | 11 (69%) | |
| Yes | 3 (14%) | 61 (69%) | 5 (31%) | |
| Smoking history | <0.01 | |||
| Never smoker | 22 (100%) | 62 (70%) | 2 (13%) | |
| Current smoker | 0 (0%) | 16 (18%) | 8 (50%) | |
| Ex-smoker | 0 (0%) | 10 (11%) | 6 (38%) | |
| Medications | ||||
| Oral corticosteroid | 0 (0%) | 0 (0%) | 0 (0%) | |
| Inhaled corticosteroid | 0 (0%) | 29 (33%) | 5 (31%) | <0.01 |
| Long-acting beta2-agonist | 0 (0%) | 31 (35%) | 7 (44%) | <0.01 |
| Long-acting muscarinic antagonist | 0 (0%) | 4 (5%) | 2 (13%) | 0.20 |
| Leukotriene receptor antagonist | 0 (0%) | 22 (25%) | 2 (13%) | 0.02 |
| Intranasal steroids | 0 (0%) | 8 (9%) | 0 (0%) | 0.16 |
| Theophylline | 0 (0%) | 2 (2%) | 2 (13%) | 0.06 |
| Eosinophil percentage (%) | 2 ± 1 | 4 ± 3 | 3 ± 2 | <0.01 |
| Eosinophil count (/μL) | 105.67 ± 76.2 | 299.05 ± 270.23 | 192.89 ± 116.47 | <0.01 |
| Eosinophil cationic protein | 0.02 | |||
| <24 μg/L | 22 (100%) | 66 (75%) | 14 (88%) | |
| ≥24 μg/L | 0 (0%) | 22 (25%) | 2 (13%) | |
| IgE (IU/mL) | 30.93 ± 26.45 | 485.49 ± 592.8 | 166.41 ± 165.69 | <0.01 |
| FEV1 (%predicted) | <0.01 | |||
| ≥80% | 22 (100%) | 76 (86%) | 4 (25%) | |
| <80% | 0 (0%) | 12 (14%) | 12 (75%) | |
| FVC (%predicted) | <0.01 | |||
| ≥80% | 22 (100%) | 74 (84%) | 8 (50%) | |
| <80% | 0 (0%) | 14 (16%) | 8 (50%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-J.; Tsai, Y.-C.; Chang, W.-A.; Lin, Y.-S.; Tsai, P.-H.; Sheu, C.-C.; Kuo, P.-L.; Hsu, Y.-L. Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease—A Next-Generation Sequencing-Guided Bioinformatic Approach. Int. J. Mol. Sci. 2019, 20, 553. https://doi.org/10.3390/ijms20030553
Tsai M-J, Tsai Y-C, Chang W-A, Lin Y-S, Tsai P-H, Sheu C-C, Kuo P-L, Hsu Y-L. Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease—A Next-Generation Sequencing-Guided Bioinformatic Approach. International Journal of Molecular Sciences. 2019; 20(3):553. https://doi.org/10.3390/ijms20030553
Chicago/Turabian StyleTsai, Ming-Ju, Yu-Chen Tsai, Wei-An Chang, Yi-Shiuan Lin, Pei-Hsun Tsai, Chau-Chyun Sheu, Po-Lin Kuo, and Ya-Ling Hsu. 2019. "Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease—A Next-Generation Sequencing-Guided Bioinformatic Approach" International Journal of Molecular Sciences 20, no. 3: 553. https://doi.org/10.3390/ijms20030553