Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury
Abstract
:1. Introduction
2. Results
2.1. Anti-Inflammatory Activity of RA and its Eluted Fractions
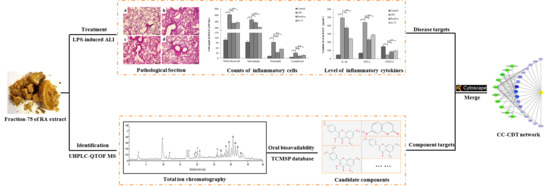
2.2. Protective Effects of Fraction-75 on LPS-Induced ALI
2.2.1. Histopathological Changes, MPO Level, and W/D Weight Ratio in Lung Tissues
2.2.2. Content of Inflammatory Cells in BALF
2.3. Identification of the Candidate Components
2.3.1. The Major Constituents of Fraction-75
2.3.2. Acquisition of the Candidate Components
2.4. Construction of the Network and ELISA Verification of the Key Targets
2.4.1. Candidate Components-Disease Targets Network
2.4.2. Biological Process of the Components’ Targets
2.4.3. ELISA of the Key Targets
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Sample Preparation
4.3. Animals
4.4. Establishment of Mouse Ear Edema Model and Screening of the Effective Fraction
4.5. Set Up of Acute Lung Injury (ALI) Model
4.6. Evaluation of the Histopathological and Wet/Dry Weight Ratio of Lung Tissues
4.7. Measurement of the MPO and Inflammatory Cells
4.8. UHPLC-QTOF MS Analysis of Fraction-75
4.9. Investigations of the Network Pharmacology
4.10. Determination of the IL-1β, TNF-α, IL-6, and VEGFA
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, D.D.; Ren, W.Y.; Jiang, Z.L.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.D.; Frutos-Vivar, F.; Esteban, A.; Fernandez-Segoviano, P.; Aramburu, J.A.; Najera, L.; Stewart, T.E. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit. Care Med. 2005, 33, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, L.; Feng, J.; Wang, Y.; Han, Z.; Meng, J. Downregulation of Paralemmni-3 ameliorates lipopolysaccharide-induced acute lung injury in rats by regulating inflammatory response and inhibiting formation of TLR4/MyD88 and TLR4/TRIF Complexes. Inflammation 2017, 40, 1983–1999. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.F.; Wang, Y.; Li, W.F.; Mu, Q.L.; Li, H.N.; Yao, H.; Zhang, H. Protective effects of isofraxidin against lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015, 24, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Righetti, R.F.; Santos, T.M.D.; Camargo, L.D.N.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; Souza, F.C.R.D.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Leick, E.A. Protective effects of anti-IL17 on acute lung injury induced by LPS in mice. Front. Pharmacol. 2018, 9, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Gohen, P.L. IL-17 stimulates differentiation of human anti-inflammatory macrophages and phagocytosis of apoptotic neutrophils in response to IL-10 and glucocorticoids. J. Immunol. 2013, 190, 5237–5246. [Google Scholar] [CrossRef]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis. Markers 2016, 3501373–3501393. [Google Scholar] [CrossRef]
- Yu, P.; Cheng, S.; Xiang, J.; Yu, B.; Zhang, M.; Zhang, C.F.; Xu, X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015, 164, 328–333. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, Q.S.; Wang, T.; Zhang, H.K.; Tian, Y.; Luo, H.; Yang, S.; Wang, Y.; Huang, X. Inhibition of human gastric carcinoma cell growth in vitro by a polysaccharide from Aster tataricus. Int. J. Biol. Macromol. 2012, 51, 509–513. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Yao, K.J.; Hu, Z.T. Protective effect of Aster tataricus extract on retinal damage on the virtue of its antioxidant and anti-inflammatory effect in diabetic rat. Biomed. Pharmacother. 2017, 89, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Tian, M.; He, Q.W.; Chi, N.; Xiu, C.M.; Wang, Y.B. Effect of Aster tataricus on production of inflammatory mediators in LPS stimulated rat astrocytoma cell line (C6) and THP-1 cells. Saudi Pharm. J. 2017, 25, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Xi, R.G.; Zhang, Z.R.; Li, W.P.; Liu, Y.; Jin, F.G.; Wang, X. 4-Hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1 alpha in seawater aspiration-induced lung injury in rats. Int. J. Mol. Sci. 2014, 15, 12861–12884. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, G.; Xiong, Y.A.; Zhao, H.P.; Yang, M. Effect of shionone on IL-1β, TNF-α and NO release of macrophages induced by lipopolysaccharide. Chinese J. Exp. Tradit. Med. Formulae 2015, 21, 123–125. [Google Scholar]
- Yuan, H.D.; Ma, Q.Q.; Gui, H.Y.; Liu, G.C.; Zhao, X.Y.; Li, W.; Piao, G. How can synergism of traditional medicines benefit from network pharmacology? Molecules 2017, 22, 1135. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Zhang, Y.S.; Li, S.; Tan, H.Y.; Wang, N.; Mu, S.Z.; Hao, X.; Feng, Y. A network pharmacology-based study on the hepatoprotective effect of Fructus Schisandrae. Molecules 2017, 22, 1617. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Xu, X.; Wang, X.; Yu, H.; Li, X.X.; Tao, W.Y.; Wang, Y.; Yang, L. A systems biology approach to understanding the mechanisms of action of Chinese herbs for treatment of cardiovascular disease. Int. J. Mol. Sci. 2012, 13, 13501–13520. [Google Scholar] [CrossRef]
- Hong, M.; Li, S.; Wang, N.; Tan, H.Y.; Cheung, F.; Feng, Y.B. A biomedical investigation of the hepatoprotective effect of Radix salvia miltiorrhizae and network pharmacology-based prediction of the active compounds and molecular targets. Int. J. Mol. Sci. 2017, 18, 620. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Hong, M.; Tan, H.Y.; Pan, G.F.; Feng, Y.B. Hepatoprotective effects of a functional formula of three Chinese medicinal herbs: experimental evidence and network pharmacology-based identification of mechanism of action and potential bioactive components. Molecules 2018, 23, 352. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, K.F.; Wu, H.C.; Yang, C.; Yang, Y.P.; Yang, J.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kB and MAPK activation. Front. Pharmacol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.W.; Yuan, Y.F. Juglanin suppresses fibrosis and inflammation response caused by LPS in acute lung injury. Int. J. Mol. Med. 2018, 41, 3353–3365. [Google Scholar] [CrossRef] [PubMed]
- Akindele, A.J.; Adeyemi, O.O. Antiinflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia 2007, 78, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Tasaka, S.; Fukunaga, K.; Shiraishi, Y.; Moriyama, K.; Miyamoto, K.; Nakano, Y.; Matsunaga, N.; Takashima, K.; Matsumoto, T.; et al. Effect of Toll-like receptor 4 inhibitor on LPS-induced lung injury. Inflamm. Res. 2010, 59, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.V.; Wilson, M.R.; O’Dea, K.P.; Takata, M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J. Immunol. 2013, 190, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Karanikolas, M.; Scolletta, S.; Karamouzos, V.; Velissaris, D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J. Clin. Med. Res. 2012, 4, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Myoshi, K.; Yanagi, S.; Kawahara, K.; Nishio, M.; Tsubouchi, H.; Imazu, Y.; Koshida, R.; Matsumoto, N.; Taguchi, A.; Yamashita, S. Epithelial Pten controls acute lung injury and fibrosis by regulating alveolar epithelial cell integrity. Am. J. Respir. Crit. Care Med. 2013, 187, 262–275. [Google Scholar] [CrossRef]
- Ward, P.A.; Grailer, J.J. Acute lung injury and the role of histones. Transl. Resp. Med. 2014, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Gao, F.Y.; Sun, B.; Hao, J.; Liu, Z.W. Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cell during acute lung injury through suppressing SMAD2 phosphorylation. Cell. Physiol. Biochem. 2015, 37, 759–767. [Google Scholar] [CrossRef]
- Liu, S.; Tang, J.; Huang, L.; Xu, Q.; Ling, X.; Liiu, J. Cordyceps militaris alleviates severity of murine acute lung injury through miRNA-mediated CXCR2 inhibition. Cell. Physiol. Biochem. 2015, 36, 2003–2011. [Google Scholar] [CrossRef]
- Williams, A.E.; Chambers, R.C. The mercurial nature of neutrophils: still an enigma in ARDS? Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Loza, R.C.; Romero-Dapueto, C. Pathophysiological approaches of acute respiratory distress syndrome: novel bases for study of lung injury. Open Resp. Med. J. 2015, 9, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Jandl, K.; Stacher, E.; Balint, Z.; Sturm, E.M.; Maric, J.; Peinhaupt, M.; Luschning, P.; Aringer, I.; Fauland, A.; Konya, V.; et al. Activated prostaglandin D2 receptors on macrophages enhance neutrophil recruitment into the lung. J. Allergy Clin. Immunol. 2016, 137, 833–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck-Schimmer, B.; Schwendener, R.; Pasch, T.; Reyes, L.; Booy, C.; Schimmer, R.C. Alveolar macrophages regulate neutrophil recruitment in endotoxin-induced lung injury. Resp. Res. 2005, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Dudek, S.M.; Jacobson, J.R.; Mereno-Vinasco, L.; Huang, L.S.; Abassi, T.; Mathew, B.; Zhao, Y.; Wang, L.; Bittman, R.; et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir Cell Mol. Biol. 2013, 49, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am. J. Respir Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fu, K.; Lv, X.; Wang, Y.; Wang, J.; Li, H.; Tian, W.; Cao, R. Lactoferrin suppresses lipopolysaccharide-induced endometritis in mice via down-regulation of the NF-kB pathway. Int. Immunopharmacol. 2015, 28, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNF alpha in pulmonary pathophysiology. Resp. Res. 2006, 7, 125. [Google Scholar] [CrossRef]
- Li, T.; Luo, N.; Du, L.; Zhou, J.; Zhang, J.; Gong, L.; Jiang, N. Tumor necrosis factor-alpha plays an initiating role in extracorporeal circulation-induced acute lung injury. Lung 2013, 191, 207–214. [Google Scholar] [CrossRef]
- Tang, M.; Tian, Y.; Li, D.; Lv, J.; Li, Q.; Kuang, C.; Hu, P.; Wang, Y.; Wang, J.; Su, K.; et al. TNF-alpha mediated increase of HIF-1alpha inhibit VASP expression, which reduces alveolar capillary barrier function during acute lung injury (ALI). PLoS ONE 2014, 9, e102967. [Google Scholar]
- Pauwels, N.S.; Bracke, K.R.; Dupont, L.L.; Van-Pottelberge, G.R.; Provoost, S.; Vandenabeele, P.; Lambrecht, B.N.; Joos, G.F.; Brusselle, G.G. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur. Respir. J. 2011, 38, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Shin, J.S.; Lee, J.; Lee, I.H.; Lee, S.K.; Ha, I.H.; Chung, H.J. Anti-inflammatory effect of Cortex Eucommiae via modulation of the toll-like receptor 4 pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 209, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.G.; Kanamarlapudi, V.; Thornton, C.A.; Sheldon, I.M. Signal transducer and activator of transcription-3 licenses toll-like receptor 4-dependent interleukin (IL)-6 and IL-8 production via IL-6 receptor-positive feedback in endometrial cells. Mucosal Immunol. 2016, 9, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lu, H.; Zheng, X.; Huang, X. Effects of vascular endothelial growth factor in recovery phase of acute lung injury in mice. Lung 2015, 193, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Chen, L.M.; Wei, Z.Y.; Wang, P.Q.; Liu, J.; Dong, J.J.; Jia, Z.X.; Yang, J.; Ma, Z.C.; Su, R.B.; et al. Identifying the molecular targets of Salvia miltiorrhiza (SM) in ox-LDL induced macrophage-derived form cells based on the integration of metabolomics s and network pharmacology. RSC Adv. 2018, 8, 3760–3767. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, L.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and Kaempferol cause inhibition of inducible nitric oxide, synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver Cells. Eur. J. Pharmacol 2007, 557, 221–229. [Google Scholar]
- Wall, C.; Lim, R.; Poljak, M.; Lappas, M. Dietary flavonoids as therapeutics for preterm birth: Luteotin and Kaempferol suppress inflammation in human gestational tissues in vitro. Oxid. Med. Cell Longev. 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Man, M.Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical Apigenin alleviates cutaneous inflammation in murine models. Evid.-Based Compl. Alt. Med. 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Hua, F.Z.; Chen, J.; Xu, Z.P.; Sun, H.B.; Qian, Y.N. Protective effects of pretreatment with Oleanolic acid in rats in the acute phase of hepatic ischemia-reperfusion injury: role of the PI3K/Akt pathway. Mediat. Inflamm. 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Haacikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin derivatives effectively suppress inflammation in vitro and in vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Fu, Y.; Wei, Z.; Zhou, E.; Li, Y.; Yao, M.; Song, X.; Yang, Z.; Zhang, N. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-kB signaling pathway. Eur. J. Pharmacol. 2014, 729, 54–58. [Google Scholar]
- Han, J.W.; Shim, D.W.; Shin, W.Y.; Heo, K.H.; Kwak, S.B.; Sim, E.J.; Jeong, J.H.; Kang, T.B.; Lee, K.H. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation. Int. J. Mol. Sci. 2015, 16, 8102–8109. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.J.; Luo, F.; Lu, Q.F.; Liu, J.Y.; Li, P.J.; Wang, X.F.; Fu, Y.; Hao, K.; Yan, T.; Ding, X. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem. Biol. Interact. 2016, 243, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Lin, Y.C.; Wang, H.M.; Chou, T. Chong. Baicalein, an active component of Scutellaria baicalensis, protects against lipopolysaccharide-induced acute lung injury in rats. J. Ethnopharmacol. 2014, 153, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.J.; Jin, H. Oxymatrine attenuates lipopolysaccharide-induced acute lung injury by activating the epithelial sodium channel and suppressing the JNK signaling pathway. Pharmacology 2018, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jian, Z.; Guo, J.; Ning, X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 2014, 117, 19–23. [Google Scholar] [CrossRef] [PubMed]








| Peak No. | RT (min) | [M-H]- (m/z) | Formula | Assignment | RPA a (%) | Error (ppm) | MS/MS Fragments (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | 2.63 | 301.0723 | C16H1406 | Quercetin b | 4.43 | −1.79 | 178.9970, 151.0021, 121.0283, 65.0024 |
| 2 | 9.82 | 285.0411 | C15H10O6 | Kaempferol b | 14.63 | −2.24 | 255.0269, 227.0321, 183.0420, 117.0337 |
| 3 | 11.81 | 315.0514 | C16H12O7 | Isorhamnetin b | 4.03 | −1.19 | 300.0246, 151.0021, 107.0124 |
| 4 | 17.33 | 285.0411 | C15H10O6 | Luteolin b | 3.41 | −2.24 | 267.1959, 178.9063, 131.8974, 67.0191 |
| 5 | 19.12 | 353.0886 | C16H18O9 | 5-Caffeoylquinic acid b | 1.89 | −2.25 | 191.0559, 179.0352, 135.0448 |
| 6 | 19.56 | 353.0887 | C16H18O9 | 4-Caffeoylquinic acid b | 1.73 | −2.53 | 191.0560, 179.0352, 173.0450, 135.0444 |
| 7 | 20.02 | 353.0868 | C16H18O9 | Chlorogenic acid b | 5.85 | 2.57 | 191.0559, 85.0293 |
| 8 | 20.73 | 353.0872 | C16H18O9 | 1-Caffeoylquinic acid b | 3.26 | 1.72 | 191.0566 |
| 9 | 25.92 | 269.0449 | C15H10O5 | Emodin b | 3.05 | 2.40 | 241.0122, 213.0183, 197.0226, 161.0273 |
| 10 | 25.93 | 431.0991 | C21H20O10 | Apigenin 7-glucoside | 1.89 | −1.69 | 277.2140, 171.0044, 152.9944, 96.9689 |
| 11 | 28.37 | 441.3748 | C30H50O2 | Betulin | 5.03 | −2.26 | 167.0002, 122.9745, 96.9591, 79.9567 |
| 12 | 29.29 | 269.0462 | C15H10O5 | Apigenin b | 7.94 | −2.43 | 225.0510, 117.0331, 107.0121, 83.0123 |
| 13 | 30.42 | 161.0242 | C9H6O3 | hydroxycoumarin | 12.81 | 1.35 | 117.0704, 91.0545, 62.0163 |
| 14 | 31.12 | 425.3795 | C30H50O | Taraxerol b | 7.83 | −1.43 | 392.2623, 211.0295, 174.8617, 96.9593 |
| 15 | 31.77 | 455.3542 | C30H48O3 | Oleanolic Acid | 8.28 | −2.48 | 407.1013, 391.4235, 377.2357, 363.0071 |
| 16 | 32.83 | 435.3126 | C26H44O5 | Terpene c | 1.76 | −2.3 | 152.9946, 78.9585 |
| Compound | OB (%) | RPA (%) | CAS |
|---|---|---|---|
| Quercetin | 46.43 | 4.43 | 117-39-5 |
| Kaempferol | 41.88 | 14.63 | 520-18-3 |
| Isorhamnetin | 49.60 | 3.03 | 480-19-3 |
| Luteolin | 36.16 | 3.41 | 491-70-3 |
| Chlorogenic acid | 24.50 | 5.85 | 327-97-9 |
| Emodin | 24.40 | 2.05 | 518-82-1 |
| Betulin | 20.48 | 4.03 | 473-98-3 |
| Apigenin | 23.06 | 7.94 | 520-36-5 |
| Hydroxycoumarin | 25.36 | 12.81 | 93-35-6 |
| Oleanolic acid | 29.02 | 8.28 | 508-02-1 |
| Compound | Key Relevant Targets | Biological Process |
|---|---|---|
| Quercetin | IL6, IL1B, TNF, PTGS2 | regulation of chemokine biosynthetic process (56.25%) |
| TNF, IL10, VEGFA | regulation of chronic inflammatory response to antigenic stimulus (18.75%) | |
| BAX, VEGFA | positive regulation of B cell apoptotic process (12.5%) | |
| CCL2, ICAM1 | negative regulation of vascular endothelial cell proliferation (9.38%) | |
| BAX | retinal cell programmed cell death (3.12%) | |
| Kaempferol | PPARG, HMOX1, TNF | regulation of vascular smooth muscle cell proliferation (33.33%) |
| CAT, CYP1A1, PPARG | response to hyperoxia (26.67%) | |
| IL4, NFE2L2, TNF | endothelial cell apoptotic process (20.0%) | |
| TXN, BAX, BCL2 | homeostasis of number of cells within tissue (20.0%) | |
| Isorhamnetin | TNF, NFE2L2 | regulation of removal of superoxide radicals (40.0%) |
| TNF, HMOX1 | positive regulation of chemokine biosynthetic process (40.0%) | |
| HMOX1, NFE2L2 | regulation of transcription from RNA polymerase II promoter in response to oxidative stress (20.0%) | |
| Luteolin | TNF, IL4, IL1B, TGFB1, HMOX1 | cytokine production involved in immune response (75.93%) |
| TNF, IL6, HMOX1 | regulation of chemokine biosynthetic process (18.52%) | |
| VEGFA, HMOX1, PPARG, TGFB1 | regulation of blood vessel endothelial cell migration (3.7%) | |
| VEGFA, TGFB1, BCL2 | branching involved in ureteric bud morphogenesis (1.85%) | |
| Chlorogenic acid | IL1B, BCL2, BAX | programmed cell death involved in cell development (55.56%) |
| ALB, CAT, GSR, NFE2L2 | cellular oxidant detoxification (33.33%) | |
| VEGFA, PTGS2, NFE2L2 | positive regulation of blood vessel endothelial cell migration (11.11%) | |
| Emodin | TNF, IL1B, IL6, PTGS2 | positive regulation of acute inflammatory response (68.09%) |
| TNF, IL10, VEGFA | regulation of chronic inflammatory response to antigenic stimulus (19.15%) | |
| PPARG, TGFB1 | negative regulation of vascular endothelial cell proliferation (12.77%) | |
| Betulin | BCL2, BAX, FASLG | retinal cell programmed cell death (33.33%) |
| VEGFA | monocyte differentiation (25.0%) | |
| VEGFA, BAX | post-embryonic camera-type eye development (16.67%) | |
| FAS, BAX | positive regulation of cysteine-type endopeptidase activity involved in apoptotic signaling pathway (16.67%) | |
| FASLG, FAS | necroptotic signaling pathway (8.33%) | |
| Apigenin | TNF, IL6, HMOX1 | regulation of chemokine biosynthetic process (42.86%) |
| VEGFA, HMOX1 | positive regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis (14.29%) | |
| VEGFA | positive regulation of transcription from RNA polymerase II promoter in response to hypoxia (14.29%) | |
| BAX | retinal cell programmed cell death (14.29%) | |
| VEGFA, BAX | post-embryonic camera-type eye development (14.29%) | |
| Hydroxycoumarin | NFE2L2 | response to oxygen radical (41.67%) |
| CYP1A1, CAT | response to hyperoxia (25.0%) | |
| F3 | positive regulation of coagulation (25.0%) | |
| F3, CYP1A1 | response to iron ion (8.33%) | |
| Oleanolic acid | IL1B, TNF, IL6, HMOX1 | regulation of chemokine biosynthetic process (83.33%) |
| BAX, FAS, ICAM1 | retinal cell programmed cell death (13.89%) | |
| CAT, PPARG | response to vitamin E (2.78%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dong, J.; Liu, J.; Xu, W.; Wei, Z.; Li, Y.; Wu, H.; Xiao, H. Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 543. https://doi.org/10.3390/ijms20030543
Chen Y, Dong J, Liu J, Xu W, Wei Z, Li Y, Wu H, Xiao H. Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury. International Journal of Molecular Sciences. 2019; 20(3):543. https://doi.org/10.3390/ijms20030543
Chicago/Turabian StyleChen, Yijun, Jiaojiao Dong, Jie Liu, Wenjuan Xu, Ziyi Wei, Yueting Li, Hao Wu, and Hongbin Xiao. 2019. "Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury" International Journal of Molecular Sciences 20, no. 3: 543. https://doi.org/10.3390/ijms20030543