The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock
Abstract
:1. Introduction
2. Results
2.1. Mast Cells Express Functional REV-ERBs
2.2. SR9009 and Other Synthetic Agonists of REV-ERBs Inhibit IgE- and IL-33-Mediated Mast Cell Activation
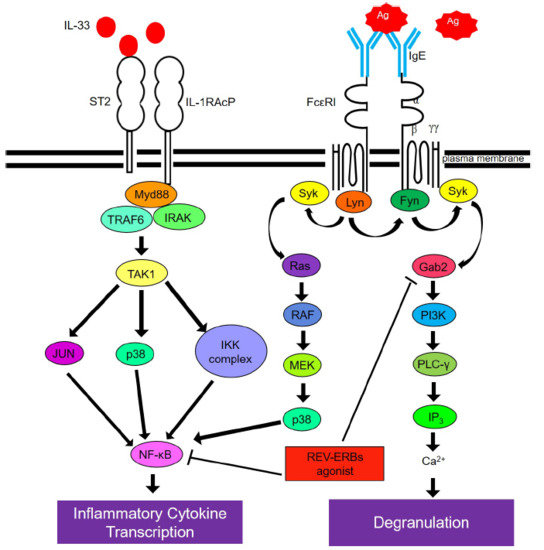
2.3. SR9009 Inhibits IgE- and IL-33-Mediated Activation of the Gab2/PI3K and NF-κB Pathways in Mast Cells
2.4. SR9009 May Inhibit IgE- and IL-33-Mediated Mast Cell Activation Independently of REV-ERBs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mice
4.3. Preparation of Bone Marrow-Derived Mast Cells (BMMCs) and Fetal Skin-Derived Mast Cells (FSMCs)
4.4. Quantitative Real-Time PCR
4.5. Measurement of Bioluminescence in BMMCs Generated from Per2Luc Mice
4.6. Flow Cytometry Analysis
4.7. Stimulations of BMMCs or FSMCs
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. β-Hexosaminidase Release Assay
4.10. Western Blot Analysis
4.11. Reporter Assays
4.12. Cell Viability Assay
4.13. Passive Cutaneous Anaphylaxis
4.14. siRNAs Experiments
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor κB |
References
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Nakano, N.; Ishimaru, K.; Hara, M.; Ikegami, T.; Tahara, Y.; Katoh, R.; Ogawa, H.; Okumura, K.; Shibata, S.; et al. Circadian regulation of allergic reactions by the mast cell clock in mice. J. Allergy Clin. Immunol. 2014, 133, 568–575. [Google Scholar] [CrossRef]
- Kawauchi, T.; Ishimaru, K.; Nakamura, Y.; Nakano, N.; Hara, M.; Ogawa, H.; Okumura, K.; Shibata, S.; Nakao, A. Clock-dependent temporal regulation of IL-33/ST2-mediated mast cell response. Allergol. Int. 2017, 66, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic diseases. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Morita, H.; Arae, K.; Matsumoto, K.; Nakae, S. Interleukin-33 in allergy. Allergy 2012, 67, 1203–1214. [Google Scholar] [CrossRef] [Green Version]
- Christ, P.; Sowa, A.S.; Froy, O.; Lorentz, A. The Circadian Clock Drives Mast Cell Functions in Allergic Reactions. Front Immunol. 2018, 9, 1526. [Google Scholar] [CrossRef] [Green Version]
- Nakao, A. Clockwork allergy: How the circadian clock underpins allergic reactions. J. Allergy Clin. Immunol. 2018, 142, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef]
- Lam, M.T.; Cho, H.; Lesch, H.P.; Gosselin, D.; Heinz, S.; Tanaka-Oishi, Y.; Benner, C.; Kaikkonen, M.U.; Kim, A.S.; Kosaka, M.; et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature 2013, 498, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wang, Y.; Solt, L.A.; Griffett, K.; Kazantzis, M.; Amador, A.; El-Gendy, B.M.; Huitron-Resendiz, S.; Roberts, A.J.; Shin, Y.; et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat. Commun. 2014, 5, 5759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Nat. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.J.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Harama, D.; Shimokawa, N.; Hara, M.; Suzuki, R.; Tahara, Y.; Ishimaru, K.; Katoh, R.; Okumura, K.; Ogawa, H.; et al. Circadian clock gene Period2 regulates a time-of-day-dependent variation in cutaneous anaphylactic reaction. J. Allergy Clin. Immun. 2011, 127, 1038–1045. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; King, D.P.; Chang, A.M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef] [Green Version]
- Yoo, O.J.; Menaker, M.; Takahashi, J.S. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Nat. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.K.; Yoo, S.H.; Liu, A.C.; Takahashi, J.S.; Kay, S.A. Bioluminescence imaging of individual fibroblasts reveals persistent independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004, 14, 2289–2295. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, D.D.; Peavey, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Takatori, H.; Makita, S.; Ito, T.; Matsuki, A.; Nakajima, H. Regulatory Mechanisms of IL-33-ST2-Mediated Allergic Inflammation. Front. Immunol. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Saito, K.; Klaman, L.D.; Shen, J.; Fleming, T.; Wang, Y.; Pratt, J.C.; Lin, G.; Lim, B.; Kinet, J.P.; et al. Essential role for Gab2 in the allergic response. Nature 2001, 412, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Sulli, G.; Manoogian, E.N.C.; Taub, P.R.; Panda, S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci. 2018, 39, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Shirato, K.; Ishibashi, Y.; Oh-ishi, S.; Imaizumi, K.; Haga, S.; Hitomi, Y.; Izawa, T.; et al. Direct and indirect suppression of interleukin-6 gene expression in murine macrophages by nuclear orphan receptor REV-ERBα. Sci. World J. 2014, 2014, 685854. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Lin, Y.; Yuan, X.; Li, F.; Guo, L.; Wu, B. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat. Commun. 2018, 9, 4246. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Tan, P.; Zhang, Z.; Wu, W.; Dong, Y.; Zhao, L.; Liu, H.; Guan, H.; Li, F. REV-ERB agonism suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss partially via FABP4 upregulation. FASEB J. 2018, 32, 3215–3228. [Google Scholar] [CrossRef] [Green Version]
- Dierickx, P.; Emmett, M.J.; Jiang, C.; Uehara, K.; Liu, M.; Adlanmerini, M.; Lazar, M.A. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc. Nat. Acad. Sci. USA 2019, 116, 12147–12152. [Google Scholar] [CrossRef] [Green Version]
- Matsushima, H.; Yamada, N.; Matsue, H.; Shimada, S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J. Immunol. 2004, 173, 531–541. [Google Scholar] [CrossRef] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishimaru, K.; Nakajima, S.; Yu, G.; Nakamura, Y.; Nakao, A. The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock. Int. J. Mol. Sci. 2019, 20, 6320. https://doi.org/10.3390/ijms20246320
Ishimaru K, Nakajima S, Yu G, Nakamura Y, Nakao A. The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock. International Journal of Molecular Sciences. 2019; 20(24):6320. https://doi.org/10.3390/ijms20246320
Chicago/Turabian StyleIshimaru, Kayoko, Shotaro Nakajima, Guannan Yu, Yuki Nakamura, and Atsuhito Nakao. 2019. "The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock" International Journal of Molecular Sciences 20, no. 24: 6320. https://doi.org/10.3390/ijms20246320