Pairing and Exchanging between Daypyrum villosum Chromosomes 6V#2 and 6V#4 in the Hybrids of Two Different Wheat Alien Substitution Lines
Abstract
:1. Introduction
2. Results
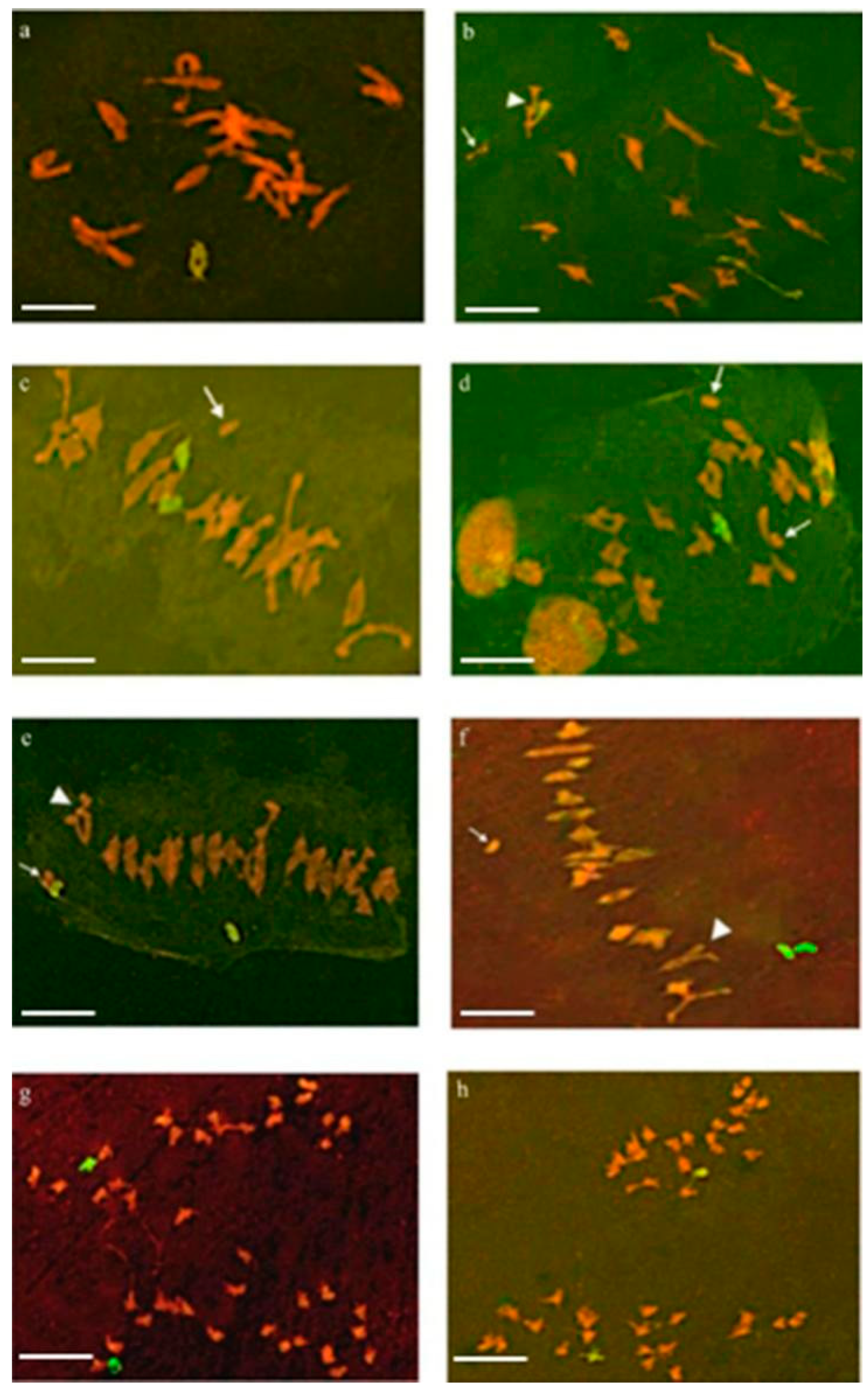
2.1. Pairing Behavior of 6V#2 and 6V#4 Chromosomes in Their F1 Hybrids
2.2. Detection of Plant Genotypes by Nine 6VS-Specific Molecular Markers in the F2 Populations
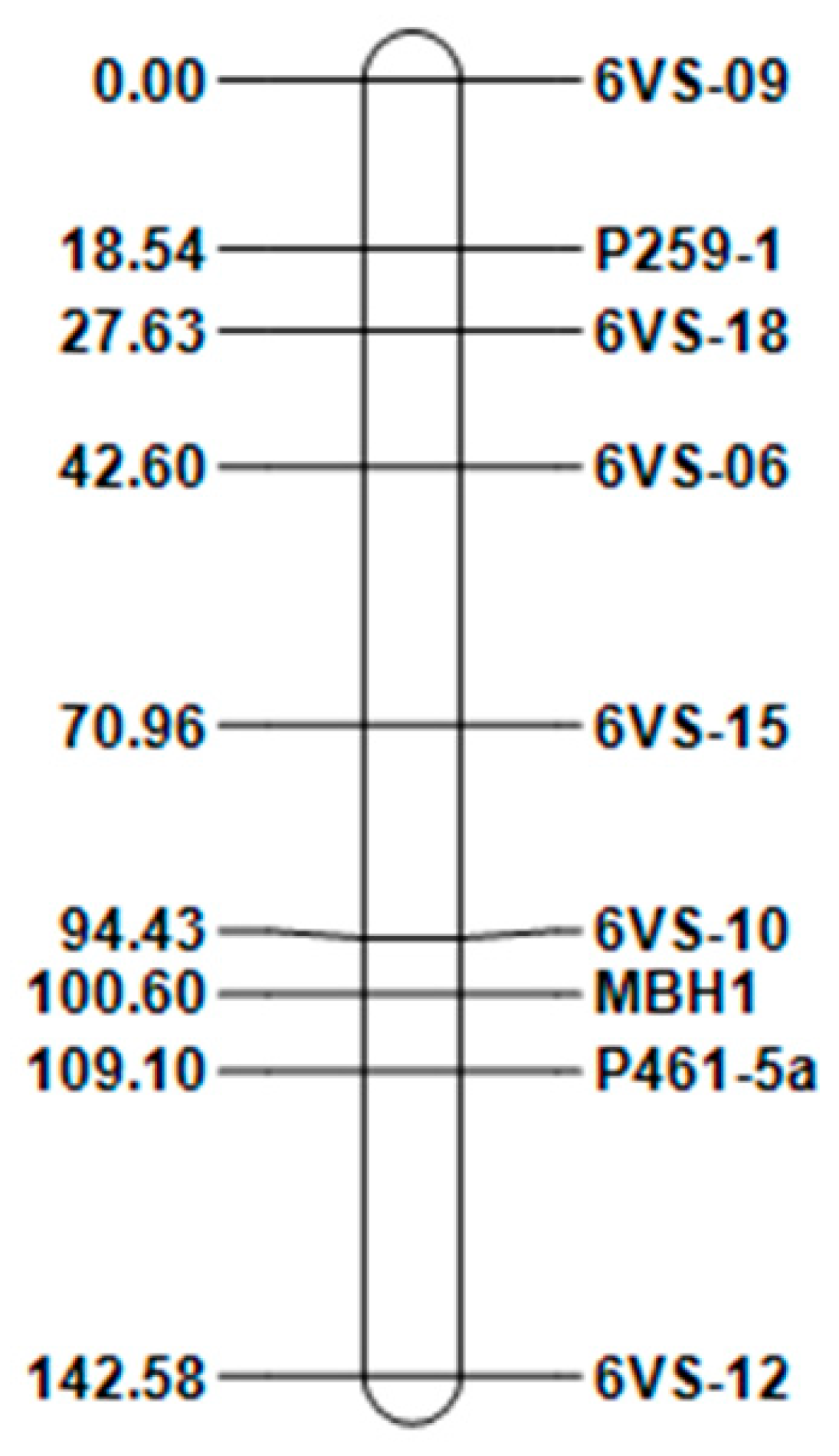
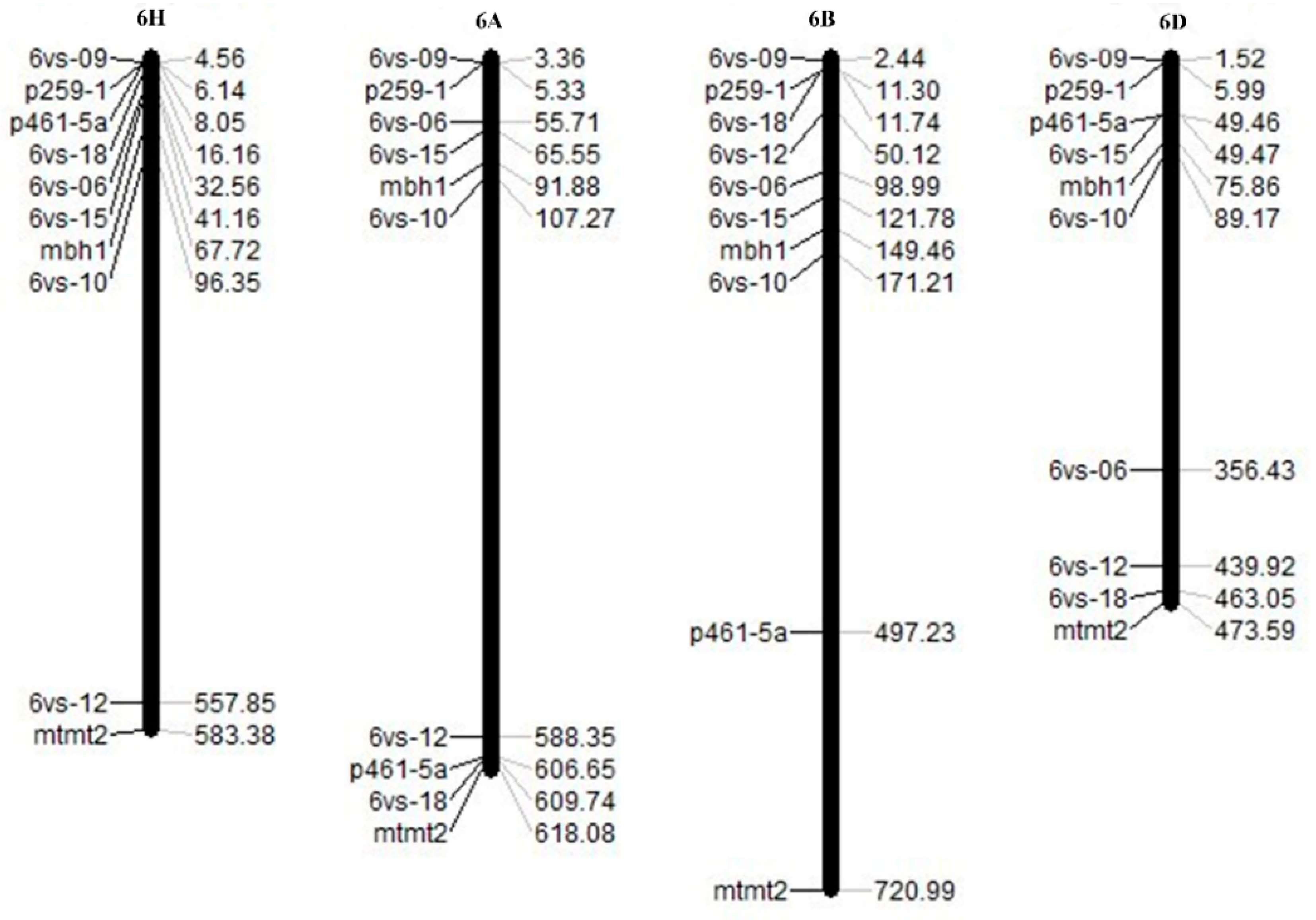
2.3. Construction of the 6VS Genetic Linkage Map and Collinearity Analysis
2.4. Development of 6A- and 6D-Specific Molecular Markers and Detection of 6A and 6D Chromosomes in the F2 Populations
2.5. Development of Pm21 and Its Allele-Specific Molecular Markers and Detection of Both Genes in the Testcross Populations
2.6. Identification of New Genotypes
3. Discussion
3.1. Distribution of Amplified Sequences on Chromosomes and Their Separation Ratios in Hybrid Progenies
3.2. Two Alien Chromosomes 6V#2 and 6V#4 Pair and Exchange in Their Hybrids
3.3. Screening of Molecular Markers for Novel Alien Substitution Lines
3.4. Importance of Genetic Linkage Map for Genera That Lack Genome Sequence Information
4. Materials and Methods
4.1. Plant Materials
4.2. Molecular Markers
4.3. DNA Extraction and PCR Amplification Conditions
4.4. Construction of a Linkage Map and Comparison Maps
4.5. Cytological Procedures
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GISH | genomic in situ hybridization |
| MAS | marker-assisted selection |
| PMCs | pollen mother cells |
| FITC | fluorescein isothiocyanate |
| PCR | polymerase chain reaction |
| PM | powdery mildew |
References
- Grądzielewska, A. The genus Dasypyrum-part 2. Dasypyrum villosum-a wild species used in wheat improvement. Euphytica 2006, 152, 441–454. [Google Scholar]
- Pace, C.D.; Snidaro, D.; Ciaffi, M.; Vittori, D.; Ciofo, A.; Cenci, A.; Tanzarella, O.A.; Qualset, C.O.; Scarascia Mugnozza, G.T. Introgression of Dasypyrum villosum chromatin into common wheat improves grain protein quality. Euphytica 2011, 117, 67–75. [Google Scholar] [CrossRef]
- Qi, L.; Friebe, B.; Zhang, P.; Gill, B.S. Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 2007, 15, 3–19. [Google Scholar] [CrossRef]
- Li, K.; Hegarty, J.; Zhang, C.; Wan, A.; Wu, J.; Guedira, G.B.; Chen, X.; Muñoz-Amatriaín, M.; Fu, D.; Dubcovsky, J. Fine mapping of barley locusRps6conferring resistance to wheat stripe rust. Theor. Appl. Genet. 2016, 129, 845–859. [Google Scholar] [CrossRef]
- Xing, L.; Hu, P.; Liu, J.; Witek, K.; Zhou, S.; Xu, J.; Zhou, W.; Gao, L.; Huang, Z.; Zhang, R.; et al. Pm21 from Haynaldia villosa Encodes a CC-NBS-LRR that Confers Powdery Mildew Resistance in Wheat. Mol. Plant. 2018, 11, 874–878. [Google Scholar] [CrossRef]
- Blanco, A.; Resta, P.; Simeone, R.; Parmar, S.; Shewry, P.R.; Sabelli, P.; Lafiandra, D. Chromosomal location of seed storage protein genes in the genome of Dasypyrum villosum (L.) Candargy. Theor. Appl. Genet. 1991, 82, 358–362. [Google Scholar] [CrossRef]
- Chen, P.; Liu, D. Cytogenetic studies of hybrid progenies between triticum aestivum and haynaldia villosa. J. Nanjing Agric. Univ. 1982, 4, 1–16. [Google Scholar]
- Blanco, A.; Simeone, R.; Resta, P. The addition of Dasypyrum villosum (L.) Candargy chromosomes to durum wheat (Triticum durum Desf.). Theor. Appl. Genet. 1987, 74, 328–333. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Li, D.; Yang, E.; Huang, Y.; Liu, C.; Yang, Z. A Novel Wheat-Dasypyrum breviaristatum Substitution Line with Stripe Rust Resistance. Cytogenet. Genome Res. 2014, 143, 280–287. [Google Scholar] [CrossRef]
- Li, S.; Lin, Z.; Liu, C.; Wang, K.; Du, L.; Ye, X. Development and comparative genomic mapping of Dasypyrum villosum 6V#4S-specific PCR markers using transcriptome data. Theor. Appl. Genet. 2017, 130, 2057–2068. [Google Scholar]
- Li, S.; Wang, J.; Wang, K.; Chen, J.; Wang, K.; Du, L.; Ni, Z.; Lin, Z.; Ye, X. Development of PCR markers specific to Dasypyrum villosum genome based on transcriptome data and their application in breeding Triticum aestivum-D. villosum#4 alien chromosome lines. BMC Genom. 2019, 20, 289. [Google Scholar]
- Qi, L.; Wang, S.; Chen, P.; Liu, D.; Gill, B.S. Identification and physical mapping of three Haynaldia villosa chromosome-6V deletion lines. Theor. Appl. Genet. 1998, 97, 1042–1046. [Google Scholar] [CrossRef]
- He, H.; Zhu, S.; Zhao, R.; Jiang, Z.; Ji, Y.; Ji, J.; Qiu, D.; Li, H.; Bie, T. Pm21 encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol. Plant 2018, 11, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, J.; Hua, W.; Yang, Z.; Sun, Q.; Liu, Z. Comparative Genomics Analysis and Constructing EST Markers Linkage Map of Powdery Mildew Resistance Gene pm42 in Wheat. Acta Agron. Sin. 2011, 37, 1569–1576. (In Chinese) [Google Scholar] [CrossRef]
- Ando, K.; Krishnan, V.; Rynearson, S.; Rouse, M.N.; Danilova, T.; Friebe, B.; See, D.; Pumphrey, M.O. Introgression of a Novel Ug99-Effective Stem Rust Resistance Gene into Wheat and Development of Dasypyrum villosum Chromosome-Specific Markers via Genotyping-by-Sequencing (GBS). Plant Dis. 2019, 103, 1068–1074. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, M.; Wang, X.; Chen, P. Introduction of chromosome segment carrying the seed storage protein genes from chromosome 1V of Dasypyrum villosum showed positive effect on bread-making quality of common wheat. Theor. Appl. Genet. 2014, 127, 523–533. [Google Scholar]
- Zhang, R.; Hou, F.; Feng, Y.; Zhang, W.; Zhang, M.; Chen, P. Characterization of a Triticum aestivum-Dasypyrum villosum T2VS·2DL translocation line expressing a longer spike and more kernels traits. Theor. Appl. Genet. 2015, 128, 2415–2425. [Google Scholar] [CrossRef]
- Zhang, J.; jiang, Y.; Wang, Y.; Guo, Y.; Long, H.; Deng, G.; Chen, Q.; Xuan, P. Molecular markers and cytogenetics to characterize a wheat-Dasypyrum villosum 3V (3D) substitution line conferring resistance to stripe rust. PLoS ONE 2018, 13, e0202033. [Google Scholar] [CrossRef]
- Wang, H.; Dai, K.; Xiao, J.; Yuan, C.; Zhao, R.; Doležel, J.; Wu, Y.; Cao, A.; Chen, P.; Zhang, S.; et al. Development of intron targeting (IT) markers specific for chromosome arm 4VS of Haynaldia villosa by chromosome sorting and next-generation sequencing. BMC Genom. 2017, 18, 167. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, B.; Chen, J.; Cao, A.; Xing, L.; Feng, Y.; Lan, C.; Chen, P. Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor. Appl. Genet. 2016, 129, 1975–1984. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, A.; Wang, X.; Chen, P. Screening and Application of EST-Based PCR Markers Specific to Individual Chromosomes of Haynaldia villosa. Acta Agron. Sin. 2009, 35, 1–10. [Google Scholar]
- Liu, C.; Li, S.; Wang, K.; Ye, X.; Lin, Z. Developing of Specific Transcription Sequences P21461 and P33259 on Dasypyrum villosum 6VS and Application of Molecular Markers in Identifying Wheat-D. villosum Breeding Materials with Powdery Mildew Resistance. Acta Agron. Sin. 2017, 43, 983–992. (In Chinese) [Google Scholar] [CrossRef]
- Bie, T.; Zhao, R.; Zhu, S. Development and characterization of marker MBH1 simultaneously tagging genes Pm21 and PmV conferring resistance to powdery mildew in wheat. Mol. Breed 2015, 35, 189. [Google Scholar] [CrossRef]
- Kreiner, J.M.; Kron, P.; Husband, B.C. Evolutionary Dynamics of Unreduced Gametes. Trends Genet. 2017, 33, 583–593. [Google Scholar] [CrossRef]
- Mason, A.S.; Pires, J.C. Unreduced gametes: meiotic mishap or evolutionary mechanism? Trends Genet. 2015, 31, 5–10. [Google Scholar] [CrossRef]
- Liu, C.; Ye, X.; Wang, M.; Li, S.; Lin, Z. Genetic behavior of Triticum aestivum-Dasypyrum villosum translocation chromosomes T6V#4S·6DL and T6V#2S·6AL carrying powdery mildew resistance. J. Integr. Agric. 2017, 16, 2136–2144. [Google Scholar]
- Zhang, X.; Chen, S. Production and Utilization of Alien Substitution Lines of Common Wheat. Hereditas 1990, 12, 40–44. (In Chinese) [Google Scholar]
- Sears, E.R. Nullisomic Analysis in Common Wheat. Am. Nat. 1953, 87, 245–252. [Google Scholar] [CrossRef]
- Ghazali, S.; Mirzaghaderi, G.; Majdi, M. Production of a novel Robertsonian translocation from Thinopyrum bessarabicum into bread wheat. Tsitol Genet 2015, 49, 38–42. [Google Scholar] [CrossRef]
- Danilova, T.V.; Friebe, B.; Gill, B.S.; Poland, J.; Jackson, E. Chromosome Rearrangements Caused by Double Monosomy in Wheat-Barley Group-7 Substitution Lines. Cytogenet. Genome Res. 2018, 154, 45–55. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Che, Y.; Liu, W.; Lu, Y.; Yang, X.; Li, X.; Jia, J.; Liu, X.; Li, L. Construction of Agropyron Gaertn. genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2018, 16, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Danilova, T.V.; Friebe, B.; Gill, B.S. Development of a wheat single gene FISH map for analyzing homoeologous relationship and chromosomal rearrangements within the Triticeae. Theor. Appl. Genet. 2014, 127, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Huang, L.; Gao, A.; Zhang, J.; Yang, X.; Liu, W.; Li, X.; Li, L. A high-density genetic map for P genome of Agropyron Gaertn. based on specific-locus amplified fragment sequencing (SLAF-seq). Planta 2015, 242, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hackett, C.A.; Bradshaw, J.E.; McNicol, J.W.; Milbourne, D. Construction of a genetic linkage map in tetraploid species using molecular markers. Genetics 2001, 157, 1369–1385. [Google Scholar]
- Qi, P.; Eudy, D.; Schnable, J.C.; Schmutz, J.; Raymer, P.L.; Devos, K.M. High Density Genetic Maps of Seashore Paspalum Using Genotyping-By-Sequencing and Their Relationship to The Sorghum Bicolor Genome. Sci. Rep. 2019, 9, 12183. [Google Scholar] [CrossRef]
- Jia, J. Molecular Germplasm Diagnostics and Molecular Marker assisted Breeding. Scientia Agricultura Sinica 1996, 29, 1–10. (In Chinese) [Google Scholar]
- Qi, L.; Echalier, B.; Chao, S.; Lazo, G.R.; Butler, G.E.; Anderson, O.D.; Akhunov, E.D.; Dvorák, J.; Linkiewicz, A.M.; Ratnasiri, A.; et al. A Chromosome Bin Map of 16,000 Expressed Sequence Tag Loci and Distribution of Genes Among the Three Genomes of Polyploid Wheat. Genetics 2004, 168, 701–712. [Google Scholar] [CrossRef]
- Zimin, A.V.; Puiu, D.; Hall, R.; Kingan, S.; Clavijo, B.J.; Salzberg, S.L. The first near-complete assembly of the hexaploid bread wheat genome, Triticum aestivum. Gigascience 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Lu, F.H.; McKenzie, N.; Kettleborough, G.; Heavens, D.; Clark, M.D.; Bevan, M.W. Independent assessment and improvement of wheat genome sequence assemblies using Fosill jumping libraries. Gigascience 2018, 7, giy053. [Google Scholar] [CrossRef]
- International Barley Genome Sequencing Consortium; Mayer, K.F.; Waugh, R.; Brown, J.W.; Schulman, A.; Langridge, P.; Platzer, M.; Fincher, G.B.; Muehlbauer, G.J.; Sato, K.; et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 2012, 491, 711–716. [Google Scholar]
- Ariyadasa, R.; Mascher, M.; Nussbaumer, T.; Schulte, D.; Frenkel, Z.; Poursarebani, N.; Zhou, R.; Steuernagel, B.; Gundlach, H.; Taudien, S.; et al. A sequence-ready physical map of barley anchored genetically by two million single-nucleotide polymorphisms. Plant Physiol. 2014, 164, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Mascher, M.; Gundlach, H.; Himmelbach, A.; Beier, S.; Twardziok, S.O.; Wicker, T.; Radchuk, V.; Dockter, C.; Hedley, P.E.; Russell, J.; et al. A chromosome conformation capture ordered sequence of the barley genome. Nature 2017, 544, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Wang, H.; Xiao, J.; Bie, T.; Cheng, S.; Jia, Q.; Yuan, C.; Zhang, R.; Cao, A.; Chen, P.; et al. Induction of 4VS chromosome recombinants using the CS ph1b mutant and mapping of the wheat yellow mosaic virus resistance gene from Haynaldia villosa. Theor. Appl. Genet. 2013, 126, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deal, K.R.; Luo, M.; Ji, W.; Distelfeld, A.; Dvorak., J. Introgression of the Aegilops speltoides Su1-Ph1 Suppressor into Wheat. Front. Plant. Sci. 2017, 8, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.H.; Qin, R.; Song, Y.C.; Ning, S.B.; Guo, L.Q.; Gu, M.G. Location and analysis of introgressed segments in the parthenogenetic progenies of Zea mays×Z. diploperennis by GISH. Acta Bot. Sin. 2002, 44, 373–376. [Google Scholar]
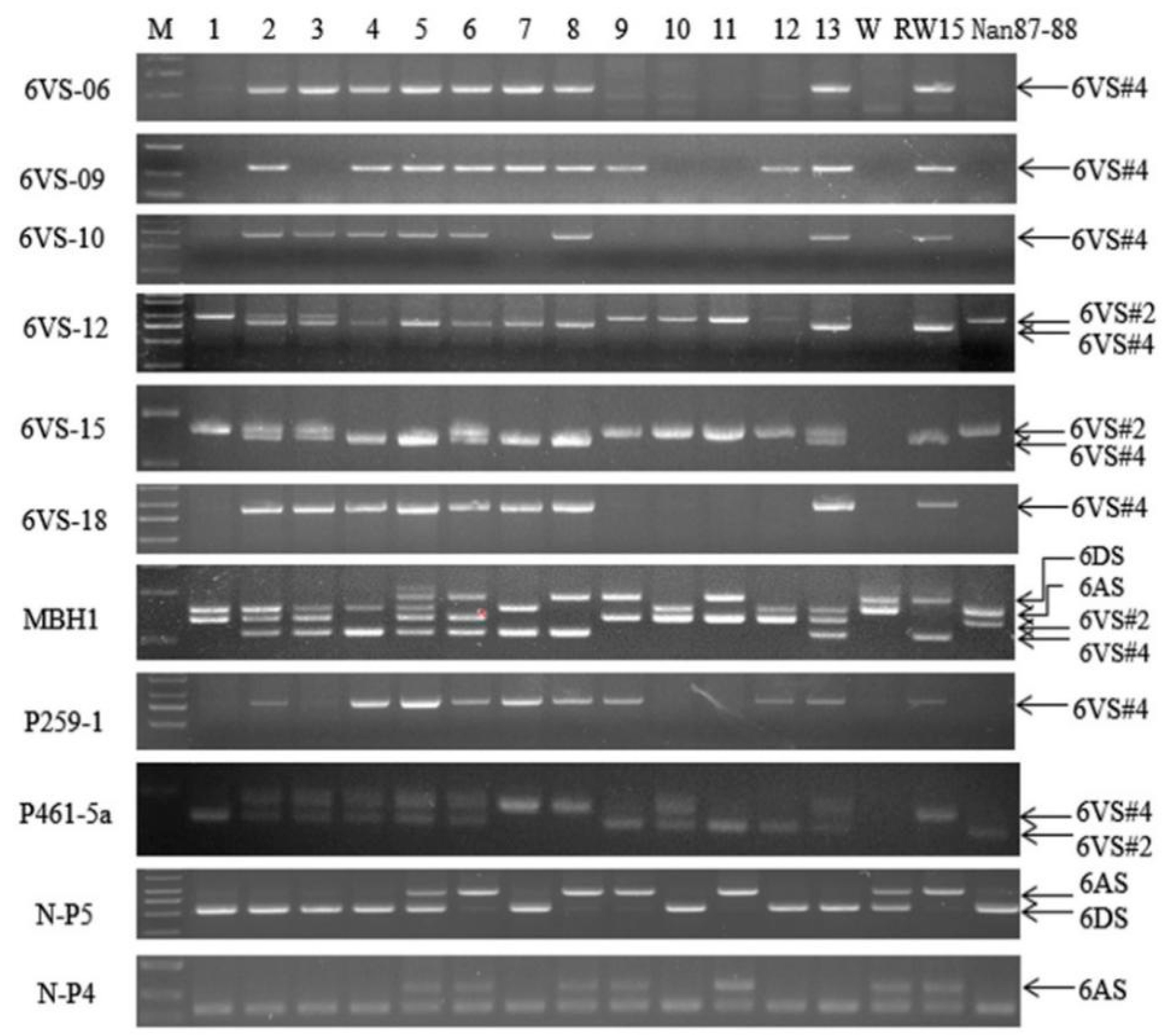

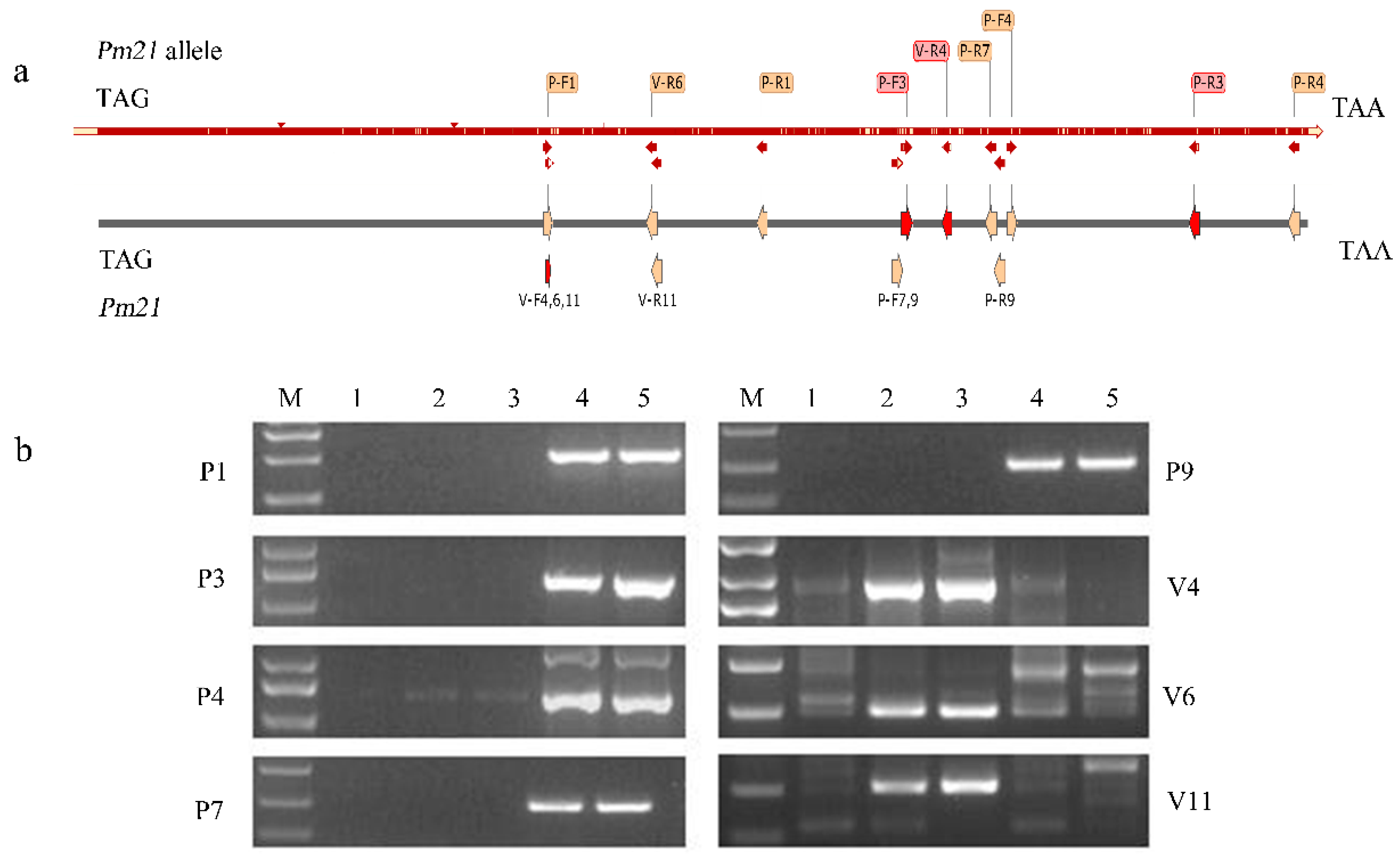
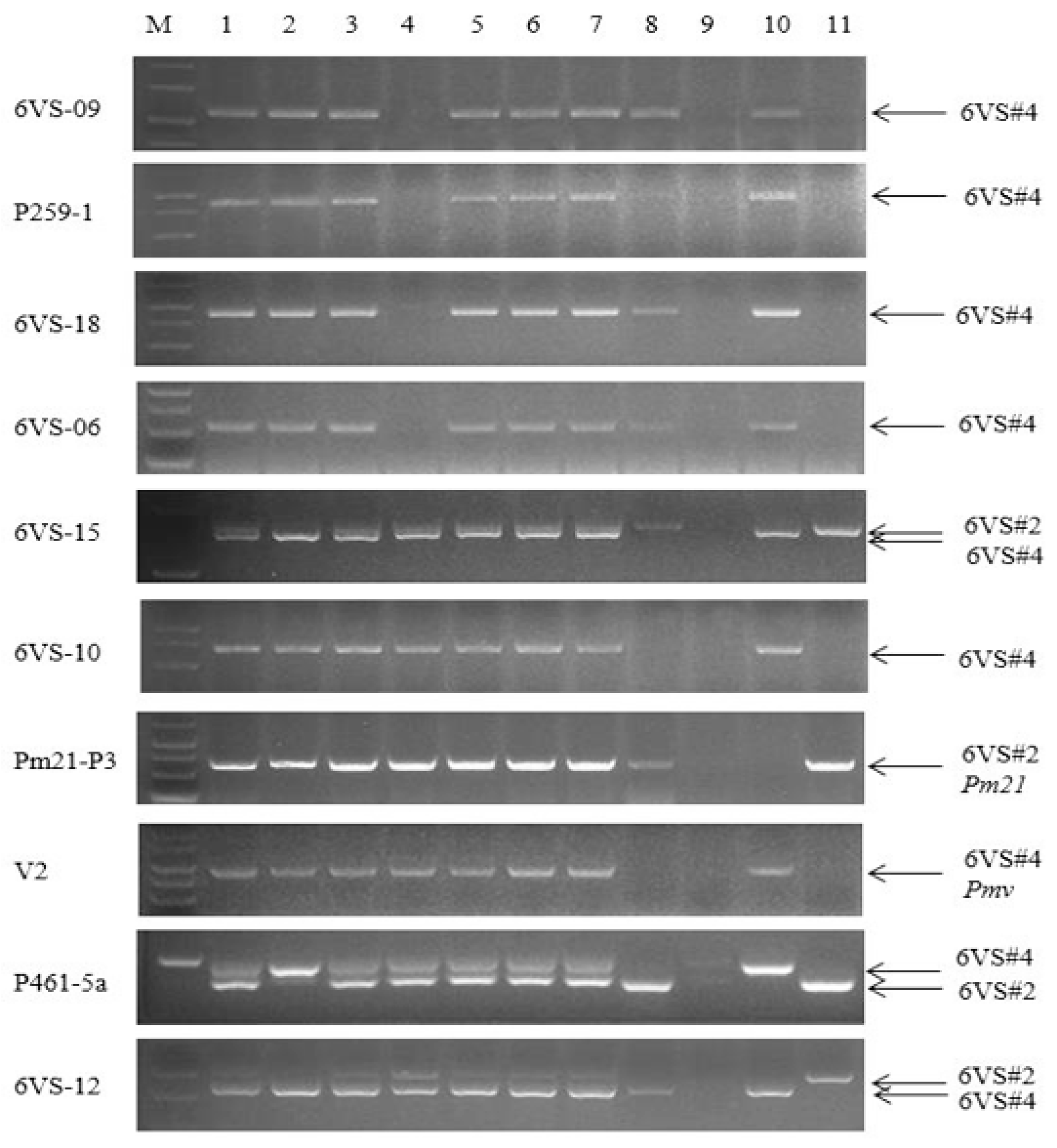

| Materials | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Wan 7107 | RW15 | Nan 87–88 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers | ||||||||||||||||
| 6VS-06 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 | - | - | - | - | 4 | - | 4 | - |
| 6VS-09 | - | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | - | - | 4 | 4 | - | 4 | - |
| 6VS-10 | - | 4 | 4 | 4 | 4 | 4 | - | 4 | - | - | - | - | 4 | - | 4 | - |
| 6VS-12 | 2 | 2, 4 | 2, 4 | 4 | 4 | 4 | 4 | 4 | 2 | 2 | 2 | - | 4 | - | 4 | 2 |
| 6VS-15 | 2 | 2, 4 | 2, 4 | 4 | 4 | 2, 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2, 4 | - | 4 | 2 |
| 6VS-18 | - | 4 | 4 | 4 | 4 | 4 | 4 | 4 | - | - | - | - | 4 | - | 4 | - |
| MBH1 | 2 | 2, 4 | 2, 4 | 4 | 2, 4 | 2, 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2, 4 | - | 4 | 2 |
| P259-1 | - | 4 | - | 4 | 4 | 4 | 4 | 4 | 4 | - | - | 4 | 4 | - | 4 | - |
| P461-5a | 2 | 2, 4 | 2, 4 | 2, 4 | 2, 4 | 2, 4 | 4 | 4 | 2 | 2,4 | 2 | 2 | 2, 4 | - | 4 | 2 |
| N-P4 | - | - | - | - | 6A | 6A | - | 6A | 6A | - | 6A | - | - | 6A | 6A | - |
| N-P5 | 6D | 6D | 6D | 6D | 6A, 6D | - | 6D | - | - | 6D | - | 6D | 6D | 6A, 6D | 6A | 6D |
| Markers | Total | #2+ | #4+ | #2+#4+ | Chi-Square Value X23:1 = 6.635, X21:2:1 = 9.210, p = 0.01 |
|---|---|---|---|---|---|
| 6VS-06 | 323 | - | 233 | - | 1.41 < X23:1 |
| 6VS-09 | 323 | - | 198 | - | 32.33 > X23:1 |
| 6VS-10 | 323 | - | 228 | - | 3.35 < X23:1 |
| 6VS-18 | 323 | - | 229 | - | 2.90 < X23:1 |
| P259-1 | 323 | - | 236 | - | 0.64 < X23:1 |
| MBH1 | 316 | 84 | 79 | 153 | 0.47 < X21:2:1 |
| 6VS-12 | 307 | 55 | 103 | 149 | 15.27 > X21:2:1 |
| 6VS-15 | 125 | 36 | 30 | 59 | 0.97 < X21:2:1 |
| P461-5a | 308 | 77 | 94 | 137 | 5.63 < X21:2:1 |
| Primer | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) | Specific Type | Reference |
|---|---|---|---|---|
| N-P4 | TTAAGAATGTAAGATCGTTGACCCGTAGAC | GGACTGTGACTTGTGAGCATGATGT | 6AS | Present study |
| N-P5 | GCGACCTGTTAGAATGCTATTACGATTAC | ATGCTACTCTACCGATGCTTTGAACC | 6AS, 6DS | Present study |
| ND-P7 | AACTCTAAGCTCCGCATCATCAATCAT | CTGCTGCCTCATCCAGTTACCAAG | 6DS | Present study |
| ND-P8 | GCAACTCTAAGCTCCGCATCATCA | CTGCTGCCTCATCCAGTTACCAAG | 6DS | Present study |
| P1 | AGAGTATTTGGTTCCGGATATG | TTTCTGCACACTTGCTGAGGAT | 6V#2 (Pm21) | Present study |
| P3 | CATACGGAATAGATTTTCCTACCGAAT | TAGCCCTATCTGAAACTGCATGTC | 6V#2 (Pm21) | Present study |
| P4 | AGTCTGAGGGAGCTGAGGCTTTACA | GACCACATTCATAGAACTGAGGGGAA | 6V#2 (Pm21) | Present study |
| P7 | GTATGTCAAGGTTTCTGCTTCATACGG | ATATCCTCTAGCGAGATGTGCTCCATA | 6V#2 (Pm21) | Present study |
| P9 | GTATGTCAAGGTTTCTGCTTCATACGG | AGCTCGCCAAGGCCATTAATATCC | 6V#2 (Pm21) | Present study |
| V4 | TTTGGTTCCGGACCCTGCCC | GACAACCGTGGCAAGCAGACAA | 6V#4 (Pm21 allele) | Present study |
| V6 | TTTGGTTCCGGACCCTGCCC | ACATGGACGGAGATGAAGAGGAAGAT | 6V#4 (Pm21 allele) | Present study |
| V11 | TTTGGTTCCGGACCCTGCCC | AGTACAGGAGACATGGACGGAGATG | 6V#4 (Pm21 allele) | Present study |
| 6VS-06 | GACAGGCAGCTATGAGGC | AATCGTCGTTTGGAGTGG | 6V#4S | [10] |
| 6VS-09 | GTAAGAACAAGAGGCTAAACAG | CCAGATGACGGTTATTACATAG | 6V#4S | [10] |
| 6VS-10 | GCCATAAGTGACGCTGAT | GCATCCTGTGAAGTTGTTG | 6V#4S | [10] |
| 6VS-12 | TGTTGCCTCTCCTCATCA | ATTGCTGTCCGCTCATAC | 6V#4S, 6V#2S | [10] |
| 6VS-15 | AGGACCATACATTCACAGAG | TTCCATGAGCAGATTAGCA | 6V#4S, 6V#2S | [10] |
| 6VS-18 | AGCCAGTAAGATTCCGTATG | TCTAACCTTCCTCACAACAC | 6V#4S | [10] |
| P461-5a | GCGTCATCCGCGCCCGTCAGGT | GAGTGCTAATGATAGATGTG | 6V#4S, 6V#2S | [22] |
| P259-1 | CGTGATTCAGGAAATGCGATAC | TTGCGCCGCCATGTTAG | 6V#4S | [22] |
| MBH1 | GCCATTATAGTCAAGAGTGCACTAGCTGT | AGCTCCTCTCGTTCTCCAATGCT | 6AS, 6DS, 6V#4S, 6V#2S | [23] |
| Total Plants Investigated | 6A+6D− | 6A−6D+ | 6A+6D+ | 6A−6D− |
|---|---|---|---|---|
| 319 | 86 | 121 | 97 | 15 |
| 100% | 26.96% | 37.93% | 30.41% | 4.70% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xu, Z.; Wang, J.; Chen, H.; Ye, X.; Lin, Z. Pairing and Exchanging between Daypyrum villosum Chromosomes 6V#2 and 6V#4 in the Hybrids of Two Different Wheat Alien Substitution Lines. Int. J. Mol. Sci. 2019, 20, 6063. https://doi.org/10.3390/ijms20236063
Ma X, Xu Z, Wang J, Chen H, Ye X, Lin Z. Pairing and Exchanging between Daypyrum villosum Chromosomes 6V#2 and 6V#4 in the Hybrids of Two Different Wheat Alien Substitution Lines. International Journal of Molecular Sciences. 2019; 20(23):6063. https://doi.org/10.3390/ijms20236063
Chicago/Turabian StyleMa, Xiaolan, Zhiying Xu, Jing Wang, Haiqiang Chen, Xingguo Ye, and Zhishan Lin. 2019. "Pairing and Exchanging between Daypyrum villosum Chromosomes 6V#2 and 6V#4 in the Hybrids of Two Different Wheat Alien Substitution Lines" International Journal of Molecular Sciences 20, no. 23: 6063. https://doi.org/10.3390/ijms20236063




