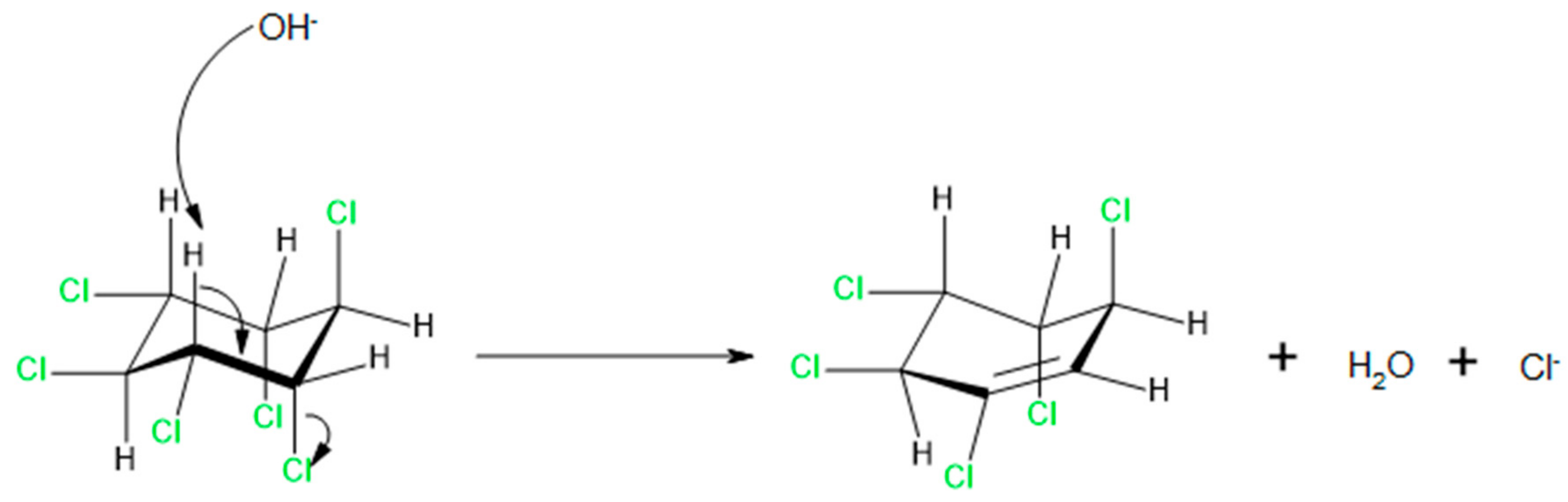
Can Alkaline Hydrolysis of γ-HCH Serve as a Model Reaction to Study Its Aerobic Enzymatic Dehydrochlorination by LinA?
Abstract
:1. Introduction
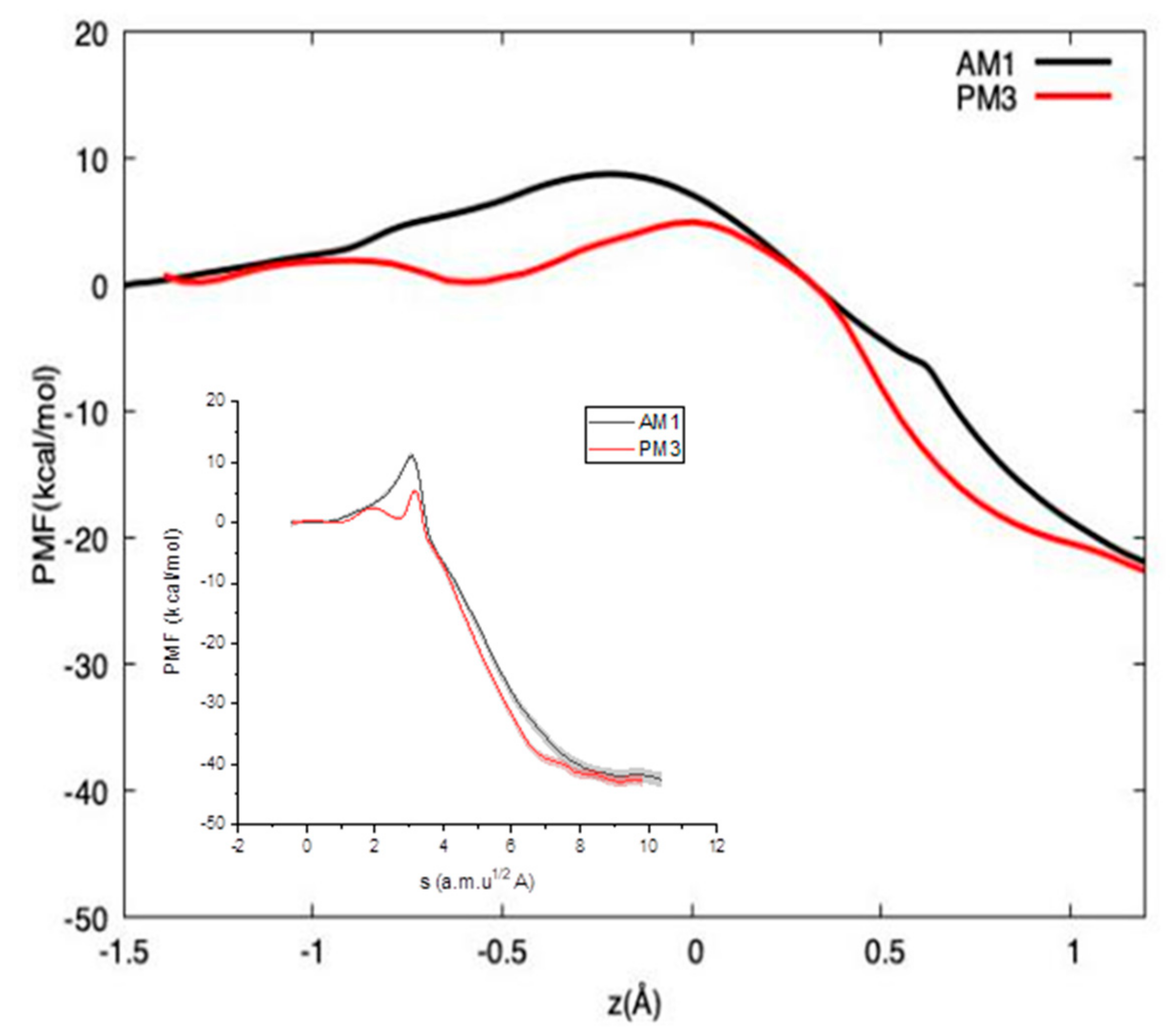
2. Results and Discussion
3. Materials and Methods
3.1. Free Energy Surfaces for the QM Microsolvation Models
3.2. PI-FEP/UM
3.3. Determination of the Experimental Isotope Enrichment Factors of γ-HCH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KIE | Kinetic isotope effect |
| PI | Path integral |
| PI-FEP/UM | Path integral combined with free energy perturbation and umbrella sampling |
| QM | Quantum mechanical |
| QM/MM | Quantum mechanics/molecular mechanics |
| HCH | Hexachlorocyclohexane |
| PMF | Potential of Mean Force |
| CSIA | Compound-specific isotope analysis |
| PCM | Polarizable continuum model |
| MD | Molecular dynamics |
References
- Agency for Toxic Substances and Disease Registry, U.S. Toxicologic Profile for α-, β-, γ- and δ-Hexachlorocyclohenxane; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2005.
- Draft Risk Management Evaluation for Lindane; Persistent Organic Pollutant Review Committee (POPRC): Geneva, Switzerland, 2007.
- Alvarez-Pedrerol, M.; Ribas-Fitó, N.; Torrent, M.; Carrizo, D.; Garcia-Esteban, R.; Grimalt, J.O.; Sunyer, J. Thyroid disruption at birth due to prenatal exposure to beta-hexachlorocyclohexane. Environ. Int. 2008, 34, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Guyton, K.; Grosse, Y.; El Ghissasi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015, 16, 891–982. [Google Scholar] [CrossRef]
- United Nations Organization. Stockholm Convention on Persistent Organic Pollutants. Adoption of Amendments to Annexes A, B and C. 2009. Available online: http://www.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 17 February 2013).
- Vijgen, J. The Legacy of Lindane HCH Isomer Production. A Global Overview of Residue Management, Formulation and Disposal; International HCH & Pesticides Association (IHPA): Copenhagen, Denmark, 2006. [Google Scholar]
- Bashir, S.; Hitzfeld, K.L.; Gehre, M.; Richnow, H.H.; Fischer, A. Evaluating degradation of hexachlorcyclohexane (HCH) isomers within a contaminated aquifer using compound-specific stable carbon isotope analysis (CSIA). Water Res. 2015, 71, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bashir, S.; Stollberg, R.; Trabitzsch, R.; Weiß, H.; Paschke, H.; Nijenhuis, I.; Richnow, H.H. Compound Specific and Enantioselective Stable Isotope Analysis as tools to monitor transformation of hexachlorocyclohexane (HCH) in a complex aquifer system. Environ. Sci. Technol. 2017, 51, 8909–8916. [Google Scholar] [CrossRef]
- Wu, L.; Moses, S.; Liu, Y.; Renpenning, J.; Richnow, H.H. A concept for studying the transformation reaction of hexachlorocyclohexanes in food webs using multi-element compound-specific isotope analysis. Anal. Chim. Acta 2019, 1046, 56–64. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Liu, X.; Bajaj, A.; Sharma, M.; Lal, R.; Richnow, H.H. Isotope fractionation approach to characterize the reactive transport processes governing the fate of hexachlorocyclohexanes at a contaminated site in India. Environ. Int. 2019, 132, 105036. [Google Scholar] [CrossRef]
- Nijenhuis, I.; Richnow, H.H. Stable isotope fractionation concepts for characterizing biotransformation of organohalides. Curr. Opin. Biotechnol. 2016, 41, 108–113. [Google Scholar] [CrossRef]
- Elsner, M.; Zwank, L.; Hunkeler, D.; Schwarzenbach, R.P. A new concept linking observable stable isotope fractionation to transformation pathways of organic pollutants. Environ. Sci. Technol. 2005, 39, 6896–6916. [Google Scholar] [CrossRef]
- Hofstetter, T.B.; Schwarzenbach, R.P.; Bernasconi, S.M. Assessing transformation processes of organic compounds using stable isotope fractionation. Environ. Sci. Technol. 2008, 42, 7737–7743. [Google Scholar] [CrossRef]
- Schilling, I.E.; Hess, R.; Bolotin, J.; Lal, R.; Hofstetter, T.B.; Kohler, H.-P.E. Kinetic Isotope Effects of the Enzymatic Transformation of γ-Hexachlorocyclohexane by the Lindane Dehydrochlorinase Variants Lin A1 and Lin A2. Environ. Sci. Technol. 2019, 53, 2353–2363. [Google Scholar] [CrossRef]
- Manna, R.N.; Zinovjev, K.; Tuñón, I.; Dybala-Defratyka, A. Dehydrochlorination of Hexachlorocyclohexanes Catalyzed by the LinA Dehydrohalogenase. A QM/MM Study. J. Phys. Chem. B 2015, 119, 15100–15109. [Google Scholar] [CrossRef] [PubMed]
- Manna, R.N. Theoretical Study on Aerobic Degradation Processes of Hexachlorocyclohexane Isomers Catalyzed by the Haloalkane Dehalogenases LinA and LinB. Ph.D. Thesis, Lodz University of Technology, Lodz, Poland, 2015. [Google Scholar]
- Szatkowski, L.; Manna, R.N.; Grzybkowska, A.; Kaminski, R.; Dybala-Defratyka, A.; Paneth, P. Measurement and Prediction of Chlorine Kinetic Isotope Effects in Enzymatic Systems in Measurement and Analysis of Kinetic Isotope Effects. Methods Enzymol. 2017, 596, 179–215. [Google Scholar] [PubMed]
- Manna, R.N.; Dybala-Defratyka, A. Insights into the elimination mechanisms employed for the degradation of different hexachlorocyclohexane isomers using kinetic isotope effects and docking studies. J. Phys. Org. Chem. 2013, 26, 797–804. [Google Scholar] [CrossRef]
- Ren, M.; Peng, P.; Huang, W.; Liu, X. Kinetics of Base-Catalyzed Dehydrochlorination of Hexachlorocyclohexanes: I. Homogeneous Systems. J. Environ. Qual. 2006, 35, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Ngabe, B.; Bidleman, T.F.; Falconer, R.L. Base Hydrolysis of α- and γ-Hexachlorcyclohexanes. Environ. Sci. Technol. 1993, 27, 1930–1933. [Google Scholar] [CrossRef]
- Marx, D.; Chandra, A.; Tuckerman, M.E. Aqueous Basic Solutions: Hydroxide Solvation, Structural Diffusion, and Comparison to the Hydrated Proton. Chem. Rev. 2010, 110, 2174–2216. [Google Scholar] [CrossRef]
- Lev, B.; Roux, B.; Noskov, S.Y. Relative Free Energies for Hydration of Monovalent Ions from QM and QM/MM Simulations. J. Chem. Theory Comput. 2013, 9, 4165–4175. [Google Scholar] [CrossRef]
- Bergstrom, P.A.; Lindgren, J.; Kristiansson, O. An IR Study of the Hydration of ClO4-, NO3-, I-, Br-, Cl-, and SO42- Anions in Aqueous Solution. J. Phys. Chem. 1991, 95, 8575–8580. [Google Scholar] [CrossRef]
- Singh, U.C.; Kollman, P.A. An approach to computing electrostatic charges for molecules. J. Comp. Chem. 1984, 5, 129–145. [Google Scholar] [CrossRef]
- Besler, B.H.; Merz, K.M., Jr.; Kollman, P.A. Atomic charges derived from semiempirical methods. J. Comp. Chem. 1990, 11, 431–439. [Google Scholar] [CrossRef]
- Zinovjev, K.; Tuñón, I. Adaptive Finite Temperature String Method in Collective Variables. J. Phys. Chem. A 2017, 121, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, R.; Zhang, Q.; Wang, W. Dehydrochlorination mechanism of g-hexachlorocyclohexane degraded by dehydrochlorinase LinA from Sphingomonas paucimobilis UT26. RSC Adv. 2016, 6, 4183–4192. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeGrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Von Ragué Schleyer, P. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first row elements, lithium to fluoride. J. Comp. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods. XXV. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of the solute with a continuum. A direct utilization of the ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comp. Chem. 1996, 17, 49–56. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F. Natural bond orbital analysis of near-Hartree-Fock water dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1972, 74, 4161–4163. [Google Scholar] [CrossRef]
- Bigeleisen, J. Statistical mechanics of isotope systems with small quantum corrections. General considerations and the rule of the geometric mean. J. Chem. Phys. 1955, 23, 2264–2267. [Google Scholar] [CrossRef]
- Bigeleisen, J. The relative reaction velocities of isotopic molecules. J. Chem. Phys. 1949, 17, 675–678. [Google Scholar] [CrossRef]
- Anisimov, V.; Paneth, P. ISOEFF98. A program for studies of isotope effects using Hessian modifications. J. Math. Chem. 1999, 26, 75–86. [Google Scholar] [CrossRef]
- Major, D.T.; Garcia-Viloca, M.; Gao, J. Path Integral Simulations of Proton Transfer Reactions in Aqueous Solution Using Combined QM/MM Potentials. J. Chem. Theory Comput. 2006, 2, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy minimization and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Monte Carlo free energy esti-mates using non-Boltzmann sampling: Application tothe sub-critical Lennard-Jones fluid. Chem. Phys. Lett. 1974, 28, 578–581. [Google Scholar] [CrossRef]
- Torrie, G.M.; Valleau, J.P. Nonphysical sampling distribu-tions in Monte Carlo free-energy estimation: Umbrellasampling. J. Comput. Phys. 1977, 23, 187–199. [Google Scholar] [CrossRef]
- Stewart, J.P.P. Optimization of parameters for semiempirical methods I. Method. J. Comp. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.P.P. Optimization of parameters for semiempirical methods II. Applications. J. Comp. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: A new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985, 10713, 3902–3909. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ferrenberg, A.M.; Swendsen, R.H. Optimized Monte Carlo data analysis. Phys. Rev. Lett. 1989, 63, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bouzida, D.; Swendsen, R.H.; Kollman, P.A.; Rosenberg, J.M. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J. Comp. Chem. 1992, 13, 1011–1021. [Google Scholar] [CrossRef]
- Sprik, M.; Klein, M.L.; Chandler, D. Staging: A sampling technique for the Monte Carlo evaluation of path integrals. Phys. Rev. B Condens. Matter 1985, 31, 4234–4244. [Google Scholar] [CrossRef]
- Major, D.T.; Gao, J. Implementation of the Bisection Sampling Method in Path Integral Simulations. J. Mol. Graph. Model. 2005, 24, 121–127. [Google Scholar] [CrossRef]
- Hwang, J.K.; Chu, Z.T.; Yadav, A.; Warshel, A. Simulations of quantum mechanical corrections for rate constants of hydride-transfer reactions in enzymes and solutions. J. Phys. Chem. 1991, 95, 8445–8448. [Google Scholar] [CrossRef]
- Hwang, J.K.; Warshel, A. A quantized classical path approach for calculations of quantum mechanical rate constants. J. Phys. Chem. 1993, 97, 10053–10058. [Google Scholar] [CrossRef]
- Hwang, J.K.; Warshel, A. How Important Are Quantum Mechanical Nuclear Motions in Enzyme Catalysis? J. Am. Chem. Soc. 1996, 118, 11745–11751. [Google Scholar] [CrossRef]
- Maragliano, L.; Fischer, A.; Vanden-Eijnden, E. String method in collective variables: Minimum free energy paths and isocommittor surfaces. J. Chem. Phys. 2006, 125, 024106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E., III; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 2018; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
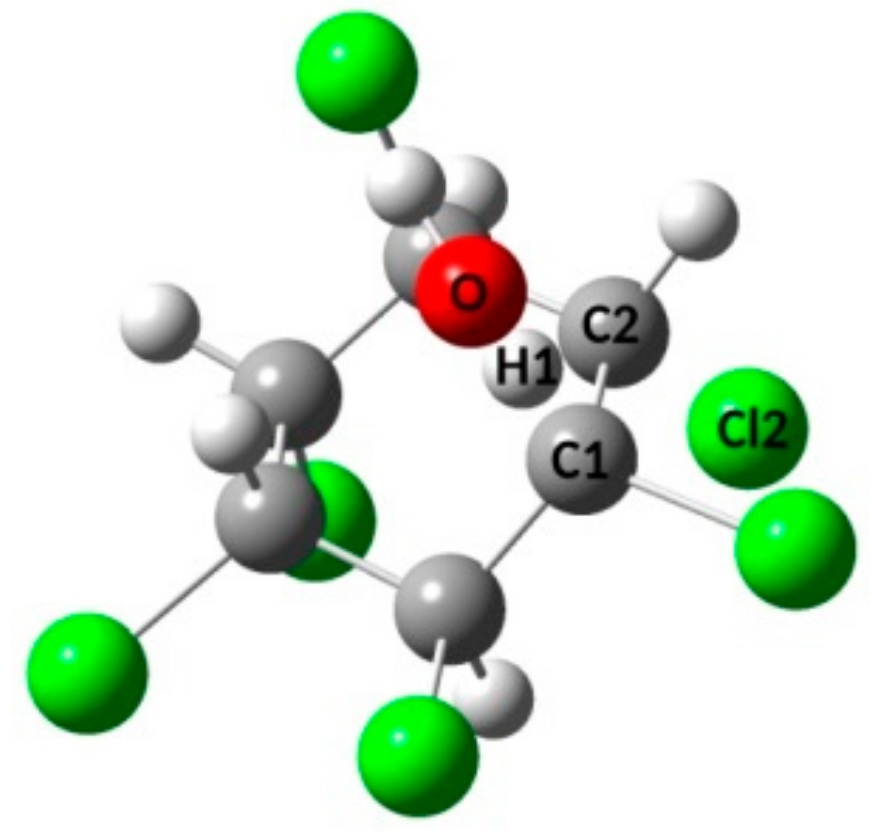
| Model | εC | εCl | εH |
|---|---|---|---|
| exp a | −7.0 ± 0.5 | −2.0 ± 0.2 | −162 ± 26 |
| H2O_2W b | −6.5 | −1.5 | −526 |
| 3W_OH | −5.7 | −0.8 | −692 |
| 4W_OH−4W_Cl | −6.0 | −0.9 | −680 |
| QM(AM1)/MM c | −7.5 (−6.7) | −1.4 (−1.2) | −481 (−463) |
| QM(PM3)/MM c | −3.6 (−2.8) | −1.0 (−0.8) | −756 (−738) |
| Model | C1 KIE | C2 KIE | Cl KIE | H1 KIE |
|---|---|---|---|---|
| H2O_2W a | 1.0182 | 1.0190 | 1.0081 | 4.0 |
| Bare model | 1.0113 | 1.0068 | 1.0018 | 5.1 |
| 3W_OH | 1.0178 | 1.0113 | 1.0029 | 5.1 |
| 4W_OH-4W_Cl | 1.0190 | 1.0144 | 1.0036 | 5.0 |
| QM(AM1)/MM | 1.0197 ± 0.0039 | 1.0204 ± 0.0010 | 1.0063 ± 0.0006 | 3.8 ± 0.2 |
| QM(PM3)/MM | 1.0083 ± 0.0019 | 1.0086 ± 0.0082 | 1.0037 ± 0.0020 | 5.5 ± 0.4 |
| Reaction | εC | εCl | εH |
|---|---|---|---|
| [14]a | −8.1 ± 0.3 (−8.3 ± 0.2) | n/d | −122 ± 6 (−160 ± 6) |
| This study | −5.3 ± 0.8 | −1.8 ± 0.4 | −119 ± 18 |
| [16,17] b | −5.0 | −0.7 | −633 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannath, S.; Adamczyk, P.; Wu, L.; Richnow, H.H.; Dybala-Defratyka, A. Can Alkaline Hydrolysis of γ-HCH Serve as a Model Reaction to Study Its Aerobic Enzymatic Dehydrochlorination by LinA? Int. J. Mol. Sci. 2019, 20, 5955. https://doi.org/10.3390/ijms20235955
Kannath S, Adamczyk P, Wu L, Richnow HH, Dybala-Defratyka A. Can Alkaline Hydrolysis of γ-HCH Serve as a Model Reaction to Study Its Aerobic Enzymatic Dehydrochlorination by LinA? International Journal of Molecular Sciences. 2019; 20(23):5955. https://doi.org/10.3390/ijms20235955
Chicago/Turabian StyleKannath, Suraj, Paweł Adamczyk, Langping Wu, Hans H. Richnow, and Agnieszka Dybala-Defratyka. 2019. "Can Alkaline Hydrolysis of γ-HCH Serve as a Model Reaction to Study Its Aerobic Enzymatic Dehydrochlorination by LinA?" International Journal of Molecular Sciences 20, no. 23: 5955. https://doi.org/10.3390/ijms20235955





