ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation
Abstract
:1. Introduction
2. Results
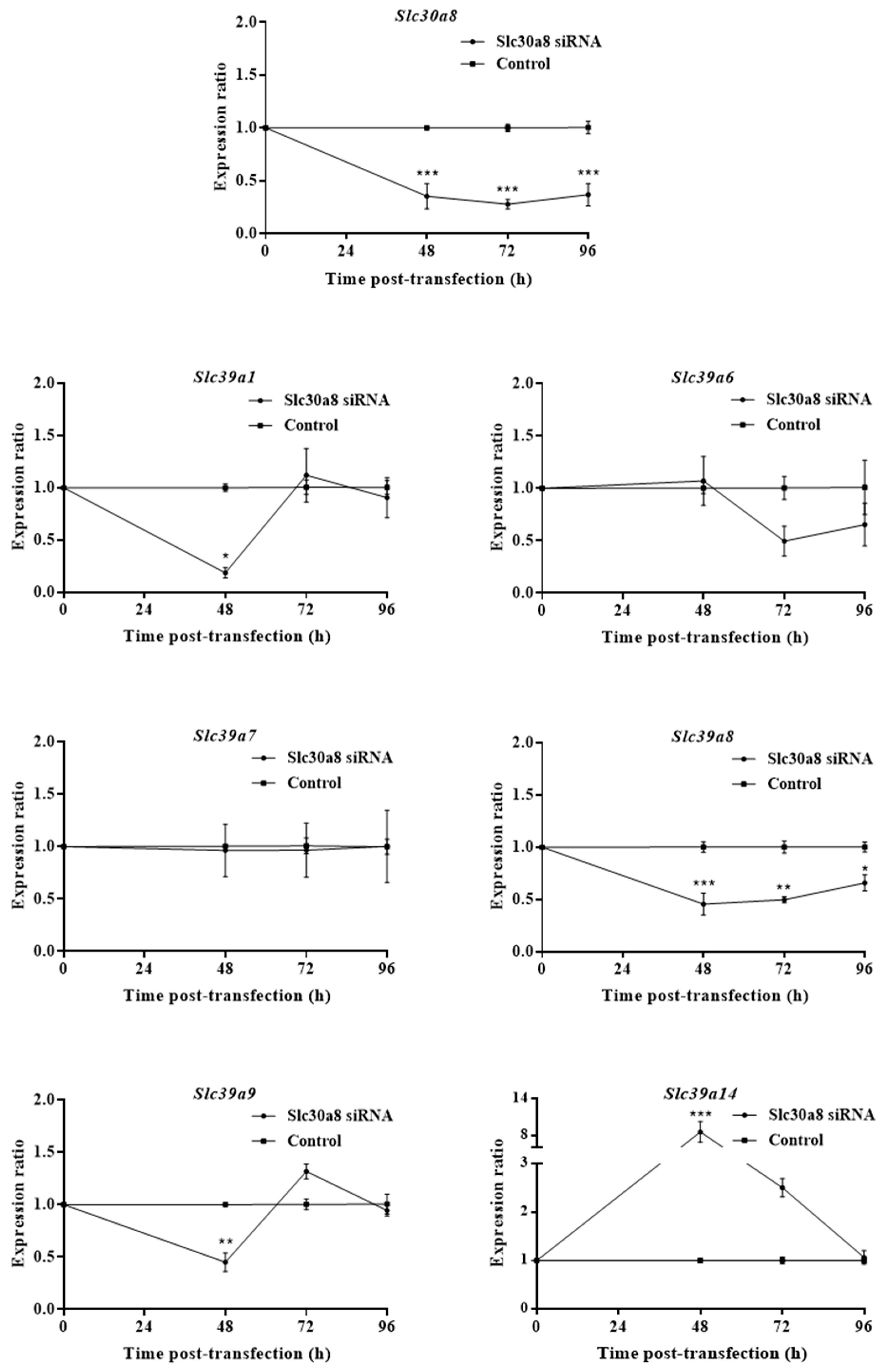
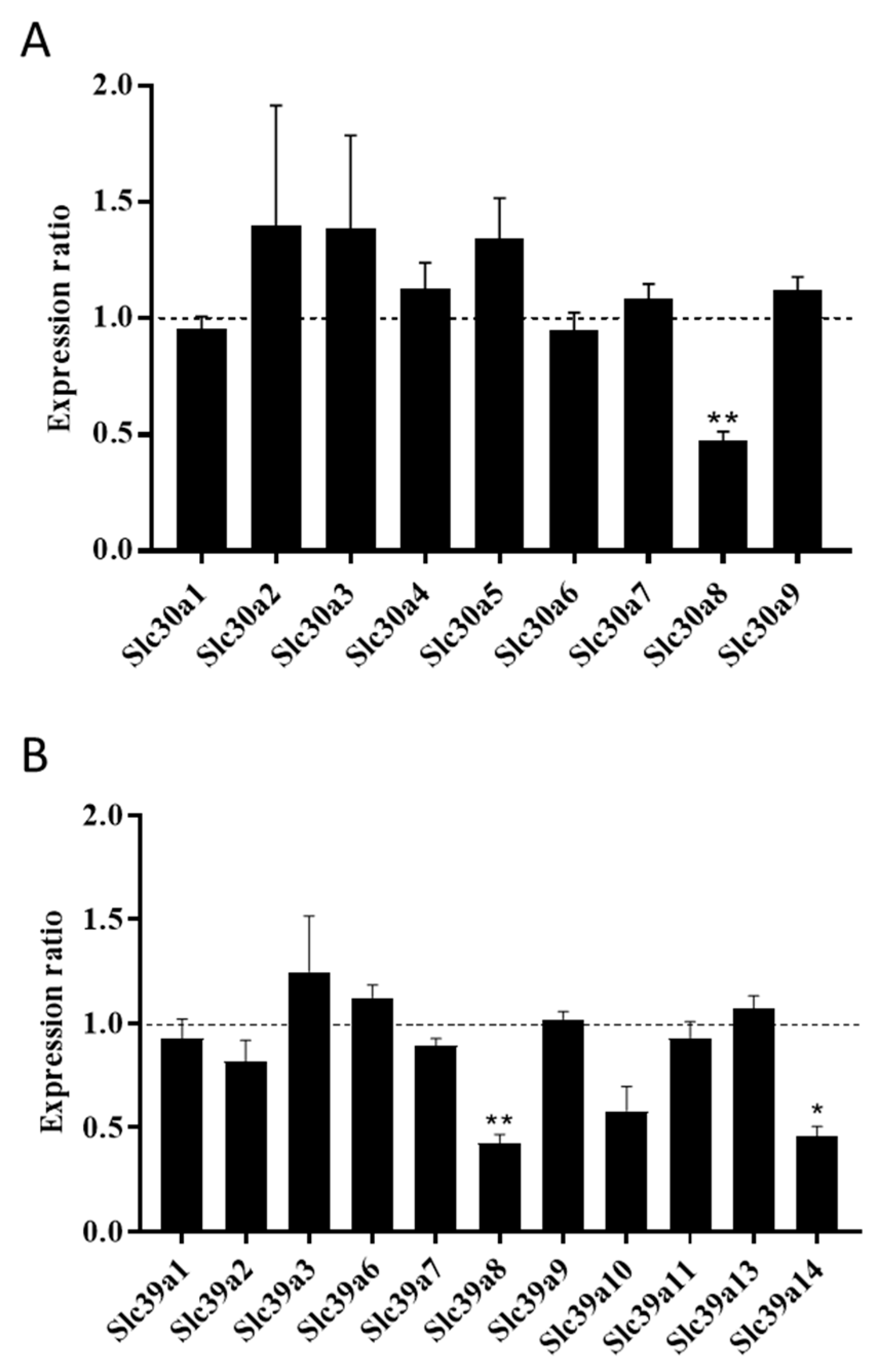
2.1. ZnT8 Expression Is Associated with Expression of ZIP8 and ZIP14
2.2. ZnT8 Haploinsufficiency Decreases Zinc Uptake into MIN6 Cells
2.3. ZnT8 Haploinsufficiency Alters Expression of Important β-Cell Markers
2.4. ZnT8 Haploinsufficiency Reduces MIN6 Cell Proliferation and Ins1 Expression and Secretion
3. Discussion
3.1. Characteristics of Zinc Metabolism in ZnT8 Haploinsufficient MIN6 Cells
3.2. Phenotypes of ZnT8 Haploinsufficient MIN6 Cells
4. Materials and Methods
4.1. Cell Line and Culture
4.2. Cell Transfection
4.3. CRISPR/Cas9 Gene Editing
4.4. Gene Expression Analysis
4.5. Determination of Cellular Zinc, Iron and Manganese Levels
4.6. Cellular Proliferation and Apoptosis
4.7. Immunoblotting
4.8. Insulin Secretion Assays
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Vinkenborg, J.L.; Nicolson, T.J.; Bellomo, E.A.; Koay, M.S.; Rutter, G.A.; Merkx, M. Genetically encoded FRET sensors to monitor intracellular Zn2+ homeostasis. Nat. Methods 2009, 6, 737–740. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Davidson, H.W.; Wenzlau, J.M.; O’Brien, R.M. Zinc transporter 8 (ZnT8) and β cell function. Trends Endocrinol. Metab. 2014, 25, 415–424. [Google Scholar] [CrossRef]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Bélisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef]
- Saxena, R.; Voight, B.F.; Lyssenko, V.; Burtt, N.P.; De Bakker, P.I.W.; Chen, H.; Roix, J.J.; Kathiresan, S.; Hirschhorn, J.N.; Daly, M.J.; et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007, 316, 1331–1336. [Google Scholar]
- Boesgaard, T.W.; Zilinskaite, J.; Vanttinen, M.; Laakso, M.; Jansson, P.-A.; Hammarstedt, A.; Smith, U.; Stefan, N.; Fritsche, A.; Häring, H.; et al. The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients—The EUGENE2 study. Diabetologia 2008, 51, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kirschke, C. Down-regulation of zinc transporter 8 (SLC30A8) in pancreatic beta-cells promotes cell survival. Austin J. Endocrinol. Diabetes 2016, 3, 1037. [Google Scholar]
- Gerber, P.A.; Bellomo, E.A.; Hodson, D.J.; Meur, G.; Solomou, A.; Mitchell, R.K.; Hollinshead, M.; Chimienti, F.; Bosco, D.; Hughes, S.J.; et al. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia 2014, 57, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Hardy, A.B.; Wijesekara, N.; Genkin, I.; Prentice, K.J.; Bhattacharjee, A.; Kong, D.; Chimienti, F.; Wheeler, M.B. Effects of high-fat diet feeding on Znt8-null mice: Differences between β-cell and global knockout of Znt8. Am. J. Physiol. Metab. 2012, 302, E1084–E1096. [Google Scholar] [CrossRef] [PubMed]
- Egefjord, L.; Jensen, J.L.; Bang-Berthelsen, C.H.; Petersen, A.B.; Smidt, K.; Schmitz, O.; Karlsen, A.E.; Pociot, F.; Chimienti, F.; Rungby, J.; et al. Zinc transporter gene expression is regulated by pro-inflammatory cytokines: A potential role for zinc transporters in beta-cell apoptosis? BMC Endocr. Disord. 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Vandewalle, B.; Balavoine, A.-S.; Queniat, G.; Moerman, E.; Vantyghem, M.-C.; Le Bacquer, O.; Gmyr, V.; Pawlowski, V.; Kerr-Conte, J.; et al. Regulation and functional effects of ZNT8 in human pancreatic islets. J. Endocrinol. 2012, 214, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimienti, F.; Devergnas, S.; Pattou, F.; Schuit, F.; Garcia-Cuenca, R.; Vandewalle, B.; Kerr-Conte, J.; Van Lommel, L.; Grunwald, D.; Favier, A.; et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J. Cell Sci. 2006, 119, 4199–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flannick, J.; Go-T2D Consortium; Thorleifsson, G.; Beer, N.L.; Jacobs, S.B.R.; Grarup, N.; Burtt, N.P.; Mahajan, A.; Fuchsberger, C.; Atzmon, G.; et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 2014, 46, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, S.; Gomez, D.; Megra, B.; Na, E.; Bhavsar, R.; Cavino, K.; Xin, Y.; Rojas, J.; Dominguez-Gutierrez, G.; Zambrowicz, B.; et al. Mice harboring the human SLC30A8 R138X loss-of-function mutation have increased insulin secretory capacity. Proc. Natl. Acad. Sci. USA 2018, 115, E7642–E7649. [Google Scholar] [CrossRef]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes–associated variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef]
- Wijesekara, N.; Dai, F.F.; Hardy, A.B.; Giglou, P.R.; Bhattacharjee, A.; Koshkin, V.; Chimienti, F.; Gaisano, H.Y.; Rutter, G.A.; Wheeler, M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010, 53, 1656–1668. [Google Scholar] [CrossRef] [Green Version]
- Pound, L.D.; Sarkar, S.A.; Benninger, R.K.P.; Wang, Y.; Suwanichkul, A.; Shadoan, M.K.; Printz, R.L.; Oeser, J.K.; Lee, C.E.; Piston, D.W.; et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 2009, 421, 371–376. [Google Scholar] [CrossRef]
- Lemaire, K.; Ravier, M.A.; Schraenen, A.; Creemers, J.W.; Van de Plas, R.; Granvik, M.; Van Lommel, L.; Waelkens, E.; Chimienti, F.; Rutter, G.A.; et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 14872–14877. [Google Scholar] [CrossRef] [Green Version]
- Merriman, C.; Huang, Q.; Rutter, G.A.; Fu, D. Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. J. Biol. Chem. 2016, 291, 26950–26957. [Google Scholar] [CrossRef]
- Parsons, D.S.; Hogstrand, C.; Maret, W. The C-terminal cytosolic domain of the human zinc transporter ZnT8 and its diabetes risk variant. FEBS J. 2018, 285, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Molina-López, J.; Parsons, D.; Corpe, C.; Maret, W.; Hogstrand, C. Differential cytolocation and functional assays of the two major human SLC30A8 (ZnT8) isoforms. J. Trace Elem. Med. Biol. 2017, 44, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Bai, S.; Sheline, C.T. hZnT8 (Slc30a8) Transgenic mice that overexpress the R325W polymorph have reduced islet Zn2+ and proinsulin levels, increased glucose tolerance after a high-fat diet, and altered levels of pancreatic zinc binding proteins. Diabetes 2017, 66, 551–559. [Google Scholar] [PubMed]
- Kirchhoff, K.; Machicao, F.; Haupt, A.; Schäfer, S.A.; Tschritter, O.; Staiger, H.; Stefan, N.; Häring, H.-U.; Fritsche, A. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 2008, 51, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Dimas, A.S.; Lagou, V.; Barker, A.; Knowles, J.W.; Mägi, R.; Hivert, M.-F.; Benazzo, A.; Rybin, D.; Jackson, A.U.; Stringham, H.M.; et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014, 63, 2158–2171. [Google Scholar] [CrossRef]
- Staiger, H.; Machicao, F.; Stefan, N.; Tschritter, O.; Thamer, C.; Kantartzis, K.; Schäfer, S.A.; Kirchhoff, K.; Fritsche, A.; Häring, H.-U. Polymorphisms within novel risk loci for type 2 diabetes determine β-cell function. PLoS ONE 2007, 2, e832. [Google Scholar] [CrossRef]
- Lawson, R.; Maret, W.; Hogstrand, C. Prolonged stimulation of insulin release from MIN6 cells causes zinc depletion and loss of β-cell markers. J. Trace Elem. Med. Biol. 2018, 49, 51–59. [Google Scholar] [CrossRef]
- Bellomo, E.A.; Meur, G.; Rutter, G.A. Glucose regulates free cytosolic Zn2⁺ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. J. Biol. Chem. 2011, 286, 25778–25789. [Google Scholar] [CrossRef]
- Lawson, R.; Maret, W.; Hogstrand, C. Expression of the ZIP/SLC39A transporters in β-cells: A systematic review and integration of multiple datasets. BMC Genom. 2017, 18, 719. [Google Scholar] [CrossRef]
- Liu, Y.; Batchuluun, B.; Ho, L.; Zhu, D.; Prentice, K.J.; Bhattacharjee, A.; Zhang, M.; Pourasgari, F.; Hardy, A.B.; Taylor, K.M.; et al. Characterization of zinc influx transporters (ZIPs) in pancreatic beta cells: Roles in Regulating Cytosolic Zinc Homeostasis and Insulin Secretion. J. Biol. Chem. 2015, 290, 18757–18769. [Google Scholar] [CrossRef]
- Pound, L.D.; Sarkar, S.A.; Ustione, A.; Dadi, P.K.; Shadoan, M.K.; Lee, C.E.; Walters, J.A.; Shiota, M.; McGuinness, O.P.; Jacobson, D.A.; et al. The physiological effects of deleting the mouse Slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS ONE 2012, 7, e40972. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Fujitani, Y.; Hara, A.; Uchida, T.; Tamura, Y.; Takeno, K.; Kawaguchi, M.; Watanabe, T.; Ogihara, T.; Fukunaka, A.; et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J. Clin. Investig. 2013, 123, 4513–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; MacKenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiber, I.F.; Wu, Y.; Morgan, S.E.; Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019, 294, 9147–9160. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [Green Version]
- Elghazi, L.; Bernal-Mizrachi, E. Akt and PTEN: Beta-cell mass and pancreas plasticity. Trends Endocrinol. Metab. 2009, 20, 243–251. [Google Scholar] [CrossRef]
- Nimmanon, T.; Ziliotto, S.; Morris, S.; Flanagan, L.; Taylor, K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics 2017, 9, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekera, P.C. Of rodents and men: Species-specific glucose regulation and type 2 diabetes research. ALTEX 2014, 31, 157–176. [Google Scholar] [CrossRef]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar]
- Thomas, P.; Pang, Y.; Dong, J.; Berg, A.H. Identification and characterization of membrane androgen receptors in the ZIP9 zinc transporter subfamily: II. Role of human ZIP9 in testosterone-induced prostate and breast cancer cell apoptosis. Endocrinology 2014, 155, 4250–4265. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Schmitt-Ulms, G.; Ehsani, S.; Watts, J.C.; Westaway, D.; Wille, H. Evolutionary descent of prion genes from the ZIP family of metal ion transporters. PLoS ONE 2009, 4, e7208. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef] [Green Version]
- El Muayed, M.; Raja, M.R.; Zhang, X.; MacRenaris, K.W.; Bhatt, S.; Chen, X.; Urbanek, M.; O’Halloran, T.V.; Lowe, W.L.; Lowe, J.W.L. Accumulation of cadmium in insulin-producing β cells. Islets 2012, 4, 405–416. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef]
- Kim, G.; Shin, K.H.; Pae, E.K. Zinc up-regulates insulin secretion from β cell-like cells derived from stem cells from human exfoliated deciduous tooth (SHED). Int. J. Mol. Sci. 2016, 17, 2092. [Google Scholar] [CrossRef]
- Brun, T.; Franklin, I.; St-Onge, L.; Biason-Lauber, A.; Schoenle, E.J.; Wollheim, C.B.; Gauthier, B.R. The diabetes-linked transcription factor PAX4 promotes β-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004, 167, 1123–1135. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; Lan, M.S.; Zhang, S.; Fan, W.; Wang, H.; Lu, D. Pax4 paired domain mediates direct protein transduction into mammalian cells. Endocrinology 2007, 148, 5558–5565. [Google Scholar] [CrossRef]
- Van Der Meulen, T.; Huising, M.O. Role of transcription factors in the transdifferentiation of pancreatic islet cells. J. Mol. Endocrinol. 2015, 54, R103–R117. [Google Scholar] [CrossRef] [Green Version]
- Bartoov-Shifman, R.; Hertz, R.; Wollheim, C.B.; Bar-Tana, J.; Walker, M.D.; Wang, H. Activation of the insulin gene promoter through a direct effect of hepatocyte nuclear factor 4. J. Biol. Chem. 2002, 277, 25914–25919. [Google Scholar] [CrossRef]
- Gao, N.; Le Lay, J.; Qin, W.; Doliba, N.; Schug, J.; Fox, A.J.; Smirnova, O.; Matschinsky, F.M.; Kaestner, K.H. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol. Endocrinol. 2010, 24, 1594–1604. [Google Scholar] [CrossRef]
- Beyersmann, D.; Haase, H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. BioMetals 2001, 14, 331–341. [Google Scholar] [CrossRef]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Hershfinkel, M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef]
- Samet, J.M.; Graves, L.M.; Quay, J.; Dailey, L.A.; Devlin, R.B.; Ghio, A.J.; Wu, W.; Bromberg, P.A.; Reed, W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. Content 1998, 275, L551–L558. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef]
- Merriman, C.; Fu, D. Down-regulation of the islet-specific zinc transporter-8 (ZnT8) protects human insulinoma cells against inflammatory stress. J. Biol. Chem. 2019, jbc.RA119.010937. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Chang, S.-M.; Guthrie, G.J.; Maki, A.B.; Ryu, M.-S.; Karabiyik, A.; Cousins, R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (Endotoxemia). PLoS ONE 2012, 7, e48679. [Google Scholar]
- Liu, M.-J.; Bao, S.; Gálvez-Peralta, M.; Pyle, C.J.; Rudawsky, A.C.; Pavlovicz, R.E.; Killilea, D.W.; Li, C.; Nebert, D.W.; Wewers, M.D.; et al. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Rep. 2013, 3, 386–400. [Google Scholar] [CrossRef]
- Maxel, T.; Smidt, K.; Petersen, C.C.; Honoré, B.; Christensen, A.K.; Jeppesen, P.B.; Brock, B.; Rungby, J.; Palmfeldt, J.; Larsen, A. The zinc transporter Zip14 (SLC39a14) affects beta-cell function: proteomics, gene expression, and insulin secretion studies in INS-1E cells. Sci. Rep. 2019, 9, 8589. [Google Scholar] [CrossRef]
- Deniro, M.; Al-Mohanna, F.A. Zinc transporter 8 (ZnT8) expression is reduced by ischemic insults: A potential therapeutic target to prevent ischemic retinopathy. PLoS ONE 2012, 7, e50360. [Google Scholar] [CrossRef]
- Solomou, A.; Meur, G.; Bellomo, E.; Hodson, D.J.; Tomas, A.; Li, S.M.; Philippe, E.; Herrera, P.L.; Magnan, C.; Rutter, G.A. The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic α-cells for hypoglycemia-induced glucagon secretion. J. Biol. Chem. 2015, 290, 21432–21442. [Google Scholar] [CrossRef]
- Giacconi, R.; Malavolta, M.; Chiodi, L.; Boccoli, G.; Costarelli, L.; Bonfigli, A.; Galeazzi, R.; Piacenza, F.; Basso, A.; Gasparini, N.; et al. ZnT8 Arg325Trp polymorphism influences zinc transporter expression and cytokine production in PBMCs from patients with diabetes. Diabetes Res. Clin. Pr. 2018, 144, 102–110. [Google Scholar] [CrossRef]
- Miyazaki, J.-I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.I. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; A Scott, D.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [Green Version]
- Gasteiger, E. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, R.; Maret, W.; Hogstrand, C. ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation. Int. J. Mol. Sci. 2019, 20, 5485. https://doi.org/10.3390/ijms20215485
Lawson R, Maret W, Hogstrand C. ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation. International Journal of Molecular Sciences. 2019; 20(21):5485. https://doi.org/10.3390/ijms20215485
Chicago/Turabian StyleLawson, Rebecca, Wolfgang Maret, and Christer Hogstrand. 2019. "ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation" International Journal of Molecular Sciences 20, no. 21: 5485. https://doi.org/10.3390/ijms20215485
APA StyleLawson, R., Maret, W., & Hogstrand, C. (2019). ZnT8 Haploinsufficiency Impacts MIN6 Cell Zinc Content and β-Cell Phenotype via ZIP-ZnT8 Coregulation. International Journal of Molecular Sciences, 20(21), 5485. https://doi.org/10.3390/ijms20215485






