Glucagon Regulation of Energy Expenditure
Abstract
:1. Introduction
2. The Effect Size of Glucagon-Induced Energy Expenditure in Humans
3. Glucagon-Induced Energy Expenditure: Acute vs. Chronic Effects
4. Mediators of the Acute Effect of Glucagon on Energy Expenditure
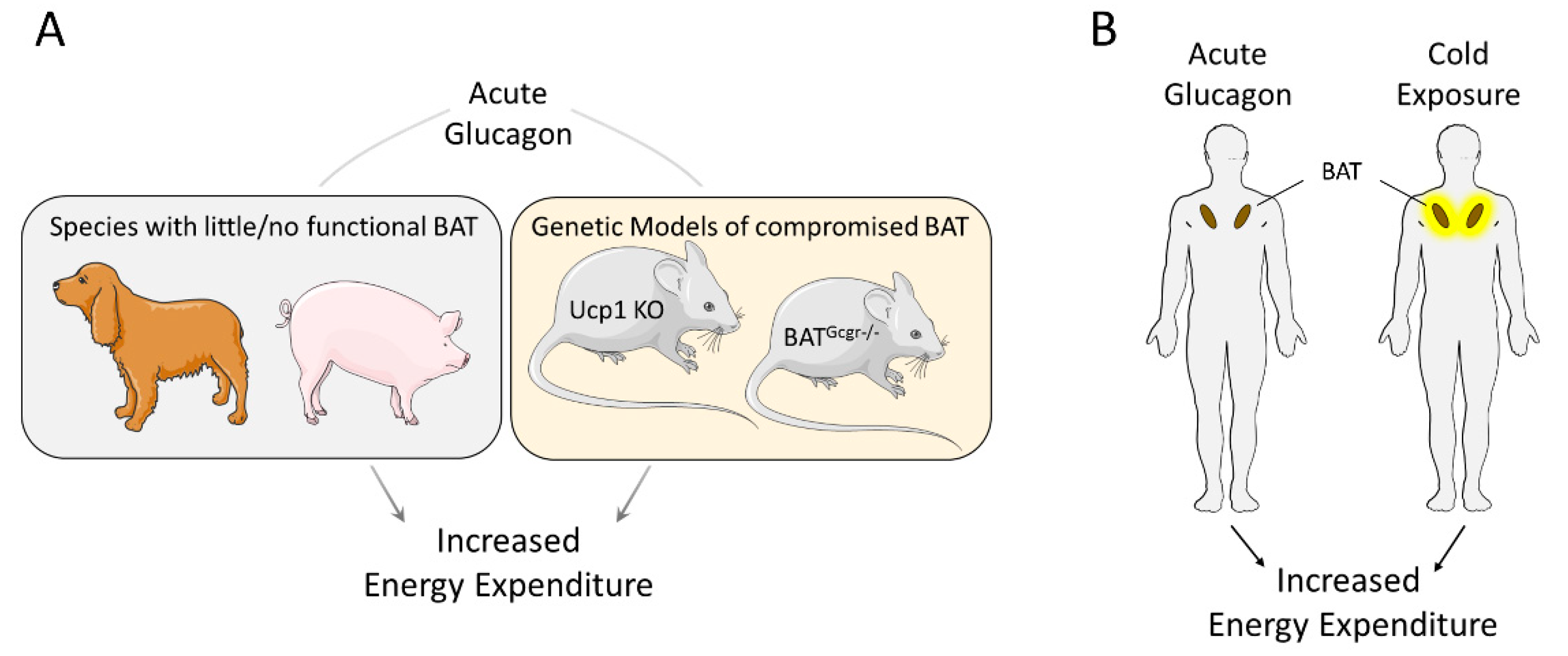
4.1. Role of the Brown Adipose Tissue
4.2. Potential Mechanisms for Acute Glucagon-Induced Energy Expenditure
5. Mediators of the Chronic Effect of Glucagon on Energy Expenditure
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Campbell, J.E.; Drucker, D.J. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat. Rev. Endocrinol. 2015, 11, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhang, B.B. Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E671–E678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedia, N. Treatment of severe diabetic hypoglycemia with glucagon: An underutilized therapeutic approach. Diabetes Metab. Syndr. Obes. Targets Ther. 2011, 4, 337. [Google Scholar] [CrossRef]
- Woods, S.C.; Lutz, T.A.; Geary, N.; Langhans, W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1219–1235. [Google Scholar] [CrossRef] [Green Version]
- Galsgaard, K.D.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Wewer Albrechtsen, N.J. Glucagon Receptor Signaling and Lipid Metabolism. Front. Physiol. 2019, 10, 413. [Google Scholar] [CrossRef]
- Finan, B.; Capozzi, M.E.; Campbell, J.E. Repositioning Glucagon Action in the Physiology and Pharmacology of Diabetes. Diabetes 2019. [Google Scholar] [CrossRef]
- Jones, B.J.; Tan, T.; Bloom, S.R. Minireview: Glucagon in stress and energy homeostasis. Endocrinology 2012, 153, 1049–1054. [Google Scholar] [CrossRef]
- Geary, N.; Smith, G. Selective hepatic vagotomy blocks pancreatic glucagon’s satiety effect. Physiol. Behav. 1983, 31, 391–394. [Google Scholar] [CrossRef]
- Capozzi, M.E.; Wait, J.B.; Koech, J.; Gordon, A.N.; Coch, R.W.; Svendsen, B.; Finan, B.; D’Alessio, D.A.; Campbell, J.E. Glucagon lowers glycemia when β cells are active. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Carr, R.D.; Larsen, M.O.; Jelic, K.; Lindgren, O.; Vikman, J.; Holst, J.J.; Deacon, C.F.; Ahrén, B. Secretion and Dipeptidyl Peptidase-4-Mediated Metabolism of Incretin Hormones after a Mixed Meal or Glucose Ingestion in Obese Compared to Lean, Nondiabetic Men. J. Clin. Endocrinol. Metab. 2010, 95, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Rauch, T.; Graefe-Mody, U.; Deacon, C.F.; Ring, A.; Holst, J.J.; Woerle, H.-J.; Dugi, K.A.; Heise, T. Linagliptin Increases Incretin Levels, Lowers Glucagon, and Improves Glycemic Control in Type 2 Diabetes Mellitus. Diabetes Ther. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Langhans, W.; Zeiger, U.; Scharrer, E.; Geary, N. Stimulation of feeding in rats by intraperitoneal injection of antibodies to glucagon. Science 1982, 218, 894–896. [Google Scholar] [CrossRef] [PubMed]
- . Unger, R.H.; Orci, L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975, 1, 14–16. [Google Scholar] [CrossRef]
- Johnson, D.G.; Goebel, C.U.; Hruby, V.J.; Bregman, M.D.; Trivedi, D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science 1982, 215, 1115–1116. [Google Scholar] [CrossRef]
- Liang, Y.; Osborne, M.C.; Monia, B.P.; Bhanot, S.; Gaarde, W.A.; Reed, C.; She, P.; Jetton, T.L.; Demarest, K.T. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004, 53, 410–417. [Google Scholar] [CrossRef]
- Sørensen, H.; Brand, C.L.; Neschen, S.; Holst, J.J.; Fosgerau, K.; Nishimura, E.; Shulman, G.I. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 2006, 55, 2843–2848. [Google Scholar] [CrossRef]
- Mu, J.; Jiang, G.; Brady, E.; Dallas-Yang, Q.; Liu, F.; Woods, J.; Zycband, E.; Wright, M.; Li, Z.; Lu, K.; et al. Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia 2011, 54, 2381–2391. [Google Scholar] [CrossRef]
- Petersen, K.F.; Sullivan, J.T. Effects of a novel glucagon receptor antagonist (Bay 27–9955) on glucagon-stimulated glucose production in humans. Diabetologia 2001, 44, 2018–2024. [Google Scholar] [CrossRef]
- Kazda, C.M.; Ding, Y.; Kelly, R.P.; Garhyan, P.; Shi, C.; Lim, C.N.; Fu, H.; Watson, D.E.; Lewin, A.J.; Landschulz, W.H.; et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY2409021 in Patients with Type 2 Diabetes: 12- and 24-Week Phase 2 Studies. Diabetes Care 2016, 39, 1241–1249. [Google Scholar] [CrossRef]
- Guzman, C.B.; Zhang, X.M.; Liu, R.; Regev, A.; Shankar, S.; Garhyan, P.; Pillai, S.G.; Kazda, C.; Chalasani, N.; Hardy, T.A. Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes: Guzman et al. Diabetes Obes. Metab. 2017, 19, 1521–1528. [Google Scholar] [CrossRef]
- Guan, H.-P.; Yang, X.; Lu, K.; Wang, S.-P.; Castro-Perez, J.M.; Previs, S.; Wright, M.; Shah, V.; Herath, K.; Xie, D.; et al. Glucagon receptor antagonism induces increased cholesterol absorption. J. Lipid Res. 2015, 56, 2183–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, I.W.F.; Salter, J.M.; Best, C.H. The Effect of Glucagon on the Metabolic Rate of Rats. Am. J. Clin. Nutr. 1960, 8, 540–546. [Google Scholar] [CrossRef]
- Nair, K.S. Hyperglucagonemia Increases Resting Metabolic Rate in Man During Insulin Deficiency *. J. Clin. Endocrinol. Metab. 1987, 64, 896–901. [Google Scholar] [CrossRef]
- Bagger, J.I.; Holst, J.J.; Hartmann, B.; Andersen, B.; Knop, F.K.; Vilsbøll, T. Effect of Oxyntomodulin, Glucagon, GLP-1, and Combined Glucagon + GLP-1 Infusion on Food Intake, Appetite, and Resting Energy Expenditure. J. Clin. Endocrinol. Metab. 2015, 100, 4541–4552. [Google Scholar] [CrossRef]
- Tan, T.M.; Field, B.C.T.; McCullough, K.A.; Troke, R.C.; Chambers, E.S.; Salem, V.; Gonzalez Maffe, J.; Baynes, K.C.R.; De Silva, A.; Viardot, A.; et al. Coadministration of Glucagon-Like Peptide-1 During Glucagon Infusion in Humans Results in Increased Energy Expenditure and Amelioration of Hyperglycemia. Diabetes 2013, 62, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Salem, V.; Izzi-Engbeaya, C.; Coello, C.; Thomas, D.B.; Chambers, E.S.; Comninos, A.N.; Buckley, A.; Win, Z.; Al-Nahhas, A.; Rabiner, E.A.; et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes. Metab. 2016, 18, 72–81. [Google Scholar] [CrossRef]
- Calles-Escandón, J. Insulin dissociates hepatic glucose cycling and glucagon-induced thermogenesis in man. Metabolism 1994, 43, 1000–1005. [Google Scholar] [CrossRef]
- Stahel, P.; Lee, S.J.; Sud, S.K.; Floh, A.; Dash, S. Intranasal glucagon acutely increases energy expenditure without inducing hyperglycaemia in overweight/obese adults. Diabetes Obes. Metab. 2019, 21, 1357–1364. [Google Scholar] [CrossRef]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elía, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Salter, J.M. Metabolic Effects of Glucagon in the Wistar Rat. Am. J. Clin. Nutr. 1960, 8, 535–539. [Google Scholar] [CrossRef]
- Beaudry, J.L.; Kaur, K.D.; Varin, E.M.; Baggio, L.L.; Cao, X.; Mulvihill, E.E.; Stern, J.H.; Campbell, J.E.; Scherer, P.E.; Drucker, D.J. The brown adipose tissue glucagon receptor is functional but not essential for control of energy homeostasis in mice. Mol. Metab. 2019, 22, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.L.; Kaciuba-Uscilko, H. Metabolic effects of glucagon in the young pig. Horm. Metab. Res. 1980, 12, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Weiser, P.C.; Grande, F. Calorigenic Effects of Glucagon and Epinephrine in Anesthetized Dogs. Exp. Biol. Med. 1974, 145, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Stemmer, K.; Cheng, C.; Muller, T.D.; Heppner, K.M.; Ottaway, N.; Holland, J.; Hembree, J.L.; Smiley, D.; Gelfanov, V.; et al. Fibroblast Growth Factor 21 Mediates Specific Glucagon Actions. Diabetes 2013, 62, 1453–1463. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Nason, S.; Holleman, C.; Pepin, M.; Wilson, L.; Berryhill, T.F.; Wende, A.R.; Steele, C.; Young, M.E.; Barnes, S.; et al. Glucagon Receptor Signaling Regulates Energy Metabolism via Hepatic Farnesoid X Receptor and Fibroblast Growth Factor 21. Diabetes 2018, 67, 1773–1782. [Google Scholar] [CrossRef] [Green Version]
- Tschöp, M.H.; Speakman, J.R.; Arch, J.R.S.; Auwerx, J.; Brüning, J.C.; Chan, L.; Eckel, R.H.; Farese, R.V.; Galgani, J.E.; Hambly, C.; et al. A guide to analysis of mouse energy metabolism. Nat. Methods 2012, 9, 57–63. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. New Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Joel, C.D. Stimulation of metabolism of rat brown adipose tissue by addition of lipolytic hormones in vitro. J. Biol. Chem. 1966, 241, 814–821. [Google Scholar]
- Kuroshima, A.; Yahata, T. Thermogenic Responses of Brown Adipocytes to Noradrenaline and Glucagon in Heat-acclimated and Cold-acclimated Rats. Jpn. J. Physiol. 1979, 29, 683–690. [Google Scholar] [CrossRef]
- Dicker, A.; Zhao, J.; Cannon, B.; Nedergaard, J. Apparent thermogenic effect of injected glucagon is not due to a direct effect on brown fat cells. Am. J. Physiol. 1998, 275, R1674–R1682. [Google Scholar] [CrossRef]
- Heim, T.; Hull, D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J. Physiol. 1966, 187, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, F.; Hull, D.; Walton, I. The Effect of Lipolytic Hormones and Theophylline on Heat Production in Brown Adipose Tissue In Vivo. Br. J. Pharmacol. Chemother. 1967, 31, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Yahata, T.; Habara, Y.; Kuroshima, A. Effects of glucagon and noradrenaline on the blood flow through brown adipose tissue in temperature-acclimated rats. Jpn. J. Physiol. 1983, 33, 367–376. [Google Scholar] [CrossRef]
- Farah, A.E. Glucagon and the circulation. Pharmacol. Rev. 1983, 35, 181–217. [Google Scholar]
- Hernández-Cascales, J. Does glucagon have a positive inotropic effect in the human heart? Cardiovasc. Diabetol. 2018, 17, 148. [Google Scholar] [CrossRef]
- Parmley, W.W.; Glick, G.; Sonnenblick, E.H. Cardiovascular Effects of Glucagon in Man. New Engl. J. Med. 1968, 279, 12–17. [Google Scholar] [CrossRef]
- Doi, K.; Kuroshima, A. Thermogenic response to glucagon in cold-acclimated mice. Jpn. J. Physiol. 1982, 32, 377–385. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Billington, C.J.; Briggs, J.E.; Link, J.G.; Levine, A.S. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1991, 261, R501–R507. [Google Scholar] [CrossRef]
- Ramnanan, C.J.; Edgerton, D.S.; Kraft, G.; Cherrington, A.D. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes. Metab. 2011, 13, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.W.; McGarry, J.D. The regulation of ketogenesis. Ciba Found. Symp. 1982, 87, 120–131. [Google Scholar] [PubMed]
- Charlton, M.R.; Adey, D.B.; Nair, K.S. Evidence for a catabolic role of glucagon during an amino acid load. J. Clin. Investig. 1996, 98, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.O.; Robl, H.; Schwandt, P. Human glucagon and vasoactive intestinal polypeptide (VIP) stimulate free fatty acid release from human adipose tissue in vitro. Peptides 1989, 10, 333–335. [Google Scholar] [CrossRef]
- Lefebvre, P.; Luyckx, A.; Bacq, Z.M. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Horm. Metab. Res. 1973, 5, 245–250. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am. J. Clin. Nutr. 2009, 90, 519–526. [Google Scholar] [CrossRef] [Green Version]
- McGuinness, O.P.; Murrell, S.; Moran, C.; Bracy, D.; Cherrington, A.D. The effect of acute glucagon removal on the metabolic response to stress hormone infusion in the conscious dog. Metabolism 1994, 43, 1310–1317. [Google Scholar] [CrossRef]
- Magnusson, I.; Rothman, D.L.; Gerard, D.P.; Katz, L.D.; Shulman, G.I. Contribution of Hepatic Glycogenolysis to Glucose Production in Humans in Response to a Physiological Increase in Plasma Glucagon Concentration. Diabetes 1995, 44, 185–189. [Google Scholar] [CrossRef]
- Wu, C.; Kang, J.E.; Peng, L.-J.; Li, H.; Khan, S.A.; Hillard, C.J.; Okar, D.A.; Lange, A.J. Enhancing hepatic glycolysis reduces obesity: Differential effects on lipogenesis depend on site of glycolytic modulation. Cell Metab. 2005, 2, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Amigo, I.; Traba, J.; González-Barroso, M.M.; Rueda, C.B.; Fernández, M.; Rial, E.; Sánchez, A.; Satrústegui, J.; del Arco, A. Glucagon Regulation of Oxidative Phosphorylation Requires an Increase in Matrix Adenine Nucleotide Content through Ca 2+ Activation of the Mitochondrial ATP-Mg/P i Carrier SCaMC-3. J. Biol. Chem. 2013, 288, 7791–7802. [Google Scholar] [CrossRef]
- López-Moratalla, N.; Funes, T.; Garrido, B.; de Manzanos, T.F.; Santiago, E. Effect of injected glucagon or fatty acids on mitochondrial ATPase. Arch. Biochem. Biophys. 1984, 229, 194–201. [Google Scholar] [CrossRef]
- Barre, H.; Berne, G.; Brebion, P.; Cohen-Adad, F.; Rouanet, J.L. Loose-coupled mitochondria in chronic glucagon-treated hyperthermic ducklings. Am. J. Physiol. 1989, 256, R1192–R1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.H.; Spathis, G.S. Intramuscular glucagon as a provocative stimulus for the assessment of pituitary function: Growth hormone and cortisol responses. Metabolism 1987, 36, 658–663. [Google Scholar] [CrossRef]
- Brillon, D.J.; Zheng, B.; Campbell, R.G.; Matthews, D.E. Effect of cortisol on energy expenditure and amino acid metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 1995, 268, E501–E513. [Google Scholar] [CrossRef]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jørgensen, J.O.L.; Møller, N. Effects of Cortisol on Carbohydrate, Lipid, and Protein Metabolism: Studies of Acute Cortisol Withdrawal in Adrenocortical Failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Cherrington, A.D.; Neal, D.N.; McGuinness, O.P. Role of cortisol in the metabolic response to stress hormone infusion in the conscious dog. Metabolism 1996, 45, 571–578. [Google Scholar] [CrossRef]
- Miller, R.A.; Birnbaum, M.J. Glucagon: acute actions on hepatic metabolism. Diabetologia 2016, 59, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.K.; Mackey, M.A.; Snover, D.C.; Schneider, P.D.; Rucker, R.D.; Eugene Allen, C.; Buchwald, H. Suppression of weight gain by glucagon in obese Zucker rats. Exp. Mol. Pathol. 1984, 40, 320–327. [Google Scholar] [CrossRef]
- Billington, C.J.; Bartness, T.J.; Briggs, J.; Levine, A.S.; Morley, J.E. Glucagon stimulation of brown adipose tissue growth and thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1987, 252, R160–R165. [Google Scholar] [CrossRef]
- Day, J.W.; Ottaway, N.; Patterson, J.T.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757. [Google Scholar] [CrossRef]
- Pocai, A.; Carrington, P.E.; Adams, J.R.; Wright, M.; Eiermann, G.; Zhu, L.; Du, X.; Petrov, A.; Lassman, M.E.; Jiang, G.; et al. Glucagon-Like Peptide 1/Glucagon Receptor Dual Agonism Reverses Obesity in Mice. Diabetes 2009, 58, 2258–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, A.; Haack, T.; Lorenz, M.; Bossart, M.; Elvert, R.; Henkel, B.; Stengelin, S.; Kurz, M.; Glien, M.; Dudda, A.; et al. Design of Novel Exendin-Based Dual Glucagon-like Peptide 1 (GLP-1)/Glucagon Receptor Agonists. J. Med. Chem. 2017, 60, 4293–4303. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.J.; Konkar, A.; Hornigold, D.C.; Trevaskis, J.L.; Jackson, R.; Fritsch Fredin, M.; Jansson-Löfmark, R.; Naylor, J.; Rossi, A.; Bednarek, M.A.; et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016, 18, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Ambery, P.; Parker, V.E.; Stumvoll, M.; Posch, M.G.; Heise, T.; Plum-Moerschel, L.; Tsai, L.-F.; Robertson, D.; Jain, M.; Petrone, M.; et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: A randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet 2018, 391, 2607–2618. [Google Scholar] [CrossRef]
- Finan, B.; Clemmensen, C.; Zhu, Z.; Stemmer, K.; Gauthier, K.; Müller, L.; De Angelis, M.; Moreth, K.; Neff, F.; Perez-Tilve, D.; et al. Chemical Hybridization of Glucagon and Thyroid Hormone Optimizes Therapeutic Impact for Metabolic Disease. Cell 2016, 167, 843–857.e14. [Google Scholar] [CrossRef]
- Cyphert, H.A.; Alonge, K.M.; Ippagunta, S.M.; Hillgartner, F.B. Glucagon Stimulates Hepatic FGF21 Secretion through a PKA- and EPAC-Dependent Posttranscriptional Mechanism. PLoS ONE 2014, 9, e94996. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Giralt, M.; Gavaldà-Navarro, A.; Villarroya, F. Fibroblast growth factor-21, energy balance and obesity. Mol. Cell. Endocrinol. 2015, 418, 66–73. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [Green Version]
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.R.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.A.; Ornitz, D.M.; Potthoff, M.J. FGF21 Regulates Metabolism Through Adipose-Dependent and -Independent Mechanisms. Cell Metab. 2017, 25, 935–944.e4. [Google Scholar] [CrossRef] [Green Version]
- Douris, N.; Stevanovic, D.M.; Fisher, F.M.; Cisu, T.I.; Chee, M.J.; Nguyen, N.L.; Zarebidaki, E.; Adams, A.C.; Kharitonenkov, A.; Flier, J.S.; et al. Central Fibroblast Growth Factor 21 Browns White Fat via Sympathetic Action in Male Mice. Endocrinology 2015, 156, 2470–2481. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Ding, X.; Morgan, D.A.; Coate, K.C.; Bookout, A.L.; Rahmouni, K.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 Acts Centrally to Induce Sympathetic Nerve Activity, Energy Expenditure, and Weight Loss. Cell Metab. 2014, 20, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodoro, J.S.; Rolo, A.P.; Palmeira, C.M. Hepatic FXR: Key regulator of whole-body energy metabolism. Trends Endocrinol. Metab. 2011, 22, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J. Peptides and the blood-brain barrier: Lipophilicity as a predictor of permeability. Brain Res. Bull. 1985, 15, 287–292. [Google Scholar] [CrossRef]
- Hoosein, N.M.; Gurd, R.S. Identification of glucagon receptors in rat brain. Proc. Natl. Acad. Sci. USA 1984, 81, 4368–4372. [Google Scholar] [CrossRef]
- Sasaki, H.; Ebitani, I.; Tominaga, M.; Yamatani, K.; Yawata, Y.; Hara, M. Glucagon-like substance in the canine brain. Endocrinol. Jpn. 1980, 27 (Suppl. 1), 135–140. [Google Scholar] [CrossRef]
- Atrens, D.M.; Menéndez, J. Glucagon and the paraventricular hypothalamus: Modulation of energy balance. Brain Res. 1993, 630, 245–251. [Google Scholar] [CrossRef]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct Control of Brown Adipose Tissue Thermogenesis by Central Nervous System Glucagon-Like Peptide-1 Receptor Signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef] [Green Version]
- Mighiu, P.I.; Yue, J.T.Y.; Filippi, B.M.; Abraham, M.A.; Chari, M.; Lam, C.K.L.; Yang, C.S.; Christian, N.R.; Charron, M.J.; Lam, T.K.T. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat. Med. 2013, 19, 766–772. [Google Scholar] [CrossRef]
- Abraham, M.A.; Yue, J.T.Y.; LaPierre, M.P.; Rutter, G.A.; Light, P.E.; Filippi, B.M.; Lam, T.K.T. Hypothalamic glucagon signals through the KATP channels to regulate glucose production. Mol. Metab. 2014, 3, 202–208. [Google Scholar] [CrossRef]
- LaPierre, M.P.; Abraham, M.A.; Yue, J.T.; Filippi, B.M.; Lam, T.K. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep. 2015, 16, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Habegger, K.M.; Heppner, K.M.; Geary, N.; Bartness, T.J.; DiMarchi, R.; Tschöp, M.H. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 2010, 6, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Glucagon Administration | Increase in Circulating Glucagon | Co-Infusion | Prandial State | Delta EE (kcal/day) | Ref. |
|---|---|---|---|---|---|
| 6 ng/kg/min; infused | 3.5-fold | Somatostatin; Insulin (0.15 mU/kg/min) | Overnight fasted | +75 | [22] |
| 6 ng/kg/min; infused | 3.5-fold | Somatostatin; Insulin (0.45 mU/kg/min) | Overnight fasted | No effect | [22] |
| 3 ng/kg/min; infused | 5-fold | Somatostatin | Overnight fasted | +240 | [18] |
| 3 ng/kg/min; infused | 5–6-fold | -- | Meal right before infusion | No effect | [19] |
| 0.7 mg; intranasal | Transient 2-fold | -- | Overnight fasted | +207 | [23] |
| 50 ng/kg/min; infused | 25-fold | -- | Meal 2 h before infusion | +150 | [20] |
| 50 ng/kg/min; infused | not shown | -- | Overnight fasted | +230 | [21] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinert, M.; Sachs, S.; Habegger, K.M.; Hofmann, S.M.; Müller, T.D. Glucagon Regulation of Energy Expenditure. Int. J. Mol. Sci. 2019, 20, 5407. https://doi.org/10.3390/ijms20215407
Kleinert M, Sachs S, Habegger KM, Hofmann SM, Müller TD. Glucagon Regulation of Energy Expenditure. International Journal of Molecular Sciences. 2019; 20(21):5407. https://doi.org/10.3390/ijms20215407
Chicago/Turabian StyleKleinert, Maximilian, Stephan Sachs, Kirk M. Habegger, Susanna M. Hofmann, and Timo D. Müller. 2019. "Glucagon Regulation of Energy Expenditure" International Journal of Molecular Sciences 20, no. 21: 5407. https://doi.org/10.3390/ijms20215407




