G-Protein Coupled Estrogen Receptor in Breast Cancer
Abstract
:1. Introduction
2. G-Protein Coupled Estrogen Receptor Expression in Breast Cancer
3. G-Protein Coupled Estrogen Receptor Functions in Breast Cancer
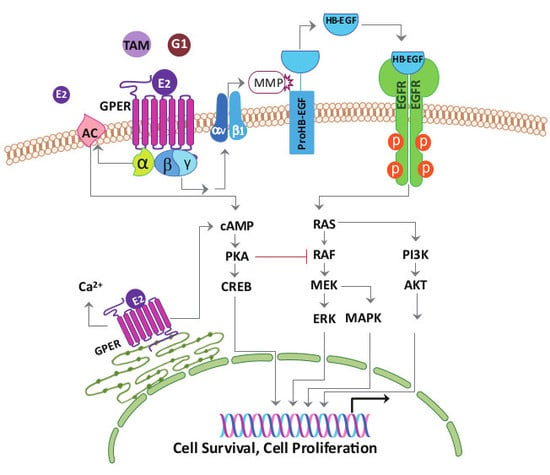
3.1. Tamoxifen-Resistant ERα Positive Cells
3.2. Triple-Negative Breast Cancer Cells
3.3. Cancer-Associated Fibroblasts
3.4. Controversies about G-Protein Coupled Estrogen Receptor Function in Breast Cancer
4. G-protein Coupled Estrogen Receptor Knockout Mice
5. Interactions between Cancer Cells and Cancer-Associated Fibroblasts through G-Protein Coupled Estrogen Receptor
6. Future Perspectives in the Study of the G-Protein Coupled Estrogen Receptor in Breast Cancer
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Ak strain transforming murine thymoma viral oncogene |
| AP-1 | activator protein 1 |
| CAF | cancer-associated fibroblast |
| cAMP | cyclic AMP |
| E2 | 17β-estradiol |
| EGFR | epidermal growth factor receptor |
| ER | estrogen receptor |
| ERα | estrogen receptor α |
| ERβ | estrogen receptor β |
| ERK | extracellular regulated protein kinase |
| GPER | G-protein coupled estrogen receptor |
| HB-EGF | heparin-binding EGF-like growth factor |
| HER2/neu | human epidermal growth factor receptor 2 |
| HIF1α | hypoxia-inducible transcription factor-1α |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor-kappa B |
| PI3K | Phosphoinositide 3-kinase |
| PKA | protein kinase A |
| PR | progesterone receptor |
| SERM | selective estrogen receptor modulator |
| SRC | steroid receptor coactivator |
| TNBC | triple-negative breast cancer |
| VEGF | vascular endothelial growth factor |
References
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan Cancer Registry Annual Report, 2015, Taiwan. Available online: http://www.hpa.gov.tw (accessed on 21 November 2018).
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer Risk Reduction. Available online: http://www.nccn.org (accessed on 21 November 2018).
- Carmeci, C.; Thompson, D.A.; Ring, H.Z.; Francke, U.; Weigel, R.J. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2005, 146, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef]
- Dennis, M.K.; Burai, R.; Ramesh, C.; Petrie, W.K.; Alcon, S.N.; Nayak, T.K.; Bologa, C.G.; Leitao, A.; Brailoiu, E.; Deliu, E.; et al. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 2009, 5, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Pupo, M.; Maggiolini, M.; Musti, A.M. GPER mediates non-genomic effects of estrogen. Methods Mol. Biol. 2016, 1366, 471–488. [Google Scholar]
- Rae, J.M.; Johnson, M.D. What does an orphan G-protein-coupled receptor have to do with estrogen? Breast Cancer Res. 2005, 7, 243–244. [Google Scholar] [CrossRef]
- Lappano, R.; Rosano, C.; Santolla, M.F.; Pupo, M.; De Francesco, E.M.; De Marco, P.; Ponassi, M.; Spallarossa, A.; Ranise, A.; Maggiolini, M. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets 2012, 12, 531–542. [Google Scholar] [CrossRef]
- Méndez-Luna, D.; Bello, M.; Correa-Basurto, J. Understanding the molecular basis of agonist/antagonist mechanism of GPER1/GPR30 through structural and energetic analyses. Steroid Biochem. Mol. Biol. 2016, 158, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Carullo, G.; Giordano, F.; Spina, E.; Nigro, A.; Garofalo, A.; Tassini, S.; Costantino, G.; Vincetti, P.; Bruno, A.; et al. Identification of breast cancer inhibitors specific for G protein-coupled estrogen receptor (GPER)-expressing cells. ChemMedChem 2017, 12, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Graeber, C.T.; Quinn, J.A.; Resnick, M.B.; Giri, D.; DeLellis, R.A.; Steinhoff, M.M.; Sabo, E. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin. Cancer Res. 2006, 12, 6359–6366. [Google Scholar] [CrossRef] [PubMed]
- Marot, D.; Bieche, I.; Aumas, C.; Esselin, S.; Bouquet, C.; Vacher, S.; Lazennec, G.; Perricaudet, M.; Kuttenn, F.; Lidereau, R.; et al. High tumoral levels of Kiss1 and G-protein-coupled receptor 54 expression are correlated with poor prognosis of estrogen receptor-positive breast tumors. Endocr. Relat. Cancer 2007, 14, 691–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prossnitz, E.R.; Hathaway, H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol. 2015, 153, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Marjon, N.A.; Hu, C.; Hathaway, H.J.; Prossnitz, E.R. G protein-coupled estrogen receptor regulates mammary tumorigenesis and metastasis. Mol. Cancer Res. 2014, 12, 1644–1654. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Noske, A.; Meisel, A.; Varga, Z.; Fink, D.; Imesch, P. The G protein-coupled estrogen receptor (GPER) is expressed in two different subcellular localizations reflecting distinct tumor properties in breast cancer. PLoS ONE 2014, 9, e83296. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, M.; Hartman, L.; Grabau, D.; Fornander, T.; Malmström, P.; Nordenskjöld, B.; Sgroi, D.C.; Skoog, L.; Stål, O.; Leeb-Lundberg, L.M.; et al. Lack of G protein-coupled estrogen receptor (GPER) in the plasma membrane is associated with excellent long-term prognosis in breast cancer. Breast Cancer Res. Treat. 2014, 145, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J. A role for G-protein coupled estrogen receptor (GPER) in estrogen-induced carcinogenesis: Dysregulated glandular homeostasis, survival and metastasis. J. Steroid Biochem. Mol. Biol. 2018, 176, 38–48. [Google Scholar] [CrossRef]
- Wang, D.; Hu, L.; Zhang, G.; Zhang, L.; Chen, C. G protein-coupled receptor 30 in tumor development. Endocrine 2010, 38, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Pisano, A.; Maggiolini, M. GPER function in breast cancer: An overview. Front. Endocrinol. 2014, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Cygankiewicz, A.I.; Krajewska, W.M. The G protein-coupled estrogen receptor as a modulator of neoplastic transformation. Mol. Cell Endocrinol. 2016, 429, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Molina, L.; Figueroa, C.D.; Bhoola, K.D.; Ehrenfeld, P. GPER-1/GPR30 a novel estrogen receptor sited in the cell membrane: Therapeutic coupling to breast cancer. Expert Opin. Ther. Targets 2017, 21, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ignatov, T.; Roessner, A.; Costa, S.D.; Kalinski, T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res. Treat. 2010, 123, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ignatov, T.; Weissenborn, C.; Eggemann, H.; Bischoff, J.; Semczuk, A.; Roessner, A.; Costa, S.D.; Kalinski, T. G-protein-coupled estrogen receptor GPR30 and tamoxifen resistance in breast cancer. Breast Cancer Res. Treat. 2011, 128, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Liu, M.; Yang, F.; Luo, H.; Li, Z.; Tu, G.; Yang, G. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 2013, 15, R114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; He, Y.; Shang, D.; Pan, J.; Wang, H.; Chen, H.; Zhu, Z.; Wan, L.; Wang, X. Estrogen and pure antiestrogen fulvestrant (ICI 182 780) augment cell-matrigel adhesion of MCF-7 breast cancer cells through a novel G protein coupled estrogen receptor (GPR30)-to-calpain signaling axis. Toxicol. Appl. Pharmacol. 2014, 275, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Catalano, S.; Giordano, C.; Panza, S.; Chemi, F.; Bonofiglio, D.; Lanzino, M.; Rizza, P.; Romeo, F.; Fuqua, S.A.; Maggiolini, M.; et al. Tamoxifen through GPER upregulates aromatase expression: A novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res. Treat. 2014, 146, 273–285. [Google Scholar] [CrossRef]
- Nass, N.; Kalinski, T. Tamoxifen resistance: From cell culture experiments towards novel biomarkers. Pathol. Res. Pract. 2015, 211, 189–197. [Google Scholar] [CrossRef]
- Rondón-Lagos, M.; Villegas, V.E.; Rangel, N.; Sánchez, M.C.; Zaphiropoulos, P.G. Tamoxifen resistance: Emerging molecular targets. Int. J. Mol. Sci. 2016, 17, 1357. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, T.; Eggemann, H.; Semczuk, A.; Smith, B.; Bischoff, J.; Roessner, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. Role of GPR30 in endometrial pathology after tamoxifen for breast cancer. Am. J. Obstet. Gynecol. 2010, 203, 595. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Hilakivi-Clarke, L.; Clarke, R. Molecular mechanisms of tamoxifen-associated endometrial cancer. Oncol. Lett. 2015, 9, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; De Marco, P.; De Francesco, E.M.; Pezzi, V.; Maggiolini, M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol. Cell Endocrinol. 2010, 320, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. Inactivation of GPR30 reduces growth of triple-negative breast cancer cells: Possible application in targeted therapy. Breast Cancer Res. Treat. 2012, 134, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Steiman, J.; Peralta, E.A.; Louis, S.; Kamel, O. Biology of the estrogen receptor, GPR30, in triple negative breast cancer. Am. J. Surg. 2013, 206, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, M.; Luo, H.; Wu, C.; Tang, X.; Tang, S.; Hu, P.; Yan, Y.; Wang, Z.; Tu, G. GPER mediates enhanced cell viability and motility via non-genomic signaling induced by 17β-estradiol in triple-negative breast cancer (TNBC) cells. J. Steroid Biochem. Mol. Biol. 2014, 143, 392–403. [Google Scholar] [CrossRef]
- Zhou, K.; Sun, P.; Zhang, Y.; You, X.; Li, P.; Wang, T. Estrogen stimulated migration and invasion of estrogen receptor-negative breast cancer cells involves an ezrin-dependent crosstalk between G protein-coupled receptor 30 and estrogen receptor beta signaling. Steroids 2016, 111, 113–120. [Google Scholar] [CrossRef]
- Albanito, L.; Sisci, D.; Aquila, S.; Brunelli, E.; Vivacqua, A.; Madeo, A.; Lappano, R.; Pandey, D.P.; Picard, D.; Mauro, L.; et al. Epidermal growth factor induces G protein-coupled receptor 30 expression in estrogen receptor-negative breast cancer cells. Endocrinology 2008, 149, 3799–3808. [Google Scholar] [CrossRef]
- Lapensee, E.W.; Tuttle, T.R.; Fox, S.R.; Ben-Jonathan, N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and -negative breast cancer cells. Environ. Health Perspect. 2009, 117, 175–180. [Google Scholar] [CrossRef]
- Dong, S.; Terasaka, S.; Kiyama, R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ. Pollut. 2011, 159, 212–218. [Google Scholar] [CrossRef]
- Yu, X.; Filardo, E.J.; Shaikh, Z.A. The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Toxicol. Appl. Pharmacol. 2010, 245, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, T.; Yang, P.; Li, M.; Yang, Y.; Wang, Y.; Du, J.; Pan, K.; Zhang, K. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals. Toxicol. Vitro 2015, 30, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Peng, W.; Xu, G.; Wu, M.; Wen, Y.; Xu, J.; Yu, Z.; Wang, Y. Activation of G protein-coupled receptor 30 by thiodiphenol promotes proliferation of estrogen receptor α-positive breast cancer cells. Chemosphere 2017, 169, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, J.S.; Ishizawar, R.C.; Silva, C.M.; Parsons, S.J. Tyrosine kinase signalling in breast cancer: Epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000, 2, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Quinn, J.A.; Bland, K.I.; Frackelton, A.R., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000, 14, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: A novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 231–238. [Google Scholar] [CrossRef]
- Levin, E.R. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol. 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Filardo, E.J.; Thomas, P. GPR30: A seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol. Metab. 2005, 16, 362–367. [Google Scholar] [CrossRef]
- Quinn, J.A.; Graeber, C.T.; Frackelton, A.R., Jr.; Kim, M.; Schwarzbauer, J.E.; Filardo, E.J. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30. Mol. Endocrinol. 2009, 23, 1052–1064. [Google Scholar] [CrossRef]
- Zekas, E.; Prossnitz, E.R. Estrogen-mediated inactivation of FOXO3a by the G protein-coupled estrogen receptor GPER. BMC Cancer 2015, 15, 702. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Pellegrino, M.; Santolla, M.F.; Lappano, R.; Ricchio, E.; Abonante, S.; Maggiolini, M. GPER mediates activation of HIF1α/VEGF signaling by estrogens. Cancer Res. 2014, 74, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Santolla, M.F.; Lappano, R.; De Marco, P.; Pupo, M.; Vivacqua, A.; Sisci, D.; Abonante, S.; Iacopetta, D.; Cappello, A.R.; Dolce, V.; et al. G protein-coupled estrogen receptor mediates the up-regulation of fatty acid synthase induced by 17β-estradiol in cancer cells and cancer-associated fibroblasts. J. Biol.Chem. 2012, 287, 43234–43245. [Google Scholar] [CrossRef] [PubMed]
- Broselid, S.; Cheng, B.; Sjöström, M.; Lövgren, K.; Klug-De Santiago, H.L.; Belting, M.; Jirström, K.; Malmström, P.; Olde, B.; Bendahl, P.O.; et al. G protein-coupled estrogen receptor is apoptotic and correlates with increased distant disease-free survival of estrogen receptor-positive breast cancer patients. Clin. Cancer Res. 2013, 19, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Poola, I.; Abraham, J.; Liu, A.; Marshalleck, J.J.; Dewitty, R.L. The cell surface estrogen receptor, G protein-coupled receptor 30 (GPR30), is markedly down regulated during breast tumorigenesis. Breast Cancer 2008, 1, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.H.; Chang, L.Y.; Liu, D.L.; Hwa, H.L.; Lin, J.J.; Lee, P.H.; Chen, C.N.; Lien, H.C.; Yuan, R.H.; Shun, C.T.; et al. The interactions between GPR30 and the major biomarkers in infiltrating ductal carcinoma of the breast in an Asian population. Taiwan J. Obstet. Gynecol. 2007, 46, 135–145. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R., Jr.; Bland, K.I. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef]
- Ariazi, E.A.; Brailoiu, E.; Yerrum, S.; Shupp, H.A.; Slifker, M.J.; Cunliffe, H.E.; Black, M.A.; Donato, A.L.; Arterburn, J.B.; Oprea, T.I.; et al. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 2010, 70, 1184–1194. [Google Scholar] [CrossRef]
- Weißenborn, C.; Ignatov, T.; Poehlmann, A.; Wege, A.K.; Costa, S.D.; Zenclussen, A.C.; Ignatov, A. GPER functions as a tumor suppressor in MCF-7 and SK-BR-3 breast cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 663–671. [Google Scholar] [CrossRef]
- Weißenborn, C.; Ignatov, T.; Ochel, H.J.; Costa, S.D.; Zenclussen, A.C.; Ignatova, Z.; Ignatov, A. GPER functions as a tumor suppressor in triple-negative breast cancer cells. J. Cancer Res. Clin. Oncol. 2014, 140, 713–723. [Google Scholar] [CrossRef]
- Chen, Z.J.; Wei, W.; Jiang, G.M.; Liu, H.; Wei, W.D.; Yang, X.; Wu, Y.M.; Liu, H.; Wong, C.K.; Du, J.; et al. Activation of GPER suppresses epithelial mesenchymal transition of triple negative breast cancer cells via NF-κB signals. Mol. Oncol. 2016, 10, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Chen, Z.; Jiang, G.; Zhou, Y.; Liu, Q.; Su, Q.; Wei, W.; Du, J.; Wang, H. Activation of GPER suppresses migration and angiogenesis of triple negative breast cancer via inhibition of NF-κB/IL-6 signals. Cancer Lett. 2017, 386, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Mizukami, Y. GPER negatively regulates TNFα-induced IL-6 production in human breast cancer cells via NF-κB pathway. Endocr. J. 2016, 63, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Xuan, J.; Liu, Y.; Shi, G. Function of G-protein-coupled estrogen receptor-1 in reproductive system tumors. J. Immunol. Res. 2016, 2016, 7128702. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yang, G.; Yu, T.; Luo, S.; Wu, C.; Sun, Y.; Liu, M.; Tu, G. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr. Relat. Cancer 2014, 21, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Liu, M.; Luo, S.; Yu, T.; Wu, C.; Yang, G.; Tu, G. Dynamic monitoring of GPER-mediated estrogenic effects in breast cancer associated fibroblasts: An alternative role of estrogen in mammary carcinoma development. Steroids 2016, 112, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Fuchs, I.; Kauselmann, G.; Kern, H.; Zevnik, B.; Andreasen, P.; Schwarz, G.; Altmann, H.; Klewer, M.; Schoor, M.; et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol. Reprod. 2009, 80, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Martensson, U.E.; Salehi, S.A.; Windahl, S.; Gomez, M.F.; Sward, K.; Daszkiewicz-Nilsson, J.; Wendt, A.; Andersson, N.; Hellstrand, P.; Grande, P.O.; et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 2009, 150, 687–698. [Google Scholar] [CrossRef]
- Isensee, J.; Meoli, L.; Zazzu, V.; Nabzdyk, C.; Witt, H.; Soewarto, D.; Effertz, K.; Fuchs, H.; Gailus-Durner, V.; Busch, D.; et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 2009, 150, 1722–1730. [Google Scholar] [CrossRef]
- Wang, C.; Dehghani, B.; Magrisso, I.J.; Rick, E.A.; Bonhomme, E.; Cody, D.B.; Elenich, L.A.; Subramanian, S.; Murphy, S.J.; Kelly, M.J.; et al. GPR30 contributes to estrogen-induced thymic atrophy. Mol. Endocrinol. 2008, 22, 636–648. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Yang, G.; Hou, Y.; Tang, X.; Wu, C.; Wu, X.A.; Guo, L.; Zhu, Q.; Luo, H.; Du, Y.E.; et al. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene 2017, 36, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Whitaker-Menezes, D.; Castello-Cros, R.; Pavlides, S.; Pestell, R.G.; Fatatis, A.; Witkiewicz, A.K.; Vander Heiden, M.G.; Migneco, G.; Chiavarina, B.; et al. The reverse Warburg effect: Glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle 2010, 9, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Lin, Z.; Ertel, A.; Flomenberg, N.; Witkiewicz, A.K.; Birbe, R.C.; Howell, A.; Pavlides, S.; Gandara, R.; et al. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011, 10, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Maggiolini, M. GPER is involved in the functional liaison between breast tumor cells and cancer-associated fibroblasts (CAFs). J. Steroid Biochem. Mol. Biol. 2018, 176, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: From discovery to functions in vivo. Endocr. J. 2010, 57, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Bilancio, A.; Bontempo, P.; Di Donato, M.; Conte, M.; Giovannelli, P.; Altucci, L.; Migliaccio, A.; Castoria, G. Bisphenol A induces cell cycle arrest in primary and prostate cancer cells through EGFR/ERK/p53 signaling pathway activation. Oncotarget 2017, 8, 115620–115631. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Wang, Z.; Feng, X.; Liu, P.; Lv, X.B.; Li, F.; Yu, F.X.; Sun, Y.; Yuan, H.; et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J. Clin. Investig. 2015, 125, 2123–2135. [Google Scholar] [CrossRef] [Green Version]
- Pupo, M.; Pisano, A.; Abonante, S.; Maggiolini, M.; Musti, A.M. GPER activates Notch signaling in breast cancer cells and cancer-associated fibroblasts (CAFs). Int. J. Biochem. Cell Biol. 2014, 46, 56–67. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, M.; Yang, L.; Tu, G.; Zhu, Q.; Chen, M.; Cheng, H.; Luo, H.; Fu, W.; Li, Z.; et al. Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: A new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and β1-integrin signaling pathway in tumor cells. Breast Cancer Res. 2015, 17, 69. [Google Scholar]
- Pandey, D.P.; Lappano, R.; Albanito, L.; Madeo, A.; Maggiolini, M.; Picard, D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009, 28, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.; He, H.; Chen, Q.; Yue, W. GPER mediated estradiol reduces miR-148a to promote HLA-G expression in breast cancer. Biochem. Biophys. Res. Commun. 2014, 451, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, A.; De Marco, P.; Santolla, M.F.; Cirillo, F.; Pellegrino, M.; Panno, M.L.; Abonante, S.; Maggiolini, M. Estrogenic GPER signaling regulates miR144 expression in cancer cells and cancer-associated fibroblasts (CAFs). Oncotarget 2015, 6, 16573–16587. [Google Scholar] [CrossRef] [PubMed]
- Meijer, D.; van Agthoven, T.; Bosma, P.T.; Nooter, K.; Dorssers, L.C. Functional screen for genes responsible for tamoxifen resistance in human breast cancer cells. Mol. Cancer Res. 2006, 4, 379–386. [Google Scholar] [CrossRef] [PubMed]
- van Agthoven, T.; Sieuwerts, A.M.; Meijer-van Gelder, M.E.; Look, M.P.; Smid, M.; Veldscholte, J.; Sleijfer, S.; Foekens, J.A.; Dorssers, L.C. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J. Clin. Oncol. 2009, 27, 542–549. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P.; Bartella, V.; Vivacqua, A.; Lappano, R.; Santolla, M.F.; Morcavallo, A.; Pezzi, V.; Belfiore, A.; Maggiolini, M. Insulin-like growth factor-I regulates GPER expression and function in cancer cells. Oncogene 2013, 32, 678–688. [Google Scholar] [CrossRef]
- Recchia, A.G.; De Francesco, E.M.; Vivacqua, A.; Sisci, D.; Panno, M.L.; Andò, S.; Maggiolini, M. The G protein-coupled receptor 30 is up-regulated by hypoxia-inducible factor-1alpha (HIF-1alpha) in breast cancer cells and cardiomyocytes. J. Biol. Chem. 2011, 286, 10773–10782. [Google Scholar] [CrossRef]
- Manjegowda, M.C.; Gupta, P.S.; Limaye, A.M. Hyper-methylation of the upstream CpG island shore is a likely mechanism of GPER1 silencing in breast cancer cells. Gene 2017, 614, 65–73. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Giraldi, T.; Migliaccio, A.; Castoria, G.; Auricchio, F. Targeting rapid action of sex steroid receptors in breast and prostate cancers. Front. Biosci. 2011, 16, 2224–2232. [Google Scholar] [CrossRef]
- Cato, A.C.; Nestl, A.; Mink, S. Rapid actions of steroid receptors in cellular signaling pathways. Sci. STKE 2002, 138, re9. [Google Scholar] [CrossRef]
- Prossnitz, E.R. New developments in the rapid actions of steroids and their receptors. J. Steroid Biochem. Mol. Biol. 2018, 176, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.F.; Siu, E.R.; Esteves, C.A.; Monteiro, H.P.; Oliveira, C.A.; Porto, C.S.; Lazari, M.F. 17 beta-estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol. Reprod. 2008, 78, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| References | Materials | Methods | Subcellular Localizations | Effects on Tumor |
|---|---|---|---|---|
| Tamoxifen-resistant ERα positive cells | ||||
| Ignatov 2010 [26] | MCF-7, TAM-R MCF-7 | Western blot | membrane/endoplasmic reticulum | promoting |
| Ignatov 2011 [27] | TAM-R cancer tissue | immunohistochemistry | nucleus/cytoplasm | promoting |
| Mo 2013 [28] | MCF-7, TAM-R MCF-7 | immunohistochemistry | membrane/cytoplasm | promoting |
| TAM-R cancer tissue | immunofluorescence | |||
| TAM-R mouse xenograft | RT-PCR, Western blot | |||
| Chen 2014 [29] | MCF-7, SkBr3 cells | qRT-PCR, Western blot | non-specified | promoting |
| Catalano 2014 [30] | MCF-7, TAM-R MCF-7, SkBr3, CAF | RT-PCR, Western blot for aromatase activity | non-specified | promoting |
| Triple-negative breast cancer cells | ||||
| Lappano 2010 [35] | MCF-7, SkBr3 | RT-PCR, Western blot | non-specified | promoting |
| Girgert 2012 [36] | MDA-MB-435, HCC1806 | RT-PCR, Western blot | non-specified | promoting |
| Steiman 2013 [37] | TNBC cancer tissue | immunohistochemistry | non-specified | promoting |
| Yu 2014 [38] | MDA-MB-468, MDA-MB-436 | immunohistochemistry | nucleus/cytoplasm | promoting |
| TNBC cancer tissue | immunofluorescence | |||
| RT-PCR, Western blot | ||||
| Zhou 2016 [39] | SkBr3, MDA-MB-231 | Western blot | nucleus/cytoplasm | promoting |
| immunofluorescence | ||||
| Albanito 2008 [40] | SkBr3, BT20 | RT-PCR, Western blot | nucleus/cytoplasm | promoting |
| immunofluorescence | ||||
| Cancer-associated fibroblast | ||||
| Luo 2014, 2016 [66,67] | CAFs isolated from surgical | RT-PCR, Western blot | nucleus/cytoplasm | promoting |
| specimens | immunofluorescence | |||
| Conflicting results | ||||
| Broselid 2013 [55] | ER-(+) cancer tissue | RT-PCR, Western blot | non-specified | suppressive |
| MCF-7 ± GPER knockdown | immunofluorescence | |||
| T47D, HEK ± GPER | ||||
| Poola 2008 [56] | ER-(+)&(−) cancer tissue | qRT-PCR | non-specified | suppressive |
| Kuo 2007 [57] | ER-(+)&(−) cancer tissue | qPCR | non-specified | suppressive |
| Filardo 2002 [58] | MCF-7, SkBr3, MDA-MB-231 | Western blot | non-specified | suppressive |
| Ariazi 2010 [59] | ER-(+)/(−) cancer microarray | RT-PCR, Western blot | non-specified | suppressive |
| MCF-7, SkBr3 | Ca2+ imaging | |||
| Weißenborn 2014 [60] | MCF-7, SkBr3 | RT-PCR, Western blot | non-specified | suppressive |
| methylation PCR | ||||
| bioinformatic | ||||
| Weißenborn 2014 [61] | MDA-MB-231, MDA-MB-468 | RT-PCR, Western blot | non-specified | suppressive |
| methylation PCR | ||||
| bioinformatic | ||||
| Chen 2016 [62] | MDA-MB-231 | qRT-PCR, Western blot | nucleus/cytoplasm | suppressive |
| TNBC cancer tissue | immunofluorescence | |||
| MDA-MB-231 mice xenograft | ||||
| Liang 2017 [63] | MDA-MB-231 | qRT-PCR, Western blot | nucleus/cytoplasm | suppressive |
| TNBC tissue microarray | immunofluorescence | |||
| MDA-MB-231 mice xenograft | ||||
| Okamoto 2016 [64] | SkBr3 cells | qRT-PCR, Western blot | non-specified | suppressive |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, L.-H.; Chu, N.-M.; Lin, Y.-F.; Kao, S.-H. G-Protein Coupled Estrogen Receptor in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 306. https://doi.org/10.3390/ijms20020306
Hsu L-H, Chu N-M, Lin Y-F, Kao S-H. G-Protein Coupled Estrogen Receptor in Breast Cancer. International Journal of Molecular Sciences. 2019; 20(2):306. https://doi.org/10.3390/ijms20020306
Chicago/Turabian StyleHsu, Li-Han, Nei-Min Chu, Yung-Feng Lin, and Shu-Huei Kao. 2019. "G-Protein Coupled Estrogen Receptor in Breast Cancer" International Journal of Molecular Sciences 20, no. 2: 306. https://doi.org/10.3390/ijms20020306