Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats
Abstract
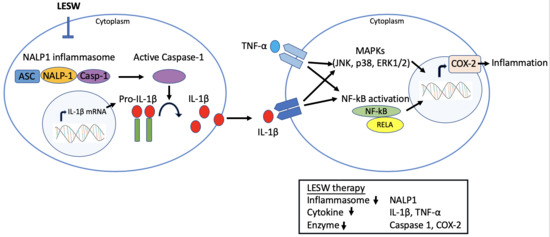
1. Introduction
2. Results
2.1. Pain Behavior Induced by Intraprostatic Capsaicin Injection Were Suppressed by LESW Treatment
2.2. Capsaicin Decrease Pain Threshold and Induce Skin Hypersensitivity, Which Were Suppressed by LESW Treatment
2.3. LESW Treatment Suppressed Capsaicin-Induced Inflammatory Reaction, and Increased COX-2 and TNF-α Immunostaining
2.4. LESW Suppressed Capsaicin-Induced Upregulation of Inflammatory Molecules
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Capsaicin-Induced Prostatitis
4.3. Low Energy Shock Wave Treatment
4.4. Assessment of Pain Behaviors
4.5. Von Frey Filament in Behavioral Testing
4.6. Histology and Immunohistochemistry
4.7. Western Blotting Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LESW | Low energy shock wave |
| CP | Chronic prostatitis |
| CPPS | Chronic pelvic pain syndrome |
| SW | Shock wave |
| VEGF | Vascular endothelial growth factor |
| eNOS | Endothelial nitric oxide synthase |
| PCNA | Proliferating cell nuclear antigen |
| HE | Haematoxylin and eosin |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor Necrosis Factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| NGF | Nerve growth factor |
| NALP1 | NAcht, Leucine-rich repeat and PYD domain containing protein 1 |
| CPSI | Chronic Prostatitis Symptom Index |
| NIK | Nuclear I kappa kinase |
| MAPKs | Mitogen-activated protein kinases |
| NF-κB | Nuclear factor-kappa B |
References
- Pontari, M.A.; Ruggieri, M.R. Mechanisms in prostatitis/chronic pelvic pain syndrome. J. Urol. 2004, 172, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Pontari, M.A.; Ruggieri, M.R. Mechanisms in prostatitis/chronic pelvic pain syndrome. J. Urol. 2008, 179, S61–S67. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.M.; Fagin, A.P.; Hariton, E.; Niska, J.R.; Pierce, M.W.; Kuriyama, A.; Whelan, J.S.; Jackson, J.L.; Dimitrakoff, J.D. Therapeutic intervention for chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS): A systematic review and meta-analysis. PLoS ONE 2012, 7, e41941. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.V.A.; Turk, T.; Jung, J.H.; Xiao, Y.T.; Iakhno, S.; Garrote, V.; Vietto, V. Non-pharmacological interventions for treating chronic prostatitis/chronic pelvic pain syndrome: A Cochrane systematic review. BJU Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Buga, S.; Sarria, J.E.; Spiess, P.E. Advancements in the management of urologic chronic pelvic pain: What is new and what do we know? Curr. Urol. Rep. 2010, 11, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Chaussy, C.; Schmiedt, E.; Jocham, D.; Brendel, W.; Forssmann, B.; Walther, V. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J. Urol. 1982, 127, 417–420. [Google Scholar] [CrossRef]
- Wang, C.J. An overview of shock wave therapy in musculoskeletal disorders. Chang Gung Med. J. 2003, 26, 220–232. [Google Scholar]
- Moayednia, A.; Haghdani, S.; Khosrawi, S.; Yousefi, E.; Vahdatpour, B. Long-term effect of extracorporeal shock wave therapy on the treatment of chronic pelvic pain syndrome due to non bacterial prostatitis. J. Res. Med. Sci. 2014, 19, 293–296. [Google Scholar]
- Vahdatpour, B.; Alizadeh, F.; Moayednia, A.; Emadi, M.; Khorami, M.H.; Haghdani, S. Efficacy of extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome: A randomized, controlled trial. ISRN Urol. 2013, 2013, 972601. [Google Scholar] [CrossRef]
- Zimmermann, R.; Cumpanas, A.; Hoeltl, L.; Janetschek, G.; Stenzl, A.; Miclea, F. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: A feasibility study and the first clinical results. BJU Int. 2008, 102, 976–980. [Google Scholar] [CrossRef]
- Zimmermann, R.; Cumpanas, A.; Miclea, F.; Janetschek, G. Extracorporeal shock wave therapy for the treatment of chronic pelvic pain syndrome in males: A randomised, double-blind, placebo-controlled study. Eur. Urol. 2009, 56, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Breser, M.L.; Salazar, F.C.; Rivero, V.E.; Motrich, R.D. Immunological Mechanisms Underlying Chronic Pelvic Pain and Prostate Inflammation in Chronic Pelvic Pain Syndrome. Front. Immunol. 2017, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Chang, P.J.; Lin, W.Y.; Huang, Y.C.; Ho, D.R. Evidences of the inflammasome pathway in chronic prostatitis and chronic pelvic pain syndrome in an animal model. Prostate 2013, 73, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, M.; Berger, I.; Madersbacher, S. Low-energy extracorporeal shock wave therapy for chronic pelvic pain syndrome: Finally, the magic bullet? Eur. Urol. 2009, 56, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Al Edwan, G.M.; Muheilan, M.M.; Atta, O.N. Long term efficacy of extracorporeal shock wave therapy [ESWT] for treatment of refractory chronic abacterial prostatitis. Ann. Med. Surg. 2017, 14, 12–17. [Google Scholar] [CrossRef]
- Butterworth, P.A.; Walsh, T.P.; Pennisi, Y.D.; Chesne, A.D.; Schmitz, C.; Nancarrow, S.A. The effectiveness of extracorporeal shock wave therapy for the treatment of lower limb ulceration: A systematic review. J. Foot Ankle Res. 2015, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, K.H.; Yin, T.C.; Huang, T.H.; Yuen, C.M.; Chung, S.Y.; Sung, P.H.; Tong, M.S.; Chen, C.H.; Chang, H.W.; et al. Extracorporeal shock wave therapy effectively prevented diabetic neuropathy. Am. J. Transl. Res. 2015, 7, 2543–2560. [Google Scholar] [PubMed]
- Cheng, J.H.; Wang, C.J. Biological mechanism of shockwave in bone. Int. J. Surg. 2015, 24, 143–146. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.N.; Lee, I.S.; Jung, H.; Lee, K.S.; Koh, S.E. Effects of Extracorporeal Shock Wave Therapy on Spasticity in Patients after Brain Injury: A Meta-analysis. J. Phys. Sci. 2014, 26, 1641–1647. [Google Scholar] [CrossRef]
- Wang, C.J. Extracorporeal shockwave therapy in musculoskeletal disorders. J. Orthop. Surg. Res. 2012, 7, 11. [Google Scholar] [CrossRef]
- Wang, C.J.; Cheng, J.H.; Kuo, Y.R.; Schaden, W.; Mittermayr, R. Extracorporeal shockwave therapy in diabetic foot ulcers. Int. J. Surg. 2015, 24, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Cheng, J.H.; Chuang, Y.C. Potential applications of low-energy shock waves in functional urology. Int. J. Urol. 2017, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.C.; Craig, K.; Tibalt, E.; Respizzi, S. Shock wave as biological therapeutic tool: From mechanical stimulation to recovery and healing, through mechanotransduction. Int. J. Surg. 2015, 24, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Stojadinovic, A.; Anam, K.; Amare, M.; Naik, S.; Peoples, G.E.; Tadaki, D.; Elster, E.A. Extracorporeal shock wave therapy suppresses the early proinflammatory immune response to a severe cutaneous burn injury. Int. Wound J. 2009, 6, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.R.; Wang, C.T.; Wang, F.S.; Yang, K.D.; Chiang, Y.C.; Wang, C.J. Extracorporeal shock wave treatment modulates skin fibroblast recruitment and leukocyte infiltration for enhancing extended skin-flap survival. Wound Repair Regen. 2009, 17, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Yang, C.C.; Sun, C.K.; Chiang, H.J.; Chen, Y.L.; Sung, P.H.; Zhen, Y.Y.; Huang, T.H.; Chang, C.L.; Chen, H.H.; et al. Extracorporeal shock wave therapy ameliorates cyclophosphamide-induced rat acute interstitial cystitis though inhibiting inflammation and oxidative stress-in vitro and in vivo experiment studies. Am. J. Transl. Res. 2014, 6, 631–648. [Google Scholar]
- Wang, H.J.; Lee, W.C.; Tyagi, P.; Huang, C.C.; Chuang, Y.C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2016, 36, 1440–1447. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Wu, M.; Chiang, P.H.; Chancellor, M.B. Intraprostatic botulinum toxin a injection inhibits cyclooxygenase-2 expression and suppresses prostatic pain on capsaicin induced prostatitis model in rat. J. Urol. 2008, 180, 742–748. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Yoshimura, N.; Wu, M.; Huang, C.C.; Chiang, P.H.; Tyagi, P.; Chancellor, M.B. Intraprostatic capsaicin injection as a novel model for nonbacterial prostatitis and effects of botulinum toxin A. Eur. Urol. 2007, 51, 1119–1127. [Google Scholar] [CrossRef]
- Watanabe, T.; Inoue, M.; Sasaki, K.; Araki, M.; Uehara, S.; Monden, K.; Saika, T.; Nasu, Y.; Kumon, H.; Chancellor, M.B. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU Int. 2011, 108, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Zhu, G.Q.; Kwon, E.B.; Lee, K.W.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Lee, J.Y.; Bae, W.J.; Kim, S.W. Extracorporeal shock wave therapy decreases COX-2 by inhibiting TLR4-NFkappaB pathway in a prostatitis rat model. Prostate 2019, 79, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, X.; Huang, Y. The effects of interleukin-10 and -8 in chronic prostatitis. Zhonghua Nan Ke Xue 2004, 10, 486–487. [Google Scholar] [PubMed]
- Nadler, R.B.; Koch, A.E.; Calhoun, E.A.; Campbell, P.L.; Pruden, D.L.; Bennett, C.L.; Yarnold, P.R.; Schaeffer, A.J. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J. Urol. 2000, 164, 214–218. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Elster, E.A.; Anam, K.; Tadaki, D.; Amare, M.; Zins, S.; Davis, T.A. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 2008, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Salvesen, G.S. Caspases and apoptosis. Essays Biochem. 2002, 38, 9–19. [Google Scholar] [CrossRef]
- Vykhovanets, E.V.; Resnick, M.I.; MacLennan, G.T.; Gupta, S. Experimental rodent models of prostatitis: Limitations and potential. Prostate Cancer Prostatic Dis. 2007, 10, 15–29. [Google Scholar] [CrossRef]






| Mean Withdrawal Threshold (g/s) Mean ± SEM | Significant (Tukey’s Multiple Comparisons Test) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (hour) | Sham | Capsaicin | Cap + LESW 100 | Cap + LESW 200 | Cap + LESW 300 | Cap vs. Sham | Cap + LESW 100 vs. Cap | Cap + LESW 200 vs. Cap | Cap + LESW 300 vs. Cap | |
| 2 h after 2nd LESW | 0.5 | 58.34 ± 3.60 | 26.32 ± 2.41 | 45.56 ± 5.13 | 49.03 ± 4.16 | 48.07 ± 3.18 | **** | * | ** | ** |
| 1 | 59.78 ± 4.43 | 30.70 ± 3.67 | 46.39 ± 1.88 | 60.42 ± 6.11 | 47.50 ± 2.03 | **** | ns | **** | * | |
| 1.5 | 58.69 ± 4.95 | 30.70 ± 4.03 | 45.97 ± 5.34 | 60.00 ± 6.68 | 52.60 ± 2.39 | *** | ns | **** | ** | |
| 2 | 63.33 ± 4.90 | 30.70 ± 2.43 | 44.86 ± 7.58 | 62.64 ± 3.49 | 55.42 ± 3.65 | **** | ns | **** | *** | |
| 24 h after 2nd LESW | 0.5 | 62.22 ± 6.34 | 30.00 ± 1.64 | 42.99 ± 4.64 | 42.57 ± 4.11 | 44.64 ± 2.15 | **** | ns | ns | ns |
| 1 | 78.61 ± 5.88 | 31.11 ± 3.53 | 50.14 ± 5.09 | 55.69 ± 1.73 | 50.94 ± 2.09 | **** | * | *** | ** | |
| 1.5 | 76.04 ± 5.54 | 29.72 ± 4.03 | 49.45 ± 3.83 | 62.64 ± 3.49 | 52.29 ± 1.47 | **** | ** | **** | *** | |
| 2 | 68.22 ± 5.77 | 31.25 ± 4.64 | 44.31 ± 3.56 | 62.00 ± 3.30 | 53.75 ± 4.40 | **** | ns | **** | *** | |
| Adjusted p-Value (Bonferroni’s Multiple Comparisons Test) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Capsaicin | Cap + LESW 100 | Cap + LESW 200 | Cap + LESW 300 | Cap vs. Sham | Cap + LESW 100 vs. Cap | Cap + LESW 200 vs. Cap | Cap + LESW 300 vs. Cap | ||
| Day 3 | IL-1β | 1.00 ± 0.00 | 2.7 ± 1.89 | 2.24 ±1.62 | 1.18 ± 0.61 | 1.53 ± 0.61 | 0.0196 | >0.9999 | 0.0418 | 0.2519 |
| TNF-α | 1.00 ± 0.00 | 1.34 ± 1.31 | 1.58 ± 1.92 | 0.92 ± 0.55 | 0.81 ± 0.38 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | |
| COX-2 | 1.00 ± 0.00 | 3.45 ± 2.11 | 1.50 ± 1.22 | 0.82 ± 0.30 | 1.01 ± 0.76 | <0.0001 | 0.0016 | <0.0001 | <0.0001 | |
| IL-6 | 1.00 ± 0.00 | 2.63 ± 1.86 | 1.50 ± 0.83 | 1.98 ± 1.40 | 2.17 ± 1.40 | 0.0286 | 0.3392 | >0.9999 | >0.9999 | |
| NGF | 1.00 ± 0.00 | 3.55 ± 1.36 | 2.80 ± 1.61 | 1.73 ± 0.86 | 1.88 ± 0.68 | 0.0004 | >0.9999 | 0.0331 | 0.0465 | |
| NALP1 | 1.00 ± 0.00 | 1.15 ± 0.97 | 0.87 ± 0.42 | 0.98 ± 0.39 | 0.85 ± 0.60 | >0.9999 | >0.9999 | >0.9999 | >0.9999 | |
| Caspase-1 | 1.00 ± 0.00 | 1.95 ± 0.95 | 0.88 ± 0.68 | 0.68 ± 0.37 | 0.57 ± 0.48 | 0.0041 | 0.0008 | 0.0001 | <0.0001 | |
| Day 7 | IL-1β | 1.00 ± 0.00 | 2.75 ± 1.97 | 2.35 ± 1.95 | 1.34 ± 1.41 | 1.33 ± 1.23 | 0.0003 | >0.9999 | 0.0415 | 0.0332 |
| TNF-α | 1.00 ± 0.00 | 2.04 ± 1.51 | 1.28 ± 1.37 | 0.71 ± 0.48 | 0.61 ± 0.65 | 0.0104 | 0.2992 | 0.0002 | <0.0001 | |
| COX-2 | 1.00 ± 0.00 | 3.18 ± 2.11 | 1.83 ± 1.41 | 0.95 ± 0.84 | 0.77 ± 0.52 | <0.0001 | 0.0173 | <0.0001 | <0.0001 | |
| IL-6 | 1.00 ± 0.00 | 3.71 ± 3.40 | 2.88 ± 2.97 | 2.69 ± 2.23 | 2.43 ± 2.79 | 0.0265 | >0.9999 | >0.9999 | >0.9999 | |
| NGF | 1.00 ± 0.00 | 2.48 ± 0.90 | 1.53 ± 0.66 | 1.62 ± 0.53 | 1.57 ± 0.65 | <0.0001 | 0.0154 | 0.0354 | 0.0507 | |
| NALP1 | 1.00 ± 0.00 | 1.43 ± 0.88 | 0.78 ± 0.46 | 0.81 ± 0.30 | 0.98 ± 0.38 | 0.0255 | 0.0233 | 0.0498 | 0.2945 | |
| Caspase-1 | 1.00 ± 0.00 | 1.74 ± 1.18 | 1.09 ± 0.45 | 0.89 ± 0.31 | 1.03 ± 0.36 | 0.0211 | 0.0581 | 0.0055 | 0.0276 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-J.; Tyagi, P.; Chen, Y.-M.; Chancellor, M.B.; Chuang, Y.-C. Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats. Int. J. Mol. Sci. 2019, 20, 4777. https://doi.org/10.3390/ijms20194777
Wang H-J, Tyagi P, Chen Y-M, Chancellor MB, Chuang Y-C. Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats. International Journal of Molecular Sciences. 2019; 20(19):4777. https://doi.org/10.3390/ijms20194777
Chicago/Turabian StyleWang, Hung-Jen, Pradeep Tyagi, Yu-Ming Chen, Michael B. Chancellor, and Yao-Chi Chuang. 2019. "Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats" International Journal of Molecular Sciences 20, no. 19: 4777. https://doi.org/10.3390/ijms20194777
APA StyleWang, H.-J., Tyagi, P., Chen, Y.-M., Chancellor, M. B., & Chuang, Y.-C. (2019). Low Energy Shock Wave Therapy Inhibits Inflammatory Molecules and Suppresses Prostatic Pain and Hypersensitivity in a Capsaicin Induced Prostatitis Model in Rats. International Journal of Molecular Sciences, 20(19), 4777. https://doi.org/10.3390/ijms20194777