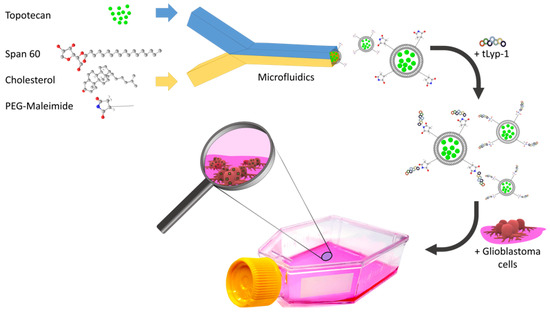
Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization and Characterization of Niosomal Formulations
2.2. Drug Release
2.3. Cellular Uptake and Internalization
2.4. Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Preparation and Optimization of Tpt Encapsulated Niosomes by Microfluidics
3.3. Conjugation of TLyp-1 to PEGNIO/TPT
3.4. Measurement of Particle Size, Distribution, and Zeta Potential
3.5. Stability
3.6. Entrapment Efficiency
3.7. Drug Release
3.8. Cell Culture
3.9. Cellular uptake and internalization
3.10. Cytotoxicity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [PubMed]
- Houghton, P.J.; Cheshire, P.J.; Hallman, J.D.; Lutz, L.; Friedman, H.S.; Danks, M.K.; Houghton, J.A. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother. Pharm. 1995, 36, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Wethington, S.L.; Wright, J.D.; Herzog, T.J. Key role of topoisomerase I inhibitors in the treatment of recurrent and refractory epithelial ovarian carcinoma. Expert Rev. Anticancer Ther. 2008, 8, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Zunino, F. Relevance of extracellular and intracellular interactions of camptothecins as determinants of antitumor activity. Biochem. Pharm. 2007, 74, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.G.; Silva, E.J.; Martins, A.L.L.; Mota, M.F.; Braga, R.C.; Lima, E.M.; Valadares, M.C.; Taveira, S.F.; Marreto, R.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Selective topotecan delivery to cancer cells by targeted pH-sensitive mesoporous silica nanoparticles. RSC Adv. 2016, 6, 50923–50932. [Google Scholar] [CrossRef] [Green Version]
- Seleci, M.; Ag Seleci, D.; Scheper, T.; Stahl, F. Theranostic Liposome-Nanoparticle Hybrids for Drug Delivery and Bioimaging. Int. J. Mol. Sci. 2017, 7, 18. [Google Scholar] [CrossRef]
- Venâncio, J.H.; Andrade, L.M.; Esteves, N.L.S.; Brito, L.B.; Valadares, M.C.; Oliveira, G.A.R.; Lima, E.M.; Marreto, R.N.; Gratieri, T.; Taveira, S.F. Topotecan-loaded lipid nanoparticles as a viable tool for the topical treatment of skin cancers. J. Pharm. Pharmacol. 2017, 69, 1318–1326. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Mauro, L.; Pellegrino, M.; Andò, S.; Picci, N. Transferrin-conjugated pluronic niosomes as a new drug delivery system for anticancer therapy. Langmuir 2013, 29, 12638–12646. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Tavano, L.; La Mesa, C. Alkyl glucopyranoside-based niosomes containing methotrexate for pharmaceutical applications: Evaluation of physico-chemical and biological properties. Int. J. Pharm. 2013, 458, 224–229. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta. Pharmaceutica. Sinica. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009, 10, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Attia, I.A.; El-Gizawy, S.A.; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech 2007, 8, E106. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel sustained release nonionic stable vesicular systems--an overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Lee, S.M.-Y.; Yi, C.; Li, C.-W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications-a review. Lab. Chip. 2017, 17, 209–226. [Google Scholar] [CrossRef]
- Obeid, M.A.; Gebril, A.M.; Tate, R.J.; Mullen, A.B.; Ferro, V.A. Comparison of the physical characteristics of monodisperse non-ionic surfactant vesicles (NISV) prepared using different manufacturing methods. Int. J. Pharm. 2017, 521, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Obeid, M.A.; Khadra, I.; Mullen, A.B.; Tate, R.J.; Ferro, V.A. The effects of hydration media on the characteristics of non-ionic surfactant vesicles (NISV) prepared by microfluidics. Int. J. Pharm. 2017, 516, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Stolzenburg, P.; Lorenz, T.; Dietzel, A.; Garnweitner, G. Microfluidic synthesis of metal oxide nanoparticles via the nonaqueous method. Chem. Eng. Sci. 2018, 191, 500–510. [Google Scholar] [CrossRef]
- Jahn, A.; Reiner, J.E.; Vreeland, W.N.; DeVoe, D.L.; Locascio, L.E.; Gaitan, M. Preparation of nanoparticles by continuous-flow microfluidics. J. Nanopart. Res. 2008, 10, 925–934. [Google Scholar] [CrossRef]
- Karnik, R.; Gu, F.; Basto, P.; Cannizzaro, C.; Dean, L.; Kyei-Manu, W.; Langer, R.; Farokhzad, O.C. Microfluidic platform for controlled synthesis of polymeric nanoparticles. Nano. Lett. 2008, 8, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-up design and synthesis of limit size lipid nanoparticle systems with aqueous and triglyceride cores using millisecond microfluidic mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Seleci, D.A.; Seleci, M.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6, 87910–87918. [Google Scholar] [CrossRef] [Green Version]
- Ciobanasu, C.; Dragomir, I.; Apetrei, A. The penetrating properties of the tumor homing peptide LyP-1 in model lipid membranes. J. Pept. Sci. 2019, 25, e3145. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Stahl, F.; Scheper, T. Tumor homing and penetrating peptide-conjugated niosomes as multi-drug carriers for tumor-targeted drug delivery. RSC Adv. 2017, 7, 33378–33384. [Google Scholar] [CrossRef] [Green Version]
- Ruoslahti, E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv. Mater. Weinheim. 2012, 24, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Lei, Y.; Li, Q.; Wu, Y.; Zhang, L.; Mu, P.-P.; Ji, G.-Q.; Tang, C.-X.; Wang, Y.-Q.; Gao, J.; et al. Neuropilin-1 is a glial cell line-derived neurotrophic factor receptor in glioblastoma. Oncotarget 2017, 8, 74019–74035. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Ernsting, M.J.; Undzys, E.; Li, S.-D. A highly tumor-targeted nanoparticle of podophyllotoxin penetrated tumor core and regressed multidrug resistant tumors. Biomaterials 2015, 52, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramishetti, S.; Kedmi, R.; Goldsmith, M.; Leonard, F.; Sprague, A.G.; Godin, B.; Gozin, M.; Cullis, P.R.; Dykxhoorn, D.M.; Peer, D. Systemic Gene Silencing in Primary T Lymphocytes Using Targeted Lipid Nanoparticles. ACS Nano. 2015, 9, 6706–6716. [Google Scholar] [CrossRef] [PubMed]
- Seleci, M.; Ag Seleci, D.; Joncyzk, R.; Stahl, F.; Blume, C.; Scheper, T. Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials 2016, 17, 410. [Google Scholar] [CrossRef]
- Hu, Q.; Gu, G.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; Yao, L.; et al. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials 2013, 34, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hayes, M.E.; Connolly-Ingram, C.; Gabriel, B.S.; Hann, B.; Liu, B.; Park, J.W.; Hong, K.; et al. Development of a highly stable and targetable nanoliposomal formulation of topotecan. J. Control Release 2010, 141, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vali, A.M.; Toliyat, T.; Shafaghi, B.; Dadashzadeh, S. Preparation, optimization, and characterization of topotecan loaded PEGylated liposomes using factorial design. Drug Dev. Ind. Pharm. 2008, 34, 10–23. [Google Scholar] [CrossRef]
- Abraham, S.A.; Edwards, K.; Karlsson, G.; Hudon, N.; Mayer, L.D.; Bally, M.B. An evaluation of transmembrane ion gradient-mediated encapsulation of topotecan within liposomes. J. Control. Release 2004, 96, 449–461. [Google Scholar] [CrossRef]
- Padhi, S.; Mirza, M.A.; Verma, D.; Khuroo, T.; Panda, A.K.; Talegaonkar, S.; Khar, R.K.; Iqbal, Z. Revisiting the nanoformulation design approach for effective delivery of topotecan in its stable form: An appraisal of its in vitro Behavior and tumor amelioration potential. Drug Deliv. 2016, 23, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.P.; Arami, H.; Banga, I.; Gupta, J.; Gandhi, S. Cell penetrating peptides in preclinical and clinical cancer diagnosis and therapy. Oncotarget 2018, 9, 37252–37267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-b.; Wang, Z.; Wang, Q.-s.; Han, Y.-j.; Wang, M.; Zhou, W.-l.; Li, H.-s. Use of Labelled tLyP-1 as a Novel Ligand Targeting the NRP Receptor to Image Glioma. PLoS ONE 2015, 10, e0137676. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Agemy, L.; Kotamraju, V.R.; Braun, G.; Teesalu, T.; Sugahara, K.N.; Hamzah, J.; Ruoslahti, E. Transtumoral targeting enabled by a novel neuropilin-binding peptide. Oncogene 2012, 31, 3754–3763. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gao, X.; Gu, G.; Kang, T.; Tu, Y.; Liu, Z.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Glioma therapy using tumor homing and penetrating peptide-functionalized PEG-PLA nanoparticles loaded with paclitaxel. Biomaterials 2013, 34, 5640–5650. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Wang, P.; Liu, Y.-H.; Liu, L.-B.; Liu, X.-B.; Li, Z.; Xue, Y.-X. Topoisomerase I inhibitors, shikonin and topotecan, inhibit growth and induce apoptosis of glioma cells and glioma stem cells. PLoS ONE 2013, 8, e81815. [Google Scholar] [CrossRef]
- Chen, L.; Miao, W.; Tang, X.; Zhang, H.; Wang, S.; Luo, F.; Yan, J. The expression and significance of neuropilin-1 (NRP-1) on glioma cell lines and glioma tissues. J. Biomed. Nanotechnol. 2013, 9, 559–563. [Google Scholar] [CrossRef]
- Hao, Y.-L.; Deng, Y.-J.; Chen, Y.; Wang, X.-M.; Zhong, H.-J.; Suo, X.-B. In vitro and in vivo studies of different liposomes containing topotecan. Arch. Pharm. Res. 2005, 28, 626–635. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Zhang, Z.; Xie, B.-B.; Zhang, H.-Y. Development and evaluation of topotecan loaded solid lipid nanoparticles: A study in cervical cancer cell lines. J. Photochem. Photobiol. B. Biol. 2016, 165, 182–188. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Amador, C.; Wang, L.C.; Mody, U.V.; Bally, M.B. What Drives Innovation: The Canadian Touch on Liposomal Therapeutics. Pharmaceutics 2019. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, M.; Lavrentieva, A.; Walter, J.-G.; Sambale, F.; Green, M.; Stahl, F.; Scheper, T. Evaluation of CdTe/CdS/ZnS core/shell/shell quantum dot toxicity on three-dimensional spheroid cultures. Toxicol. Res. (Camb) 2016, 5, 126–135. [Google Scholar] [CrossRef] [PubMed]






| Samples | EE (%) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| PEGNIO | − | 138.90 | 0.072 | −27.80 |
| PEGNIO/TPT | 39.30 | 128.47 | 0.131 | −27.00 |
| PEGNIO/TPT/tLyp-1 | 37.50 | 159.79 | 0.124 | −20.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ag Seleci, D.; Maurer, V.; Stahl, F.; Scheper, T.; Garnweitner, G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. Int. J. Mol. Sci. 2019, 20, 4696. https://doi.org/10.3390/ijms20194696
Ag Seleci D, Maurer V, Stahl F, Scheper T, Garnweitner G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. International Journal of Molecular Sciences. 2019; 20(19):4696. https://doi.org/10.3390/ijms20194696
Chicago/Turabian StyleAg Seleci, Didem, Viktor Maurer, Frank Stahl, Thomas Scheper, and Georg Garnweitner. 2019. "Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery" International Journal of Molecular Sciences 20, no. 19: 4696. https://doi.org/10.3390/ijms20194696
APA StyleAg Seleci, D., Maurer, V., Stahl, F., Scheper, T., & Garnweitner, G. (2019). Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. International Journal of Molecular Sciences, 20(19), 4696. https://doi.org/10.3390/ijms20194696