Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines
Abstract
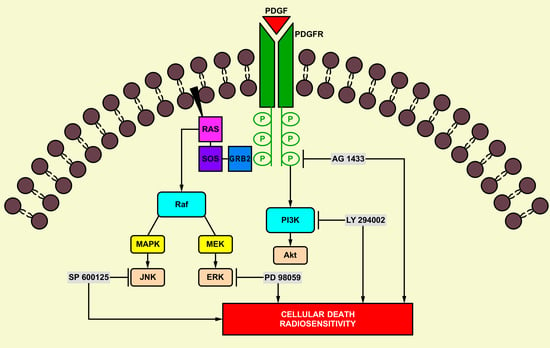
1. Introduction
2. Results
2.1. The Effect of PDGFR Inactivation on HGG Cells
2.2. The Effect of Ionizing Radiation on HGG Cellular Viability
2.3. The Effect of Combined Treatment on HGG Cell Viability
2.4. The Interaction between Combined Treatment with AG1433 and Ionizing Radiation in HGG
2.5. The Effect of Signal Transduction Inhibition on Radiation Response in HGG Cells
2.6. The Interaction between Combined Treatment with LY294002, PD 98059, and SP600125 and Ionizing Radiation in HGG Cell Lines
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Cultures and Treatment
4.3. Flow Cytometry
4.4. Irradiation
4.5. MTT Assay
4.6. Clonogenic Assay
4.7. Surviving Fraction (SF) Determination
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavence, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Hopkins, K.; Tonn, J.C.; Cohen-Jonathan-Moyal, E.; Frappaz, D.; Henriksson, R.; Balana, C. EANO Guideline for the Diagnosis and Treatment of Anaplastic Gliomas and Glioblastoma. Lancet Oncol. 2014, 15, 395–403. [Google Scholar] [CrossRef]
- Toepoel, M.; Joosten, P.H.L.J.; Knobbe, C.B.; Afink, G.B.; Zotz, R.B.; Steegers-Theunissen, R.P.; Reifenberger, G.; van Zoelen, E.J.J. Haplotype-Specific Expression of the Human PDGFRA Gene Correlates with the Risk of Glioblastomas. Int. J. Cancer 2008, 123, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Nister, M.; Heldin, C.H.; Wasteson, A.; Westmark, B. A Platelet-Derived Growth Factor Analog Produced by a Human Glioma Cell Line. Ann. N.Y. Acad. Sci. 1982, 397, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, M.; Funa, K.; Hartman, M.; Claesson-Welsh, L.; Heldin, C.-H.; Westmark, B.; Nister, M. Platelet-Derived Growth Factor and its Receptors in Human Glioma Tissue: Expression of Messenger RNA and Protein Suggests the Presence of Autocrine and Paracrine Loops. Cancer Res. 1992, 52, 3213–3219. [Google Scholar] [PubMed]
- Jackson, E.L.; Garcia-Verdugo, J.M.; Gil-Perotin, S.; Roy, M.; Quinones-Hinojosa, A.; VandenBerg, S.; Alvarez-Buylla, A. PDGFR Alpha-Positive B Cells Are Neural Stem Cells in the Adult SVZ that Form Glioma-like Growth in Response to Increased PDGF Signaling. Neuron 2006, 51, 187–199. [Google Scholar] [CrossRef]
- Cantahende, I.G.; de Oliveira, J. PDGF Family Expression in Glioblastoma Multiforme: Data Compilation from Ivy Glioblastoma Atlas Project Database. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Raymond, E.; Brandes, A.A.; Dittrich, C.; Fumoleau, P.; Coudert, B.; Clement, P.M.J.; Frenay, M.; Rampling, R.; Stupp, R.; Kros, J.M.; et al. Phase II Study of Imatinib in Patients with Recurrent Gliomas of Various Histologies: A European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J. Clin. Oncol. 2008, 26, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Razis, E.; Selviaridis, P.; Labropoulos, S.; Norris, J.L.; Zhu, M.-J.; Song, D.D.; Kalebic, T.; Torrens, M.; Kalogera-Fountzila, A.; Karkavelas, G.; et al. Phase II Study of Neoadjuvant Imatinib in Glioblastoma: Evaluation of Clinical and Molecular Effects of the Treatment. Clin. Cancer Res. 2009, 15, 6258–6266. [Google Scholar] [CrossRef]
- Roberts, W.G.; Whalen, P.M.; Soderstrome, E.; Moraski, G.; Lyssikatos, J.P.; Wang, H.F.; Cooper, B.; Baker, D.A.; Savage, D.; Dalvie, D.; et al. Antiangiogenic and Anti-tumor Activity of a Selective PDGFR Tyrosine Inhibitor, CP-673,451. Cancer Res. 2005, 65, 957–966. [Google Scholar]
- Holdhoff, M.; Kreuzer, K.A.; Appelt, C.; Scholz, R.; Na, I.K.; Hildebrandt, B.; Riess, H.; Jordan, A.; Schmidt, C.A.; Van Etten, R.A.; et al. Imatinib Mesylate Radiosensitizes Human Glioblastoma Cells Through Inhibition of Platelet-derived Growth Factor Receptor. Blood Cells Mol. Dis. 2005, 34, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Carapancea, M.; Cosaceanu, D.; Budiu, R.; Kwiecinska, A.; Tatatranu, L.; Ciubotaru, V.; Alexandru, O.; Banita, M.; Pisoschi, C.; Backlund, M.L.; et al. Dual Targeting of IGF-1R and PDGFR Inhibits Proliferation in High-grade Glioma Cells and Induces Radiosensitivity in JNK-1 Expressing Cells. J. Neurooncol. 2007, 85, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Carapancea, M.; Alexandru, O.; Fetea, A.S.; Dragutescu, L.; Castro, J.; Georgescu, A.; Popa-Wagner, A.; Backlund, M.L.; Lewensohn, R.; Dricu, A. Growth Factor Receptor Signaling in Glioblastoma Cells: Therapeutic Implications. J. Neurooncol. 2009, 92, 137–147. [Google Scholar] [CrossRef]
- Cosaceanu, D.; Carapancea, M.; Budiu, R.; Martinsson, H.-S.; Starborg, M.; Vrabete, M.; Kanter, L.; Lewensohn, R.; Dricu, A. Comparison of Three Approaches for Inhibiting Insulin-like Growth Factor I Receptor and their Effects on NSCLC Cell Lines in vitro. Growth Factors 2007, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goussia, A.C.; Agnantis, N.J.; Rao, J.S.; Kyritsis, A.P. Cytogenetic and Molecular Abnormalities in Astrocytic Gliomas (Review). Oncol. Rep. 2000, 7, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.M.; Alexandru, O.; Brindusa, C.; Purcaru, S.O.; Tache, D.E.; Tataranu, L.G.; Taisescu, C.; Dricu, A. Targeting the VEGF and PDGF Signaling Pathway in Glioblastoma Treatment. Int. J. Clin. Exp. Pathol. 2015, 8, 7825–7837. [Google Scholar] [PubMed]
- Dhermain, F. Radiotherapy of High-Grade Gliomas: Current Standards and New Concepts, Innovations in Imaging and Radiotherapy, and New Therapeutic Approaches. Chin. J. Cancer. 2014, 33, 16–24. [Google Scholar] [CrossRef]
- Khan, L.; Soliman, H.; Sahgal, A.; Perry, J.; Xu, W.; Tsao, M.N. External Beam Radiation Dose Escalation for High Grade Glioma (Protocol). Cochrane Libr. 2015, 1, CD011475. [Google Scholar]
- Zhu, H.; Ruan, S.; Jia, F.; Chu, J.; Zhu, Y.; Huang, Y.; Liu, G. In vitro and in vivo Superior Radiosensitizing Effect of Berbamine for Head and Neck Squamous Cell Carcinoma. Onco. Targets Ther. 2018, 11, 8117–8125. [Google Scholar] [CrossRef]
- Saga, R.; Matsuya, Y.; Takahashi, R.; Hasegawa, K.; Date, H.; Hosokawa, Y. Analysis of the High-dose-range Radioresistance of prostate Cancer Cells, Including Cancer Stem Cells, Based on a Stochastic Model. J. Radiat. Res. 2019, 60, 298–307. [Google Scholar] [CrossRef]
- Alexandru, O.; Purcaru, S.O.; Tataranu, L.G.; Lucan, L.; Castro, J.; Folcuti, C.; Artene, S.-A.; Tuta, C.; Dricu, A. The Influence of EGFR Inactivation on the Radiation Response in High Grade Glioma. Int. J. Mol. Sci. 2018, 19, 229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, W.; Li, N.; Neri, S.; Sharma, A.; Jiang, W.; Lin, S.H. Combining Immunotherapy and Radiotherapy for Cancer Treatment: Current Challenges and Future Directions. Front. Pharmacol. 2018, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Reardon, D.B.; Park, J.S.; Bowers, G.; Logsdon, C.; Valerie, K.; Schmidt-Ullrich, R. Radiation-induced Release of Transforming Growth Factor Alpha Activates the Epidermal Growth Factor Receptor and Mitogen-Activated Protein Kinase Pathway in Carcinoma Cells, Leading to Increased Proliferation and Protection from Radiation-Induced Cell Death. Mol. Biol. Cell 1999, 10, 2493–2506. [Google Scholar] [CrossRef] [PubMed]
- Amorino, G.P.; Hamilton, V.M.; Valerie, K.; Dent, P.; Lammering, G.; Schmidt-Ullrich, R.K. Epidermal Growth Factor Receptor Dependence of Radiation-Induced Transcription Factor Activation in Human Breast Carcinoma Cells. Mol. Biol. Cell 2002, 13, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships. The Combined Treatment of Multiple Drugs or Enzyme Inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. A Novel Approach in the Treatment of Cancer: Targeting the Epidermal Growth Factor Receptor. Clin. Cancer Res. 2001, 7, 2958–2970. [Google Scholar] [PubMed]
- Wang, M.; Xie, Y.T.; Girnita, L.; Nilsson, G.; Dricu, A.; Wejde, J.; Larsson, O. Regulatory Role of Mevalonate and N-linked Glycosylation in Proliferation and Expression of the EWS/FLI-1 Fusion Protein in Ewing’s Sarcoma Cells. Exp. Cell Res. 1999, 246, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Lewitt, M.; Massambu, C.; Dricu, A.; Grunler, J.; Axelson, M.; Biberfeld, P.; Brismar, K. Insulin-like Growth Factor-I Receptor Activity is Essential for Kaposi’s Sarcoma Growth and Survival. Br. J. Cancer 2005, 92, 1467–1474. [Google Scholar] [CrossRef]
- Suleymanova, N.; Crudden, C.; Worrall, C.; Dricu, A.; Girnita, A.; Girnita, L. Enhanced Response of Melanoma Cells to MEK Inhibitors Following Unbiased IGF-1R Down-regulation. Oncotarget 2017, 8, 82256–82267. [Google Scholar] [CrossRef]
- Hagerstrand, D.; Lindh, M.B.; Pena, C.; Garcia Ercheveria, C.; Nister, M.; Hofmann, E.; Ostman, A. PI3K/pten/akt Pathway Status Affects the Sensitivity of High-Grade Glioma Cell Cultures to the Insulin-Like Growth Factor-1 Receptor Inhibitor nvp-aew541. Neuro Oncol. 2010, 12, 967–975. [Google Scholar] [CrossRef]
- Shih, A.H.; Dai, C.; Hu, X.; Rosenblum, M.K.; Koutcher, J.A.; Holland, E.C. Dose-Dependent Effects of Platelet-Derived Growth Factor-B on Glial Tumorigenesis. Cancer Res. 2004, 64, 4783–4789. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, E.; Wu, Q.; Guryanova, O.; Hitomi, M.; Lathia, J.; Serwanski, D.; Sloan, A.; Weil, R.; Lee, J.; et al. Platelet-Derived Growth Factor Receptors Differentially Inform Intertumoral and Intratumoral Heterogeneity. Genes Dev. 2012, 26, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Langley, E.; Chao, Y.J.; Jiang, P.; Mukthavaram, R.; Sandeep, C.P.; Kim, P.; Singh, S.; Kesari, S. Mechanisms of Resistance to PDGFR Inhibition in Glioblastoma. J. Clin. Oncol. 2017, 15. [Google Scholar] [CrossRef]
- Stupp, R.; Heigi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoom, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide Versus Radiotherapy Alone on Survivalin Glioblastoma in a Randomized Phase III Study: 5-year Analysis of the EORTC-NCIC Trial. Lancet Oncol 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Kelley, K.; Knisely, J.; Symons, M.; Ruggieri, R. Radioresistance of Brain Tumors. Cancers 2016, 8, 42. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Dewire, M.; Ryall, S.; Buczkowitz, P.; Leach, J.; Miles, L.; Ramani, A.; Brudno, M.; Kumar, S.S.; Drissi, R.; et al. Spatial Genomic Heterogeneity in Diffuse Intrinsic Pontine and Midline High-grade Glioma: Implications for Diagnostic Biopsy and Targeted Therapeutics. Acta Neuropathol. Commun. 2016, 4, 1. [Google Scholar] [CrossRef]
- Wilson, G.D.; Bentzen, S.M.; Harari, P.M. Biologic Basis for Combining Drugs with Radiation. Semin Radiat. Oncol. 2006, 16, 2–9. [Google Scholar] [CrossRef]
- Steel, G.G.; Peckham, M.J. Exploitable Mechanisms in Combined Radiotherapy-Chemotherapy. The Concept of Additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef]
- Tataranu, L.G.; Ciubotaru, V.; Cazac, T.L.; Alexandru, O.; Purcaru, S.O.; Tache, D.E.; Artene, S.A. Current Trends in Glioblastoma Treatment. Tech. Open 2018. [Google Scholar] [CrossRef]
- Nakada, M.; Kita, D.; Watanabe, T.; Hayashi, Y.; Teng, L.; Pyko, I.V.; Hamada, J.I. Aberrant Signaling Pathways in Glioma. Cancers 2011, 3, 3242–3278. [Google Scholar] [CrossRef] [PubMed]








| Rad (Gy) | AG1433 (µM) | Days after the Treatment | Predicted Survival | Observed Survival | Effect |
|---|---|---|---|---|---|
| 2 | 10 | 3 | 0.7 | 0.7 | ADD |
| 7 | 0.4 | 0.5 | SUB | ||
| 20 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.3 | 0.4 | SUB | ||
| 30 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.3 | 0.4 | SUB | ||
| 4 | 10 | 3 | 0.7 | 0.7 | ADD |
| 7 | 0.4 | 0.4 | ADD | ||
| 20 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.3 | 0.4 | SUB | ||
| 30 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.3 | 0.4 | SUB | ||
| 6 | 10 | 3 | 0.7 | 0.7 | ADD |
| 7 | 0.3 | 0.4 | SUB | ||
| 20 | 3 | 0.7 | 0.8 | SUB | |
| 7 | 0.3 | 0.4 | SUB | ||
| 30 | 3 | 0.7 | 0.8 | SUB | |
| 7 | 0.2 | 0.4 | SUB | ||
| 8 | 10 | 3 | 0.7 | 0.7 | ADD |
| 7 | 0.3 | 0.3 | ADD | ||
| 20 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.3 | 0.3 | ADD | ||
| 30 | 3 | 0.6 | 0.8 | SUB | |
| 7 | 0.2 | 0.4 | SUB | ||
| 10 | 10 | 3 | 0.7 | 0.7 | ADD |
| 7 | 0.3 | 0.3 | ADD | ||
| 20 | 3 | 0.7 | 0.7 | ADD | |
| 7 | 0.2 | 0.3 | SUB | ||
| 30 | 3 | 0.6 | 0.8 | SUB | |
| 7 | 0.2 | 0.4 | SUB |
| Rad (Gy) | AG1433 (µM) | Days after the Treatment | Predicted Survival | Observed Survival | Effect |
|---|---|---|---|---|---|
| 2 | 10 | 3 | 0.6 | 0.7 | SUB |
| 7 | 0.5 | 0.6 | SUB | ||
| 20 | 3 | 0.6 | 0.7 | SUB | |
| 7 | 0.5 | 0.6 | SUB | ||
| 30 | 3 | 0.6 | 0.7 | SUB | |
| 7 | 0.5 | 0.6 | SUB | ||
| 4 | 10 | 3 | 0.5 | 0.6 | SUB |
| 7 | 0.4 | 0.4 | ADD | ||
| 20 | 3 | 0,5 | 0.7 | SUB | |
| 7 | 0.4 | 0.5 | SUB | ||
| 30 | 3 | 0.5 | 0.8 | SUB | |
| 7 | 0.4 | 0.5 | SUB | ||
| 6 | 10 | 3 | 0.5 | 0.7 | SUB |
| 7 | 0.4 | 0.4 | ADD | ||
| 20 | 3 | 0.6 | 0.7 | SUB | |
| 7 | 0.4 | 0.5 | SUB | ||
| 30 | 3 | 0.6 | 0.8 | SUB | |
| 7 | 0.4 | 0.6 | SUB | ||
| 8 | 10 | 3 | 0.5 | 0.6 | SUB |
| 7 | 0.3 | 0.4 | SUB | ||
| 20 | 3 | 0.5 | 0.7 | SUB | |
| 7 | 0.3 | 0.7 | SUB | ||
| 30 | 3 | 0.5 | 0.6 | SUB | |
| 7 | 0.3 | 0.5 | SUB | ||
| 10 | 10 | 3 | 0.5 | 0.6 | SUB |
| 7 | 0.3 | 0.4 | SUB | ||
| 20 | 3 | 0.5 | 0.8 | SUB | |
| 7 | 0.3 | 0.5 | SUB | ||
| 30 | 3 | 0.5 | 0.7 | SUB | |
| 7 | 0.3 | 0.5 | SUB |
| Cell Line | Rad (Gy) | Type of Intracellular Signaling Inhibition | Predicted Survival | Observed Survival | Effect |
|---|---|---|---|---|---|
| 11HGG | 2 | LY294002 10 µM | 0.9 | 0.9 | ADD |
| PD 98059 10 µM | 0.8 | 0.8 | ADD | ||
| SP600125 10 µM | 0.6 | 0.5 | SYN | ||
| 10 | LY294002 10 µM | 0.8 | 0.8 | ADD | |
| PD 98059 10 µM | 0.8 | 0.7 | SYN | ||
| SP600125 10 µM | 0.6 | 0.6 | ADD | ||
| 15HGG | 2 | LY294002 10 µM | 0.7 | 0.7 | ADD |
| PD 98059 10 µM | 0.6 | 0.6 | ADD | ||
| SP600125 10 µM | 0.3 | 0.3 | ADD | ||
| 10 | LY294002 10 µM | 0.7 | 0.7 | ADD | |
| PD 98059 10 µM | 0.6 | 0.6 | ADD | ||
| SP600125 10 µM | 0.3 | 0.3 | ADD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandru, O.; Sevastre, A.-S.; Castro, J.; Artene, S.-A.; Tache, D.E.; Purcaru, O.S.; Sfredel, V.; Tataranu, L.G.; Dricu, A. Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. Int. J. Mol. Sci. 2019, 20, 4663. https://doi.org/10.3390/ijms20194663
Alexandru O, Sevastre A-S, Castro J, Artene S-A, Tache DE, Purcaru OS, Sfredel V, Tataranu LG, Dricu A. Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. International Journal of Molecular Sciences. 2019; 20(19):4663. https://doi.org/10.3390/ijms20194663
Chicago/Turabian StyleAlexandru, Oana, Ani-Simona Sevastre, Juan Castro, Stefan-Alexandru Artene, Daniela Elise Tache, Oana Stefana Purcaru, Veronica Sfredel, Ligia Gabriela Tataranu, and Anica Dricu. 2019. "Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines" International Journal of Molecular Sciences 20, no. 19: 4663. https://doi.org/10.3390/ijms20194663
APA StyleAlexandru, O., Sevastre, A.-S., Castro, J., Artene, S.-A., Tache, D. E., Purcaru, O. S., Sfredel, V., Tataranu, L. G., & Dricu, A. (2019). Platelet-Derived Growth Factor Receptor and Ionizing Radiation in High Grade Glioma Cell Lines. International Journal of Molecular Sciences, 20(19), 4663. https://doi.org/10.3390/ijms20194663